Abstract
Genetic constructs must be delivered selectively to target tissues and intracellular compartments at the necessary concentrations in order to achieve the maximum therapeutic effect in gene therapy. Development of targeted carriers for non-viral delivery of nucleic acids into cells, including those in muscle, which is one of the most challenging tissues to transfect in vivo, remains a topical issue. We have studied ternary complexes of plasmid DNA and an arginine–histidine-rich peptide-based carrier coated with a glutamate–histidine-rich polymer bearing skeletal muscle targeting peptide (SMTP) for the gene delivery to muscle tissue. The relaxation of the ternary complexes after polyanion treatment was assessed using the ethidium bromide displacement assay. The developed polyplexes were used to transfect C2C12 myoblasts in full-media conditions, followed by analysis of their toxic properties using the Alamar Blue assay and expression analysis of lacZ and GFP reporter genes. After delivering plasmids containing the GFP and lacZ genes into the femoral muscles of mdx mice, which are model of Duchenne muscular dystrophy, GFP fluorescence and β-galactosidase activity were detected. We observed that the modification of ternary polyplexes with 10 mol% of SMTP ligand resulted in a 2.3-fold increase in lacZ gene expression when compared to unmodified control polyplexes in vivo. Thus, we have demonstrated that the developed DNA/carrier complexes and SMTP-modified coating are nontoxic, are stable against polyanion-induced relaxation, and can provide targeted gene delivery to muscle cells and tissues. The results of this study are useful for a range of therapeutic applications, from immunization to amelioration of inherited neuromuscular diseases.
1. Introduction
Gene therapy is a promising approach for treating various diseases by correcting genetic defects or interfering with pathogenic pathways through the introduction of DNA, RNA, or their derivatives into cells. However, the efficiency of transfection with “unprotected” nucleic acids is relatively low, and it is important to deliver genetic constructs selectively to target tissues and cellular compartments in order to achieve maximum therapeutic effects. To date, viral vectors have been widely used for gene delivery, but this approach has several disadvantages, including immunogenicity, risk of accidental integration into the genome, difficulty in large-scale production, and high cost [1]. Non-viral carriers offer an alternative to viral vectors. They are devoid of the above disadvantages, but have a low transfection efficiency in vivo [2]. Peptide-based carriers have been of particular interest due to their biodegradability and homogeneity [3]. These carriers have a structure that can easily combine modules for targeted delivery and overcome barriers to the cellular transport of nucleic acids, making them suitable for creating so-called “artificial viruses” [3,4,5]. Cationic peptide carriers interact electrostatically with negative nucleic acids due to their positive charge, but when these complexes enter the bloodstream, they are quickly bound by opsonins and other plasma proteins, leading to degradation of the genetic cargo. Adding a shielding coating to the complexes makes them inaccessible to plasma proteins and can significantly improve their efficacy in vivo [6]. Similar modifications can also be applied for improving the transfection rate of muscular tissue. Skeletal muscle, due to its unique anatomical, cellular, and physiological characteristics, has established itself as a promising target tissue for gene therapy [7]. Muscle-directed delivery has significant implications not only for the treatment of muscle-related conditions, but also for a range of other therapeutic applications. These include, for example, immunization and the production of protein-based systemic therapeutic agents [8,9]. Skeletal muscles have a dense layer of extracellular matrix that is poorly permeable, accounting for 1 to 10% of muscle mass. As a result, particles cannot be easily distributed from the circulatory system to the muscles. Thus, the prospects for widespread distribution of a non-viral vector through the systemic circulation are currently limited [10]. Therefore, it is essential to explore ways to target the delivery of genetic constructs to muscle tissue, which is considered difficult to transfect due to its mechanically robust extracellular matrix.
The direct injection of nonviral complexes into the muscle tissue can eliminate some of the challenges encountered during systemic administration. Recent studies have shown that the use of the A2G80-R9H8 peptide carrier enhances the efficiency of plasmid DNA delivery to muscle tissue of mdx mice, leading to stable gene expression. This peptide, due to inclusion of a specific ligand, is able to specifically target α-dystroglycan, which is expressed on the surface of muscle cells [11]. Another well-known source of muscle targeting peptide ligands is the phage display technology [12,13]. Recently, PAMAM-dendrimers and liposomes have been successfully modified with the skeletal muscle-targeting peptide ASSLNIA (SMTP) to ensure expression of chicken ovalbumin and microdystrophin genes in muscle tissue in vivo [14,15]. However, the efficacy of the approach based on the intramuscular administration of the complexes is still limited by the clearance by immune cells in the extracellular space of the target tissue and the limited diffusion of the complexes due to their positive charge [10]. The charge of the cationic NA/carrier complexes can be shielded to improve their in vivo biodistribution [16]. The most common approach is modification of the complexes with PEG moieties. It was demonstrated that blood serum has a relatively weak inhibitory effect on the transfection efficiency of PEG-modified complexes [17]. However, despite the fact that PEGylation can significantly increase the circulation half-life of a delivery system, numerous studies have shown a loss of long-term circulatory properties in PEG-modified complexes after repeated intravenous injections due to an accelerated blood clearance phenomenon [18]. Another approach to neutralize the positive charge of NA/carrier complexes is based on the addition of anionic polymers, e.g., glycosaminoglycans, polyglutamic acids, or anionic peptides [19,20,21].
Recently, we developed serum-resistant ternary complexes for DNA delivery to the uterine leiomyoma tissue. This benign tumor is characterized by an excessive growth of the extracellular matrix, which may account for up to 50% of its total weight [22]. Successful expression of suicide and marker genes in the target cells and tissue was achieved by means of a peptide-based DNA delivery system containing bioreducible cystine cross-linked poly-L-glutamic acid, modified with histidine residues for endosome buffering [21].
This study aims to analyze the efficacy of targeted gene delivery to myoblasts and myotubes in vitro and skeletal muscles in vivo using peptide carriers in physiologically relevant conditions. To reach this goal we designed ternary DNA complexes formed with polycondensed arginine–histidine-rich peptides and a polycondensed glutamate–histidine-rich coating modified with the targeting ligand SMTP. We evaluated the properties of three different molecular weight compositions of a glutamate–histidine-rich peptide and an SMTP ligand-conjugated peptide as carriers for targeted gene delivery to muscle cells and tissue.
2. Materials and Methods
2.1. Cell Lines
The murine myoblast C2C12 cell line was obtained from the Institute of Cytology RAS (Saint Petersburg, Russia) and maintained under mycoplasma-free conditions at 37 °C in 5% CO2. The cell line was cultivated in glutamine-supplemented DMEM (Biolot LLC, Saint Petersburg, Russia) with 10% St-Biol heat-inactivated fetal calf serum (Biolot LLC, Saint Petersburg, Russia) and 0.01% gentamicin (Biolot LLC, Saint Petersburg, Russia). C2C12 myoblasts were differentiated to myotubes by three changes of differentiation medium consisting of DMEM with 0.01% gentamicin, 1% insulin, and 2% horse serum (Sigma-Aldrich, St. Louis, MO, USA) to reach 100% confluence. After one week, most of the cells appeared to be phase-bright, fused myotubes.
2.2. Peptide and Peptide-Based Polymer Preparation
CHR6HC (R6), CHHE6HHC (E6H), and ASSLNIAXXCHHE6HHC (SMTP-E6H) peptides were produced by NPF Verta, LLC (Saint Petersburg, Russia) using solid-phase Boc chemistry with a purity of 90–95% determined by high-performance liquid chromatography, and were stored at −20 °C. The R6p, E6Hp, and SMTP-E6Hp peptide-based polymers were synthesized by oxidative polycondensation during 96 h at a 30 mM concentration, with 30% of DMSO, as described previously [21]. The obtained polycondensed R6p carrier, E6Hp and SMTP-E6Hp anionic coating were dissolved in bidistilled water at a concentration of 2 mg/mL and stored at −70 °C. Unreacted thiol groups were determined by Ellman’s assay [23].
2.3. Plasmids Preparation
The pCMV-lacZ plasmid with the β-galactosidase gene under the control of the CMV promoter (kind gift of Prof. B. Scholte, Erasmus University Rotterdam, the Netherlands) and the pEXPR-IBA5-eGFP plasmid with the green fluorescent protein (GFP) gene under the control of the same promoter (IBA GmbH, Göttingen, Germany) were isolated according to Qiagen’s Plasmid Giga kit protocol (Qiagen, Hilden, Germany) and stored at −20 °C.
2.4. Preparation of Ternary Polyplexes
DNA/R6p polyplexes were prepared at a 12 to 1 N/P ratio (peptide nitrogen/DNA phosphorus ratio) in HEPES-buffered mannitol (HBM) (5% (w/v) mannitol, 5 mM HEPES, pH 7.5), and were mixed by vortexing [24]. After 30 min, negatively charged E6Hp or SMTP-E6Hp coating was added to the complexes at various C/P ratios (peptide carboxyl group/DNA phosphorus ratio) and left for 30 min to finalize ternary complex formation.
Turbofect transfection reagent was utilized according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, 4 µL of Turbofect was taken per 1 mg of DNA, mixed by vortexing, and left for 30 min.
2.5. Relaxation of Ternary Polyplexes by Dextran Sulfate
Binding of carrier to DNA and relaxation of ternary polyplexes by polyanions were studied using the ethidium bromide (EtBr) exclusion assay on a Wallac 1420D scanning multilabel counter (PerkinElmer Wallac Oy, Turku, Finland) at 590 nm emission and 540 nm excitation. EtBr exclusion was calculated as (F − Ff)/(Fb − Ff), where Ff and Fb are the EtBr fluorescence values in the absence and presence of DNA [25]. Dextran sulfate (DS; Sigma, St. Louis, MO, USA) was added to the ternary polyplexes at a three-fold charge excess relative to the R6p carrier. At 0 min, 1 h, 3 h, 12 h, and 24 h of incubation, EtBr fluorescence intensity was monitored and the EtBr exclusion was calculated.
2.6. Gene Transfection and Cytotoxicity Assays
A day before the experiment, C2C12 cells were seeded in 48-well plates at a density of 5.0 × 104 cells per well. Polyplexes were prepared at a dose of 2 µg of DNA per well shortly before transfection and added to the cells in serum-supplemented medium within 48 h of incubation, without changing the medium. After pCMV-lacZ plasmid transfection, cells were lysed and β-galactosidase activity (mU) normalized to the total protein concentration was determined on a Wallac 1420D scanning multilabel counter (355 nm excitation, 460 nm emission) and by Bradford reagent (Helicon, Moscow, Russia) on a Multiscan plus P reader with a wavelength of 620 nm [25]. After pEXPR-IBA5-eGFP plasmid transfection, the relative number of GFP-positive cells was determined by flow cytometry with a BD FACS-Canto II cytofluorimeter.
The cytotoxicity of the polyplexes was evaluated in C2C12 cells, which were seeded in a 96-well plate a day before the experiment at a density of 1.5 × 104 cells per well. Polyplexes were prepared at a dose of 0.7 µg of DNA per well. The transfections were carried out in serum-supplemented medium within 24 h of incubation, followed by 3 h of incubation with Alamar Blue reagent (BioSources International, San Diego, CA, USA). The fluorescence was measured on a Wallac 1420D scanning multilabel counter (excitation 544 nm, emission 590 nm) and the relative fluorescence intensity was calculated as previously described [24].
2.7. Size and Zeta-Potential of Ternary Polyplexes
The size and zeta-potential of the ternary polyplexes were determined in HBM buffer using a zetasizer NANO ZS (Malvern instruments, Malvern, UK). The measurements were performed three times independently.
2.8. Cellular Uptake of Ternary Polyplexes
The day before the experiment, C2C12 myoblasts were seeded at a density of 6 × 104 cells/well in 48-well plates, whereas C2C12 myotubes were seeded three days before the transfection. DNA was pre-labeled with YOYO-1 iodide (Thermo Fisher Scientific, Waltham, MA, USA) (1 molecule of the dye per 50 base pairs) prior to the formation of polyplexes. Transfection was carried out in serum-supplemented medium, as described in Section 2.6. After 2 h of incubation, the cells were washed with 1 M NaCl (in 1× PBS; pH 7.2) and twice in 1× PBS. Then, after detachment, cells were incubated with propidium iodide solution (50 µg/mL in 1× PBS) for 15 min and then analyzed by flow cytometry. The results are presented as a relative number of YOYO-1-labeled living cells.
2.9. In Vivo Gene Transfer to Skeletal Muscle Tissue
Ten and fifteen 20-week-old C57BL/10J mdx mice (gifted by Prof. T. Partridge, Hammersmith Hospital, London, UK) were subjected to in vivo delivery of pEXPR-IBA5-eGFP and pCMV-lacZ plasmids, respectively. The mice were housed in an animal facility and supplied with water and the standard diet. Quadriceps femoris muscles of the right hindlimb were opened surgically (2 mm2 patch) under essential anesthesia and repeatedly injected using a Micro-Fine IV Insulin Syringes Needle (Becton Dickinson, Franklin Lakes, NJ, USA) with the polyplexes loaded with the plasmids (5 µg of pEXPR-IBA5-eGFP or 10 µg of pCMV-lacZ in 200 µL of sterile physiological saline) or with naked pDNA (25 µg in 200 µL of saline). The animals were sacrificed by cervical dislocation after a one week interval and quadriceps femoris muscle tissue samples (injected and contralateral) were snap-frozen in liquid nitrogen and processed for subsequent cryostat sectioning or biochemical analysis.
For visualization of GFP fluorescence, 5 µm sections were made using a Leica CM1510 cryostat (Leica Microsystems, Wetzlar, Germany). Sections were photographed under a Leica DM 2500 fluorescent microscope (Leica Microsystems, Wetzlar, Germany) at 100× magnification.
For quantification of β-galactosidase activity, the tissues were homogenized in 160 μL of complete lysis buffer (25 mM Gly–Gly, 15 mM MgSO4, 4 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol (DTT), 1 mM phenylmethanesulfonyl fluoride; pH 7.8). The suspension was then incubated for 40 min at +4 °C and centrifuged for 5 min at 6000 rpm to remove tissue debris [21]. Measurement of β-galactosidase activity was carried out as described in Section 2.6.
2.10. Statistical Analysis
Statistically significant differences were analyzed by applying Student’s t-test and one-way ANOVA with Tukey’s test for multiple comparisons (using GraphPad Prism 8 v. 8.02; GraphPad Software Inc., San Diego, CA, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Design of Carriers
Herein, we utilized an arginine–histidine-rich peptide-based oligomer, R6p, synthesized by oxidative polycondensation as cationic moiety to formulate complexes with plasmid DNA. Previously, we have demonstrated significant transfection efficiency mediated by this carrier in serum-free conditions [26]. However, to achieve successful transfection in physiologically relevant conditions we applied electrostatic stabilization of the polyplexes by anionic coating composed of glutamate-rich cysteine-flanked polycondensed peptides [21]. The addition of histidine to the peptide composition is expected to further enhance endosomal buffering [27]. Targeted delivery to muscle cells is expected through modification with the SMTP ligand [12]. We gradually increased the ratio of the targeting moiety in the composition of the polyplexes by non-covalent mixing with SMTP ligand-conjugated oligomers from 10 mol% to 100 mol%. The carriers’ design is summarized in Table 1.

Table 1.
The formulas of monomers and carriers. X—ε-aminocaproic acid.
3.2. Study of Polyplex Relaxation by Polyanions
Electrostatic interactions between the negatively charged phosphate groups of DNA and positively charged cationic peptides result in the formation of complexes that protect DNA from nuclease-mediated degradation [28]. However, serum contains negatively charged proteins and other anionic components, which can compete with DNA and thus relax polyplexes. Similarly, cellular membrane-associated glycosaminoglycans (GAGs) can induce dissociation of DNA from the carrier, which reduces transfection efficiency [29]. To address this issue, we have developed carriers that include both cationic and anionic components. The anionic component serves to shield the positive charge and stabilize the complex in the presence of the serum components and GAGs. Additionally, modification of the carrier molecules with the SMTP ligand could alter the structure of the polyplexes, potentially affecting transfection efficiency. Therefore, we aimed to investigate whether the inclusion of the ligand affects the formation of a polyplex and its stability in the presence of polyanions.
To accomplish this, we evaluated the carriers’ capacity to compact plasmid DNA and maintain the stability of the resulting complexes in the presence of polyanions. We performed tests using the ethidium bromide (EtBr) exclusion method with the ternary complexes formed at different P/N/C (phosphate/nitrogen/carboxyl) ratios ranging from 1/12/0 to 1/12/24. The ligand was included to the shielding moiety at 10 mol%, 50 mol%, and 100 mol% (Figure 1). In order to simulate the presence of negatively charged components, we added dextran sulfate (DS) to the polyplexes. The fluorescence level of EtBr dye was taken as 100% when binding to DNA without the addition of a carrier. It should be noted that without relaxation only a small amount of DNA in the polyplexes is available for EtBr binding, resulting in a low level of relative fluorescence intensity.
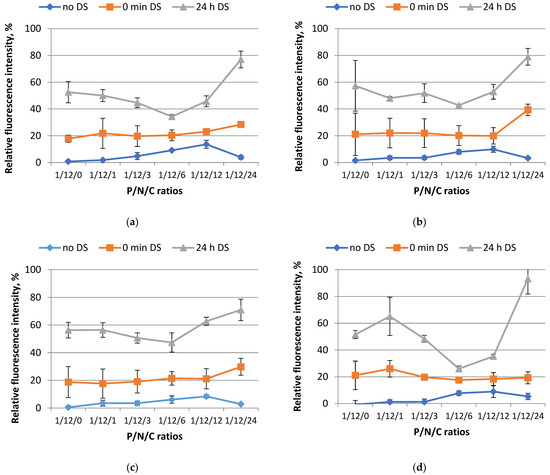
Figure 1.
The relative fluorescence intensity of ethidium bromide when binding to DNA at different charge ratios of DNA/R6p complexes and anionic coating before the addition of dextran sulfate (no DS), after the addition of DS (0 min DS), and 24 h after the addition of DS (24 h DS). (a) DNA/R6p/SMTP-E6Hp-10%, (b) DNA/R6p/SMTP-E6Hp-50%, (c) DNA/R6p/SMTP-E6Hp-100%, and (d) DNA/R6p/E6Hp polyplexes were tested. Values are the mean ± SD of n = 9 individual samples from three independent experiments.
According to the data presented in Figure 1, the R6p carrier in combination with E6pH and ligand-modified E6pH coatings, as well as their respective combinations (10 mol% and 50 mol% of SMTP-modified polymer), successfully bind and condense plasmid DNA. These findings are consistent with our previous research and the results of Jativa et al., who used a polyamidoamine dendrimer modified with the SMTP ligand and did not observe an effect of the ligand on the ability of the carrier to form DNA complexes [14,21].
The addition of DS results in a slight reduction in the degree of compaction of the complexes (Figure 1). However, at this point, there are no significant differences in the DNA compaction in the polyplexes with different SMTP contents for the ratios ranging from 1/12/1 to 1/12/12. In contrast, at a ratio of 1/12/24, there appears to be a tendency for the relaxation of ligand-modified polyplexes after DS treatment.
We also investigated the relaxation rate of the complexes after 24 h of DS treatment in order to examine the impact of prolonged exposure to polyanions on the stability of the ternary complexes. The results obtained suggest that there was no significant decrease in the DNA condensation properties of the R6p carrier as the amount of SMTP ligand in the anionic coating was increased (Figure 1). For all four complexes examined in this study, a general pattern of behavior can be observed. Specifically, the lowest levels of free DNA are found at a charge ratio of 1/12/6. Subsequently, further addition of anionic coating leads to significant destabilization of the polyplexes at a charge ratio of 1/12/24.
To summarize, based on the results of this experiment, we can conclude that the modification of the peptide-based anionic coating by the SMTP ligand does not significantly affect the stability of the resulting DNA complexes in the presence of polyanions. Charge ratios of 1/12/6 and 1/12/12 were selected for further investigation.
3.3. Evaluation of Ternary Polyplex Cytotoxicity
The broader usefulness of cationic carriers for in vivo delivery is constrained by low gene expression levels and toxicity. Intravascular administration, a promising route for systemic delivery, is especially hampered by these challenging issues [2]. Reduced transfection efficacy in vivo is partly due to the interaction of polyplexes or lipoplexes with blood components, such as serum proteins, which hinder transfection [6]. Furthermore, intravenously injected cationic DNA complexes also interact unspecifically with various cell types, including macrophages, which are potential mediators of toxicity [30]. Cytoxicity of polyplexes also greatly depends on their charge density, mostly because positively charged nanoparticles can neutralize the negative charge of cellular membrane components [31].
We studied the cytotoxic properties of ligand-modified polyplexes at different compositions and charge ratios, using a resazurin-based viability assay with Alamar Blue dye (Figure 2). Murine myoblasts, C2C12, were used as cellular model of muscle cells.
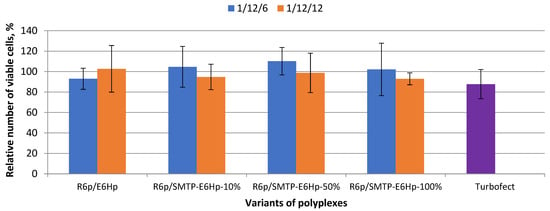
Figure 2.
The relative fluorescence intensity of ethidium bromide when binding to DNA at different charge ratios of DNA/R6p complexes and anionic coating before the addition of dextran sulfate (no DS), after the addition of DS (0 min DS), and 24 h after the addition of DS (24 h DS). Values are the mean ± SD of n = 12 individual samples from three independent experiments.
It can be seen in Figure 2 that the modification of the ternary polyplexes by the SMTP ligand at the all tested molar ratios does not significantly increase the cytotoxicity level. According to the data obtained, more than 90% of the cells survive after transfection with the DNA complexes formed at 1/12/6 and 1/12/12 ratios. Thus, we concluded that all the tested formulations do not exhibit cytotoxicity towards C2C12 cells.
3.4. Evaluation of Transfectional Properties of Ternary Polyplexes In Vitro
The relationship between varying concentrations of the SMTP ligand (10, 50, and 100 mol%) and the transfection efficiency of the ternary complexes was studied in C2C12 cells using the lacZ reporter gene. The level of β-galactosidase activity was measured to assess the transfection properties of the polyplexes at optimal P/N/C ratios. All transfections were performed in fully supplemented media. As previously reported, the addition of 10% fetal bovine serum (FBS) completely abolished the transfection activity of the DNA/R6p complex in the absence of a protective anionic coating. Here, we demonstrated that the application of DNA/R6p/E6Hp polyplexes with charge ratios of 1/12/6 and 1/12/12 resulted in a high level of transfection comparable to that of control DNA/Turbofect complexes (Figure 3a).
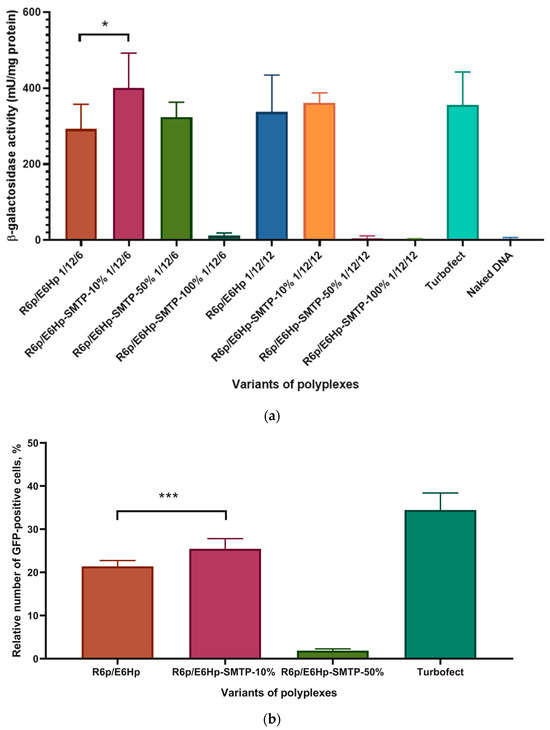
Figure 3.
Transfection efficiency evaluation in the C2C12 cells: (a) the cells were transfected in serum-supplemented medium with the ternary polyplexes at charge ratios of 1/12/6 and 1/12/12 bearing the lacZ-encoding plasmid, with Turbofect/DNA polyplexes, and with naked plasmid DNA; (b) the cells were transfected in serum-supplemented medium with R6p/SMTP-E6Hp-10% and R6p/E6Hp polyplexes formed at a 1/12/6 charge ratio bearing the GFP-encoding plasmid, and with Turbofect/DNA polyplexes. Values are the mean ± SD of n = 9 individual samples from three independent experiments. * p < 0.05, *** p < 0.001—compared to ligand-modified polyplex.
The modification of polyplexes with the SMTP ligand has an ambivalent effect on the transfection efficiency. As can be seen in Figure 3a, the transfection efficiency of the SMTP-modified polyplexes correlates inversely with the ligand content and charge ratio. Following transfection of C2C12 cells with R6p/SMTP-E6Hp-50% and R6p/SMTP-E6Hp-100% polyplexes, at a higher charge ratio of 1/12/12, a very low level of β-galactosidase activity was detected. A decrease in the amount of ligand-modified polymer in the polyplex composition resulted in an increase in the transfectional efficiency of the less-modified polyplexes. A significant difference in the transfection efficiencies was observed between R6p/E6Hp and R6p/SMTP-E6Hp-10% polyplexes formed at a charge ratio of 1/12/6 (Figure 3a). Therefore, we conclude that the addition of the SMTP ligand to ternary polyplexes at a low concentration can enhance their targeting properties toward myoblasts in vitro.
To confirm the results obtained, we performed transfection using a GFP-encoding plasmid. We used the most efficient R6p/E6Hp, R6p/SMTP-E6Hp-10%, and R6p/SMTP-E6Hp-50% polyplexes, formed at a 1/12/6 charge ratio, for the delivery of an alternate reporter gene into C2C12 cells. The results from the GFP gene transfection are presented in Figure 3b. It should be noted that we did not observe a high level of transfection when using R6p/SMTP-E6Hp-50%. This finding contradicts the data from our previous experiment. Nevertheless, we found a highly significant difference between the polyplexes coated with unmodified E6Hp and the ligand-modified polymer containing 10 mol% of the SMTP-E6Hp. Therefore, we can infer that a low concentration of the SMTP ligand in the ternary complexes is adequate for achieving myoblast-specific gene transfer in vitro. It can be hypothesized that a lower content of the SMTP ligand may result in a low ligand density in the developed nanoparticles, whereas a high ligand density would be preferable due to a higher probability of cellular binding. However, some previous studies have demonstrated that the optimal ligand density for targeted nanoparticles may be significantly lower than 100% [32]. For example, Wang et al. showed that increasing the transferrin density on polymeric nanoparticles from 0% to 25% led to an increase in cellular uptake, which plateaued thereafter, and no further increase was observed at a ligand density of 100% [33]. It can be supposed that steric interference from closely packed SMTP ligands may hinder receptor binding of the ligand-decorated polyplexes.
In order to elucidate the cause of transfection efficiency reduction induced by the SMTP-E6Hp coating at concentrations of 50 and 100 mol%, we determined the size and zeta-potential of the ternary polyplexes. These important parameters can, to some extent, determine the ways in which polyplexes enter cells [34].
Previously, we have shown that DNA/R6p polyplexes formed at a N/P ratio of 12/1 without an anionic component have a compact size of 110.3 ± 9.12 nm and a positive value of the zeta-potential of 35.5 ± 0.81 mV [35]. In this study, the DNA/R6p complexes coated with E6Hp and SMTP-E6Hp polymers showed a larger size, ranging from 359 to 2420 nm (Figure 4a). The polydispersity index (PDI) varied over a wide range, from 0.157 to 0.561. The highest size and PDI values were observed almost exclusively in cases where the polyplex contained 100 mol% of the SMTP-E6Hp polymer. The zeta potential values, as shown in Figure 4b, indicated a neutral or negative surface charge for the ternary polyplexes. It was demonstrated that all polyplexes formed with a N/P/C ratio of 1/12/6 had low zeta-potential values, while those with a ratio of 1/12/12 were negatively charged. Based on the results obtained, we found no significant difference between size and zeta-potential values of the most transfectionally active R6p/SMTP-E6Hp-10% polyplexes and control R6p/E6Hp-polyplexes formed at a charge ratio of 1/12/6. Therefore, we can deduce that the high transfection efficiency observed for the ternary polyplexes modified with 10 mol% SMTP ligand is not dependent on the size and charge characteristics, but rather is primarily attributed to the ligand modification. On the other hand, it may be proposed that the reduced activity of the other variants of polyplexes is a consequence of aggregation of the polyplexes decorated with SMTP, due to the relatively strong hydrophobicity of the ASSLNIA ligand and the spacer.
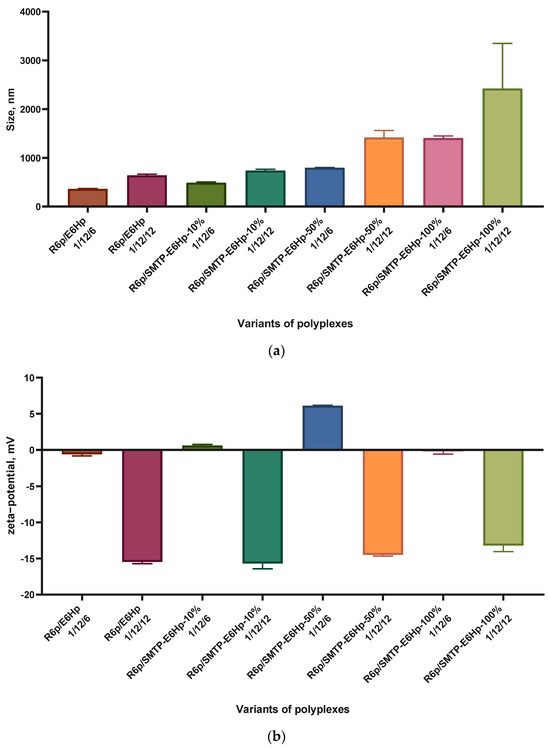
Figure 4.
Size (a) and zeta-potential (b) of the ternary polyplexes. The data are shown as the mean ± SD of n = 3 individual samples from three independent experiments.
3.5. Evaluation of Cellular Uptake of Ternary Polyplexes
It is important to note that the ASSLNIA ligand was initially identified using phage display on myotubes derived from C2C12 myoblasts [12]. Furthermore, when this sequence was incorporated into the AAV-2 capsid protein, an enhancement in transfection efficiency was observed only in myotube cultures, but not in myoblast cultures [36]. Therefore, prior to conducting experiments in vivo, it was essential to evaluate how DNA complexes formed with the SMTP ligand-modified carriers would be taken up by differentiated muscle cells.
To evaluate the cellular uptake rate, we used ternary complexes formed at both optimal charge ratios with YOYO-1-labeled DNA. An increase in the relative number of fluorescently labeled cells indicated successful uptake of the developed complexes. The cellular uptake rate was compared between C2C12 myoblasts and differentiated myotubes. As can be seen in Figure 5, a significant difference in the cellular uptake of the polyplexes formed at a 1/12/6 charge ratio was demonstrated. According to the data presented, these complexes were more efficiently taken up by myotubes. A similar trend was observed for complexes formed at a 1/12/12 charge ratio, but the overall level of cellular uptake was lower. The only exception to this relationship were R6p/SMTP-E6Hp-10% complexes formed at a 1/12/6 ratio, which had an equal uptake rate in both differentiated and undifferentiated muscle cells. It should be noted that the findings presented here are generally consistent with the results obtained from transfection experiments (Figure 3). Based on these results, it can be hypothesized that R6p/SMTP-E6Hp-10% polyplexes at a 1/12/6 ratio represent the optimal formulation for targeted and efficient delivery of pDNA to myoblasts and myotubes in vitro. The improved performance of SMTP-mediated targeting observed in myotubes may be attributed to the fact that this ligand was generated through phage display on differentiated cells [12].
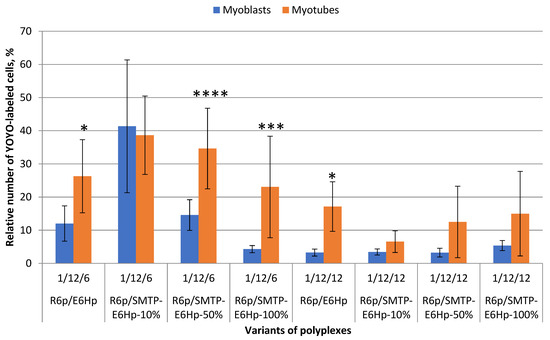
Figure 5.
Evaluation of the cellular uptake rate of the ternary polyplexes at charge ratios of 1/12/6 and 1/12/12 bearing YOYO-1-labeled plasmids in C2C12 myoblasts and C2C12-derived myotubes. Values are the mean ± SD of n = 9 individual samples from three independent experiments. * p < 0.05, *** p < 0.001, **** p < 0.0001—compared to the myoblasts’ uptake rate.
3.6. Evaluation of Transfectional Properties of Ternary Polyplexes In Vivo
With the aim to evaluate the efficiency of DNA delivery into muscle tissue in vivo using the developed delivery systems, we employed qualitative and quantitative techniques, specifically, analysis of green fluorescent protein (GFP) fluorescence on histological sections and measurement of β-galactosidase activity in muscle samples. C57BL/10J mdx mice were selected for the in vivo study as an animal model for a severe inherited neuromuscular disorder, Duchenne muscular dystrophy (DMD) [37]. If successful, the delivery system under investigation could be utilized for the development of gene therapy approaches for DMD.
The presence of GFP-expressing muscle fibers was directly assessed on histological sections following delivery of the ternary polyplexes bearing 5 µg of GFP-encoding plasmid, which were injected into the femoral muscles of mdx mice. Naked pDNA injection and Turbofect/DNA polyplexes served as controls. The muscle sections were 5 microns in thickness.
When analyzing muscle samples obtained from intact murine tissues, a small level of fluorescence was observed along the periphery of myofibrils, which was attributed to autofluorescence and was interpreted as a lack of GFP expression (Figure 6a). After delivery of GFP-encoding plasmid using Turbofect polyplexes, we also noticed a similar peripheral staining of myofibrils but no bright specific GFP fluorescence was found (Figure 6b). Muscle fibers with bright and distinct GFP fluorescence were observed in muscle sections following transfection using R6p/E6Hp ternary complexes formed at a 1/12/6 ratio. However, the number of GFP-positive fibers was low and did not exceed three per field of view (Figure 6c). A significantly higher number of GFP-positive muscle fibers was observed when using R6p/SMTP-E6Hp-10% polyplexes with the same charge ratio. Within the field of view, 4 to 10 stained fibers were observed, whereas for the control Turbofect polyplexes, no stained myofibrils were detectable, indicating the increased transfectional efficiency of the ternary complexes modified with the ASSLNIA ligand (Figure 6d). Injections of naked plasmid DNA were used as an additional positive control in in vivo transfection experiments, based on previously obtained results from skeletal muscle transfections [38]. However, during the preliminary stages of the experiment, it was observed that injection of 5 μg of plasmid did not result in the formation of GFP-positive fibers. Consequently, the plasmid dose was increased to 25 μg. According to Figure 6e, between 1 and 5 GFP-positive fibers were observed in the muscle sections following plasmid injections. Therefore, we have concluded that the modification of ternary polyplexes with the SMTP ligand results in a significant increase in transfection efficiency.
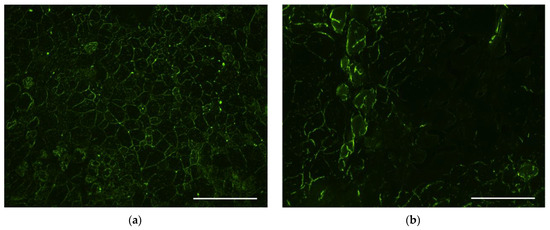
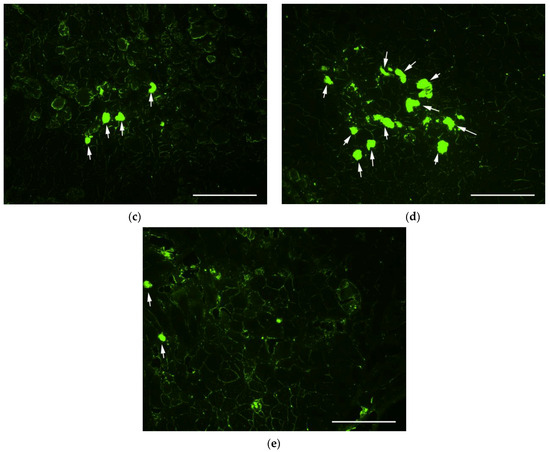
Figure 6.
Qualitative transfection efficiency evaluation in C57BL/10J mdx mice—(a) intact animals; m. quadriceps femoris was injected with (b) Turbofect polyplexes; (c) R6p/E6Hp polyplexes at a charge ratio of 1/12/6; (d) R6p/SMTP-E6Hp-10% polyplexes at a charge ratio of 1/12/6, bearing GFP-encoding plasmids (5 µg per injection); (e) naked GFP-encoding plasmid (25 µg per injection). Magnification is ×100. Arrows indicate GFP-positive myofibers. Scale bars are 100 µm. In total, ten animals were studied; n = 2 individual samples per group.
Next, we analyzed the efficiency of lacZ gene delivery by quantifying β-galactosidase activity in skeletal muscle samples following transfection mediated by the ternary polyplexes.
Based on the results of the quantitative analysis of beta-galactosidase activity following the injection of a plasmid encoding LacZ presented in Figure 7, we found no significant differences in the level of transfection of Turbofect polyplexes, which is consistent with the results of the qualitative analysis (see Figure 6b). Given that the Turbofect carrier was designed for use in vitro, we hypothesize that no activity would be expected in vivo. Additionally, we observed no significant difference in the transfection level between the injected (right) and uninjected (left) limbs after administration of R6p/E6Hp polyplexes (Figure 7). Therefore, it can be inferred that in vitro findings regarding the transfection activity of these polyplexes may not directly translate to in vivo conditions. Multiple factors may influence the transfection process within the body, and it is possible that the addition of the E6Hp coating to the ternary polyplex formulation at the specified charge ratio may not be sufficient to effectively penetrate muscle tissue. It is worth noting that recently studied ternary polyplexes R6p/E6p, when used at a charge ratio of 1/12/12, were found to be effective for gene transfection into uterine leiomyoma tissue, while they were not effective at a ratio of 1/12/6 [21]. Similarly, we observed only a tendency towards an increase in β-galactosidase activity following the injection of naked plasmid DNA (Figure 7). This may be due to the relatively high level of intrinsic β-galactosidase activity in the muscle, which may prevent the detection of low levels of transfection.
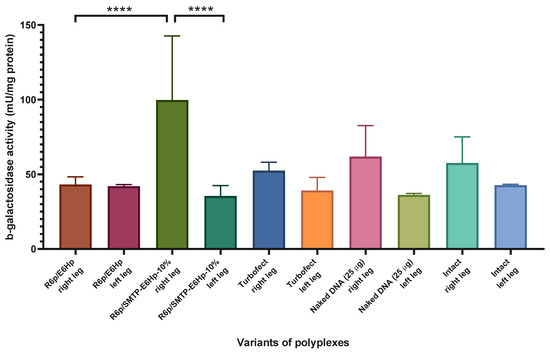
Figure 7.
Quantitative transfection efficiency evaluation in C57BL/10J mdx mice—m. quadriceps femoris (right leg; left leg served as intact control) were injected with Turbofect polyplexes, R6p/E6Hp and R6p/SMTP-E6Hp-10% polyplexes at a charge ratio of 1/12/6, bearing the GFP-encoding plasmid (10 µg per injection). Naked GFP-encoding plasmid was injected in dose of 25 µg per leg. Values are the mean ± SD of n = 3 individual samples per group. **** p < 0.0001—compared to the β-galactosidase activity in the intact left leg and compared to the β-galactosidase activity after R6p/E6Hp-mediated transfection.
We have observed a significant statistical difference in the activity of beta-galactosidase between mice that were intact and those that received intramuscular administration of DNA/R6p/SMTP-E6Hp-10% polyplexes. The level of transfection mediated by this delivery system exceeded the baseline level by more than twofold (Figure 7). Also, we found that the SMTP ligand modification of these polyplexes resulted in a 2.3-fold increase in lacZ gene expression when compared to unmodified R6p/E6Hp polyplexes. Thus, it can be concluded that the modification of ternary polyplexes with an SMTP ligand is a key factor in improving their transfection efficiency in vivo.
4. Discussion
Despite recent advances in the field of DNA delivery, the challenge of targeting specific tissues and cell types while avoiding non-specific delivery, particularly to muscle tissue, continues to be a significant obstacle. One key strategy for developing carriers that can address this challenge is the development of muscle-specific delivery systems. Examples of muscle-targeting peptides include the heptapeptide sequence ASSLNIA, which has been shown to improve specificity for binding to skeletal muscle through screening of a random phage display library [12]. More recently, it has been demonstrated that the G5-PAMAM dendrimer modified with ASSLNIA can enhance skeletal muscle gene delivery [14]. Additionally, previous research has successfully applied ASSLNIA to improve the tropism of adeno-associated viral vectors for skeletal muscle by using ligand-directed gene delivery techniques [36].
Another significant barrier is the susceptibility of DNA polyplexes to the anionic components of blood serum, which can significantly reduce their activity in vivo. After in vivo gene delivery, both serum proteins and the extracellular matrix have the ability to disrupt the nucleic acid and carrier complex [39]. The charge of cationic polyplexes can effectively be neutralized to enhance their biodistribution in vivo [16]. For this purpose, several approaches can be employed. The most common method is the chemical modification of the carrier with a hydrophilic, non-ionic polymer—poly(ethylene glycol) (PEG). For instance, modification of a peptide-based carrier, CH2R4H2C, with methoxy-PEG allowed successful systemic delivery of anti-NF-κB siRNA [40]. Another method to neutralize cationic polyplexes is to create ternary complexes by adding anionic polymers, such as glycosaminoglycans or polyglutamic acid, etc. It has been demonstrated that intravenous injection of polylysine-based polyplexes coated with polyglutamic acid into mice exhibited a high transfection efficiency and a low hepatic toxicity [41]. Recently, we have developed serum-resistant ternary DNA complexes coated with crosslinked poly-L-glutamic acid [21]. In this study, we successfully modified this anionic polymer to achieve muscle-specific delivery and demonstrate resistance of the developed polyplexes to anionic components.
In the first stage of the study, we simulated physiological conditions by adding dextran sulfate to ternary complexes (Figure 1). We have demonstrated that all complexes can withstand polyanionic treatment at optimal charge ratios and maintain colloidal stability. The cytotoxicity study also did not reveal any significant differences between cells transfected with the developed complexes and control cells, allowing us to continue using optimal charge ratios for the formulation of ternary polyplexes (Figure 2).
The developed polyplexes were applied for targeted delivery of reporter genes to a C2C12 myoblast culture (Figure 3). Based on the obtained results, the most effective formulation was identified. It is worth noting that only a low modification of ternary polyplexes with 10 mol% SMTP ligand led to successful and specific DNA delivery to muscle cells in vitro. Highly ligand-decorated complexes demonstrated an activity similar to that of unmodified control polyplexes. A possible explanation for these findings was obtained after measuring the size, which revealed a significant increase in the size of polyplexes modified with 50 and 100 mol% SMTP ligand (Figure 4a). Zeta potential measurements revealed that the most transfectionally active polyplexes had a neutral surface charge (Figure 4b). Previously, we demonstrated that certain formulations of peptide-based polyplexes coated with polyglutamic acid or anionic magnetic nanoparticles were transfectionally active at low zeta-potentials and large sizes [21,42].
The evaluation of the cellular uptake rate of ternary polyplexes in differentiated and undifferentiated C2C12 cells revealed a tendency towards better complex accumulation in myotubes compared to myoblasts. This finding may be advantageous for in vivo applications of the developed polyplexes, as it was to some extent expected, given that the ASSLNIA peptide was derived from phage display on differentiated C2C12 myotubes [12]. In vivo transfection efficacy evaluation was conducted both qualitatively and quantitatively using a mouse model of Duchenne muscular dystrophy (Figure 6 and Figure 7). The obtained results allow us to conclude that there is a beneficial effect of the SMTP ligand modification on the transfection efficacy of peptide-based polyplexes. Recent work on SMTP-mediated non-viral gene delivery into mdx muscle tissue has focused on the use of G5-PAMAM dendrimers modified with the ASSLNIA ligand [15]. The developed cationic DNA/dendrimer complexes enabled targeted delivery of the microdystrophin gene into skeletal muscle cells, although the achieved level of protein production was relatively low. A significant finding of this study is the demonstration of successful gene delivery into muscle tissue using neutrally charged polyplexes. Previously, it has been demonstrated that the delivery of naked DNA into muscles can be somewhat efficient due to its negative charge, which allows it to better penetrate the thick extracellular matrix of muscle fibers [38]. Furthermore, the use of electrically neutral polymers can significantly enhance DNA delivery to muscle tissue [43]. Therefore, based on this information, we can conclude that a peptide-based carrier modified by the ASSLNIA ligand, which is capable of forming neutrally charged nanoparticles with plasmid DNA, has the potential to be used for the targeted delivery of therapeutic genetic constructs into skeletal muscle.
5. Conclusions
As a result of our research, we have been able to demonstrate that the peptide carriers we developed are non-toxic and effectively deliver DNA to muscle cells, both in vitro and in an animal model. The R6p/SMTP-E6Hp-10% peptide complex was found to be the most efficient, with a ratio of 1/12/6, due to the inclusion of the muscle-targeting ASSLNIA ligand in its composition. We have shown that modifying the anionic peptide coating with this ligand enables transfection under physiological conditions, and provides an increased uptake efficiency of DNA into both dividing and differentiated muscle cells.
Author Contributions
Conceptualization, I.K. and A.K.; methodology, A.E.; formal analysis, A.E. and A.K.; investigation, A.E. and S.C.; resources, S.S.; writing—original draft preparation, A.E. and A.K.; writing—review and editing, A.K.; supervision, A.K. and A.E.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted as part of the fundamental research topic “Optimization of methods for prediction, prevention, and treatment of the “great obstetrical syndromes” and delivery strategies in high-risk pregnant women to improve obstetrical and perinatal outcomes” for the period 2022–2024 (state registration number: 1021062812133-0-3.2.2.2) funded by the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committee of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology (protocol 114 from 14 December 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors on request.
Acknowledgments
The authors express their gratitude to Nadezhda Krylova for excellent technical assistance and to Sofia Shtykalova for size and zeta-potential measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Butt, M.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.; Hasan, M.; Khan, Y.; Hafeez, S.; Massoud, E.; Rahman, M.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Shtykalova, S.; Deviatkin, D.; Freund, S.; Egorova, A.; Kiselev, A. Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications. Life 2023, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Xia, Z.L.; Ze, L.J.; Jing, H.; Xin, B.; Fu, S. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed. Microdevices 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; van der Aa, M.A.E.M.; Hennink, W.E.; Crommelin, D.J.A. Artificial viruses: A nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 2006, 5, 115–121. [Google Scholar] [CrossRef]
- A Egorova, A.; V Kiselev, A. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr. Top. Med. Chem. 2015, 16, 330–342. [Google Scholar] [CrossRef]
- McErlean, E.M.; McCrudden, C.M.; McCarthy, H.O. Delivery of Nucleic Acids for Cancer Gene therapy: Overcoming extra- and intra-cellular Barriers. Ther. Deliv. 2016, 7, 619–637. [Google Scholar] [CrossRef]
- Lu, Q.L.; Bou-Gharios, G.; Partridge, T.A. Non-viral gene delivery in skeletal muscle: A protein factory. Gene Ther. 2003, 10, 131–142. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Ke, H.; Guay, K.P.; Flotte, T.R.; Gierasch, L.M.; Gershenson, A.; Hebert, D.N. Secretion of functional α1-antitrypsin is cell type dependent: Implications for intramuscular delivery for gene therapy. Proc. Natl. Acad. Sci. USA 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Duan, D.; Mendell, J.R. (Eds.) Muscle Gene Therapy, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–811. [Google Scholar] [CrossRef]
- Nirasawa, K.; Hamada, K.; Naraki, Y.; Kikkawa, Y.; Sasaki, E.; Endo-Takahashi, Y.; Hamano, N.; Katagiri, F.; Nomizu, M.; Negishi, Y. Development of A2G80 peptide-gene complex for targeted delivery to muscle cells. J. Control. Release 2021, 329, 988–996. [Google Scholar] [CrossRef]
- Samoylova, T.I.; Smith, B.F. Elucidation of muscle-binding peptides by phage display screening. Muscle Nerve 1999, 22, 460–466. [Google Scholar] [CrossRef]
- Seow, Y.; Yin, H.; Wood, M.J.A. Identification of a novel muscle targeting peptide in mdx mice. Peptides 2010, 31, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Jativa, S.D.; Thapar, N.; Broyles, D.; Dikici, E.; Daftarian, P.; Jiménez, J.J.; Daunert, S.; Deo, S.K. Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharm. 2019, 16, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Hersh, J.; Condor Capcha, J.M.; Iansen Irion, C.; Lambert, G.; Noguera, M.; Singh, M.; Kaur, A.; Dikici, E.; Jiménez, J.J.; Shehadeh, L.A.; et al. Peptide-Functionalized Dendrimer Nanocarriers for Targeted Microdystrophin Gene Delivery. Pharmaceutics 2021, 13, 2159. [Google Scholar] [CrossRef]
- Harris, T.J.; Green, J.J.; Fung, P.W.; Langer, R.; Anderson, D.G.; Bhatia, S.N. Tissue-specific gene delivery via nanoparticle coating. Biomaterials 2010, 31, 998–1006. [Google Scholar] [CrossRef]
- Tang, G.P.; Zeng, J.M.; Gao, S.J.; Ma, Y.X.; Shi, L.; Li, Y.; Too, H.-P.; Wang, S. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: Effects of PEGylation extent. Biomaterials 2003, 24, 2351–2362. [Google Scholar] [CrossRef]
- Shimizu, T.; Lila, A.S.A.; Kitayama, Y.; Abe, R.; Takata, H.; Ando, H.; Ishima, Y.; Ishida, T. Peritoneal B Cells Play a Role in the Production of Anti-polyethylene Glycol (PEG) IgM against Intravenously Injected siRNA-PEGylated Liposome Complexes. Biol. Pharm. Bull. 2024, 47, b23-00733. [Google Scholar] [CrossRef]
- Iwanaga, M.; Kodama, Y.; Muro, T.; Nakagawa, H.; Kurosaki, T.; Sato, K.; Nakamura, T.; Kitahara, T.; Sasaki, H. Biocompatible complex coated with glycosaminoglycan for gene delivery. J. Drug Target. 2017, 25, 370–378. [Google Scholar] [CrossRef]
- Kodama, Y.; Tokunaga, A.; Hashizume, J.; Nakagawa, H.; Harasawa, H.; Kurosaki, T.; Nakamura, T.; Nishida, K.; Nakashima, M.; Hashida, M.; et al. Evaluation of transgene expression characteristics and DNA vaccination against melanoma metastasis of an intravenously injected ternary complex with biodegradable dendrigraft poly-L-lysine in mice. Drug Deliv. 2021, 28, 542–549. [Google Scholar] [CrossRef]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Freund, S.; Selutin, A.; Shved, N.; Selkov, S.; Kiselev, A. Serum-Resistant Ternary DNA Polyplexes for Suicide Gene Therapy of Uterine Leiomyoma. Int. J. Mol. Sci. 2023, 25, 34. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Aitken, A.; Learmonth, M. Estimation of Disulfide Bonds Using Ellman’s Reagent. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1996; pp. 1053–1055. ISBN 978-1-60327-259-9. [Google Scholar]
- Kiselev, A.; Egorova, A.; Laukkanen, A.; Baranov, V.; Urtti, A. Characterization of reducible peptide oligomers as carriers for gene delivery. Int. J. Pharm. 2013, 441, 736–747. [Google Scholar] [CrossRef]
- Egorova, A.; Kiselev, A.; Hakli, M.; Ruponen, M.; Baranov, V.; Urtti, A. Chemokine-derived peptides as carriers for gene delivery to CXCR4 expressing cells. J. Gene Med. 2009, 11, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Kiselev, A. Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells. Molecules 2022, 27, 8363. [Google Scholar] [CrossRef]
- Midoux, P.; Monsigny, M. Efficient Gene Transfer by Histidylated Polylysine/pDNA Complexes. Bioconjug. Chem. 1999, 10, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anchordoquy, T. Drug Delivery Trends in Clinical Trials and Translational Medicine: Challenges and Opportunities in the Delivery of Nucleic Acid-Based Therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef]
- Wang, T.; Upponi, J.R.; Torchilin, V.P. Design of multifunctional non-viral gene vectors to overcome physiological barriers: Dilemmas and strategies. Int. J. Pharm. 2012, 427, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; DeSimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Selutin, A.; Shved, N.; Deviatkin, D.; Selkov, S.; Baranov, V.; Kiselev, A. Polycondensed Peptide Carriers Modified with Cyclic RGD Ligand for Targeted Suicide Gene Delivery to Uterine Fibroid Cells. Int. J. Mol. Sci. 2022, 23, 1164. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Yuan, Z.; Cao, Z.; Wang, B.; Qiao, C.; Li, J.; Xiao, X. A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 2009, 16, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The Molecular Basis of Muscular Dystrophy in the mdx Mouse: A Point Mutation. Science 1989, 244, 1578–1580. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle In Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Mechtler, K.; Szoka, F.C.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Owada, M.; Iwaya, K.; Takashima, Y.; Seta, Y. Anti-Metastatic Effects on Melanoma via Intravenous Administration of Anti-NF-κB siRNA Complexed with Functional Peptide-Modified Nano-Micelles. Pharmaceutics 2020, 12, 64. [Google Scholar] [CrossRef]
- Kodama, Y.; Nakamura, T.; Kurosaki, T.; Egashira, K.; Mine, T.; Nakagawa, H.; Muro, T.; Kitahara, T.; Higuchi, N.; Sasaki, H. Biodegradable nanoparticles composed of dendrigraft poly-l-lysine for gene delivery. Eur. J. Pharm. Biopharm. 2014, 87, 472–479. [Google Scholar] [CrossRef]
- Shtykalova, S.; Egorova, A.; Maretina, M.; Baranov, V.; Kiselev, A. Magnetic Nanoparticles as a Component of Peptide-Based DNA Delivery System for Suicide Gene Therapy of Uterine Leiomyoma. Bioengineering 2022, 9, 112. [Google Scholar] [CrossRef]
- Cui, Z.; Jiao, Y.; Pu, L.; Tang, J.Z.; Wang, G. The Progress of Non-Viral Materials and Methods for Gene Delivery to Skeletal Muscle. Pharmaceutics 2022, 14, 2428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).