Abstract
Chronic obstructive pulmonary disease (COPD), characterised by persistent airflow limitation during breathing, is considered to be the third leading cause of death worldwide. Among the mechanisms involved in this pathology is the excessive generation of neutrophil extracellular traps (NETs), which can induce an unwanted inflammatory response. These traps have been reported to be generated by the enzyme peptidyl arginine deiminase 4 (PAD4). The aim of this work is therefore to evaluate the effect of the administration of a siRNA targeting PAD4 on lung damage in a COPD animal model. Wistar rats weighing 300–350 g were administered cadmium chloride (5 mg/kg i.p.) every 24 h. Then, following one week of the administration of cadmium chloride, the PAD4-targeted siRNA was administered, and at the second week, lung function was measured, as were lung and heart weights, as well as PAD4 expression by RT-PCR. Our results showed that cadmium administration generated a COPD model, which increased PAD4 expression and decreased lung and heart weights and respiratory function. SiRNA administration partially reversed the changes associated with the COPD model. In conclusion, our results suggest that administration of an siRNA targeting PAD4 could improve respiratory function by decreasing lung and heart damage.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterised by chronic respiratory symptoms (dyspnoea, cough, sputum production) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) causing persistent, often progressive, airflow obstruction [1]. The estimated prevalence of this pathology was 10.3% in people aged 30 to 79 years, equivalent to 391.9 million people [2], causing more than 3 million deaths by 2022 [1]. COPD is mainly classified into six types based on its aetiology. One of the most important types is COPD of environmental origin, which is often secondary to exposure to cigarettes, biomass, or pollution [1]. It is estimated that a quarter of the population (22.3%) uses tobacco in any form, which carries a risk of developing COPD [3], because cadmium is present in cigarettes (approximately >1 µg per cigarette) [3,4]. Cigarettes contain cadmium because tobacco plants absorb cadmium from the pesticides with which they are treated through their roots, resulting in it accumulating in the leaves [5]. These leaves are then used to make cigarettes and, consequently, are an important source of cadmium for people who smoke.
It has been reported for years that the chronic cadmium inhalation associated with cigar consumption causes lung damage such as severe pulmonary oedema, alveolar metaplasia of the lung, and pneumonitis, which can lead to death [6,7]. Cadmium inhalation has been found to induce interstitial infiltration of neutrophils and macrophages in the lung [8,9]. Cadmium has been reported to induce, through NADPH oxidase and ERK1/2 and p38 MAPK signalling pathways, the formation of neutrophil extracellular traps (NETs), which then cause lung damage [10].
NETs were described as a physiological defence mechanism that occurs when neutrophils destroy bacteria by releasing granular proteins and chromatin-forming extracellular fibres that bind to bacteria, apparently as part of the innate immune response [11]. Within these traps are citrullinated histones due to the clearance that is induced by inflammatory stimuli [10]. However, it has been found that excess NETs induce the release of proteases and reactive species, and this favours inflammation [12]. The enzyme responsible for this elimination was discovered in 1977, when it was found that proteins rich in arginine residues could undergo in situ conversion of the side chain to citrulline residues in extracts of hair follicle tissue, which contained an enzyme that acted on the trichohyalin protein (TR fraction) [13]. However, it was not until 1981 that the enzyme was identified as peptidyl arginine deiminase (PAD), which is able to use natural protein substrates such as protamine and histones to perform this conversion of arginine residues to citrulline residues, inducing a charge change in histones that allows the activation of NET formation [14,15].
PADs, known as protein-arginine deiminase enzymes or protein L-arginine amidohydrolase (EC 3.5.3.15), are widely distributed in different tissues [16,17]. To date, five PAD isotypes (PAD1, 2, 3, 4, and 6) have been identified, with transcript lengths varying according to species and subtype from 2124 to 4689 nucleotides [18]. Increased levels of PAD2 and PAD4 have been found in the lungs of COPD patients. The increase in PAD2 is related to higher levels of local expression, while PAD4 expression is due to neutrophil recruitment, where this enzyme is performed [19]. In addition, PAD4 plays a crucial role in the formation of NETs at the pulmonary level in people with COPD and actively participates in the innate immune response [20].
In light of the relationship between PAD4 and lung damage in COPD, a PAD4-targeted siRNA was designed to decrease PAD4 expression and thus reduce cadmium-associated damage in a COPD model. The aim of this study is to study the effect of the administration of a PAD4-targeted siRNA on the deterioration of respiratory function associated with COPD.
2. Materials and Methods
2.1. Selection and Care of Laboratory Animals
In this study, male Wistar rats weighing 300–500 g were used. The rats were maintained in standardised laboratory housing conditions and received ad libitum access to water and food (Purina LabDiet 5001). A light/dark cycle was established with a pattern of 12 h of light followed by 12 h of darkness, according to the guidelines established by the NORMA OFICIAL MEXICANA NOM-062-ZOO-1999, which details the technical specifications of the production, care, and use of laboratory animals [21] (Supplementary Material).
Approval by the Bioethics Committee: This research project was reviewed by the ethics committee and received the approval of the internal committee for the care and use of laboratory animals (CICUAL) of the Escuela Superior de Medicina del Instituto Politécnico Nacional. The project is registered with the number ESM-CICUAL-01/18-12-2019.
The number of animals allocated per group was based on the principle of the 3Rs (reduce, refine, and replace) and statistical parameters. A significance level of α = 0.05 was used, as this value is common in biomedical studies, to minimise the probability of type 1 errors [22]. Additionally, a statistical power (β) of 0.2 was used to detect significant differences while limiting the number of animals used to avoid unnecessary excesses. A variance of 5.985 and an allowed difference of 3 were used. Sample Size Calculations in Clinical Research were used to calculate the sample size [23]. The sample number obtained was 6. A randomisation process was employed to assign animals to groups. Random numbers were generated using the RAND function in Microsoft Excel. We ensured that there was no significant difference in the weights of the groups at the initial timepoint (0) by conducting a one-way ANOVA at a significance level of α = 0.05. We opted for a statistical power (β) of 0.2, balancing the need to detect significant differences with the limitation in the number of animals used to avoid unnecessary excesses.
2.2. Physiological Parameters—Weights of the Rats, Systolic and Diastolic Blood Pressure, and Heart Rate
Rats were weighed using a digital scale (Crow Baccara). Measurements were taken at the beginning (Week 0), at 7 days (Week 1), and at 14 days (Week 2). Systolic and diastolic blood pressure and heart rate were measured at the same timepoints using plethysmography pressure measuring equipment (IITC Life Science Inc., Woodland Hills, CA, USA).
2.3. COPD Model
The model was developed by administering cadmium chloride (5 mg/kg i.p.) every 24 h for 5 days. Two days after the last cadmium administration, siRNA administration was performed (Week 1), and the effect was evaluated 7 days after commencing siRNA administration (Week 2). The control group received the transfection vehicle (Turbofect). The groups were therefore:
- Control group: Rats not exposed to cadmium chloride. Administered only with 0.9% saline solution (1 mL) every 24 h for 5 days. n = 6 rats.
- Cadmium group: Rats exposed to cadmium chloride to induce COPD, with administrations of 5 mg/kg body weight by IP administration every 24 h for 5 days. n = 6 rats.
- Cadmium + vehicle group: Rats exposed to cadmium chloride to induce COPD with administrations of 5 mg/kg body weight by IP administration every 24 h for 5 days. On day 7, the administration of the transfection vehicle was performed. n = 6 rats.
- Cadmium + siRNA group: Rats exposed to cadmium chloride to induce COPD, with administrations of 5 mg/kg body weight by IP administration every 24 h for 5 days. On day 7, the vehicle administration was performed by administering transfection + 5 µL of the siRNA solution with the single dose turbofect. n = 6 rats.
2.4. Respiratory Function Measurement
Respiratory function was measured by means of a differential pressure transducer. The rat was placed in a sealed plethysmography chamber, where the volume of inspiration and expiration were measured. The transducer was coupled to a Grass 7D polygraph (Quincy, MA, USA), and the equipment was calibrated by volume, with a volume of 0.1 mL for calibration. Then, the analysis of the curves was performed, obtaining the area under the curve (mL/s).
2.5. Evaluation of PAD4 Expression in Lungs
Expression was measured in rat lung samples using the Trizol technique. Approximately 100 mg of tissue from the middle regions was used according to the manufacturer’s specifications (Thermofisher, Waltham, MA, USA). The purity was quantified by means of the 260/280 nm ratio with values 1.8–2.2 in a nanophotometer (IMPLEN, München, Germany). Subsequently, cDNA synthesis was performed with RNA samples at a concentration of 1µg/µL with the Improm-II Reverse Transcription System kit according to the manufacturer’s specifications (Promega, Madison, WI, USA). Quantification was performed using the SYBR green select master mix (Applied Biosystems, Waltham, MA, USA) with the primers PAD4 primer sequence Right (GGAT-TCTCATCGGGAGCAG) and PAD4 primer sequence Left (CAGGATCTGGTGCATGTCC) and using Hypoxanthine-guanine phosphoribosyl transferase (HPRT) as the constitutive. The amplification conditions were as follows: 50 °C for 2 min, 95 °C for 2 min, and then 45 cycles of 95 °C for 15 s and 60 °C for 60 s, terminating all reactions with a melting curve to rule out nonspecific amplifications. Expression levels were determined by 2 − ΔCT, normalising expression with HPRT as a constituent. We used the PCR method to evaluate expression because it is the most sensible assay to demonstrate siRNA efficiency. This approach sidesteps issues related to the half-life of the proteins that could mask the silencing efficiency of gene silencing [24].
2.6. Design and Synthesis of siRNA
We designed an siRNA targeting PAD4. Firstly, we performed a FASTA search for mRNA from PAD4 of humans and rats, which was aligned by the CRUSTAL OMEGA software (https://www.ebi.ac.uk, accessed on 12 January 2021). It was then designed using the human sequence through Wizard (https://www.invivogen.com/sirnawizard, accessed on 13 January 2021) and checked if the selected sequences could be found in the human rat. To evaluate the specificity of the siRNA and determine the probability of off-target effects, human RNA sequences were searched for similar sequences using BLAST. It was found that the probability of off-target silencing was low, which can be deduced from the ‘query cover’, the identity percentage, and particularly the E value. A high E value > 1 indicates that there is a lower probability of off-target silencing occurring. Furthermore, previous studies have shown that even a single mismatch can significantly decrease the probability of effective silencing. Therefore, we consider that a similarity percentage of less than 80% in a 20-nucleotide sequence would be indicative of a low probability of silencing occurring under these conditions. Subsequently, the selected sequences were analysed to verify that they were found in the coding regions (CDS), and the secondary structure of the transcripts was realised with RNAFOLD (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi, accessed on 22 January 2021), and the sites of hybridisation were analysed to find the regions with the greatest silencing efficiencies. To measure the effectiveness of the selected siRNAs, two algorithms were used: s-Biopredsi and Reynolds and Ui-Tei, which were evaluated on the website (https://www.med.nagoya-u.ac.jp/neurogenetics/i_Score/i_score.html, accessed on 27 April 2021). Based on the data provided, siRNA 1 had scores of 52.4 (s-Biopredsi) and 63.7 (Reynolds), while siRNA 2 had scores of 49.4 (s-Biopredsi) and 53.8 (Reynolds). The Ui-Tei algorithm classifies siRNAs according to the accessibility of the binding site and structural characteristics. It uses categories “I”, “II”, and “III”. Both siRNAs are classified as “II”, indicating moderate efficacy by this criterion [25]. The leader sequences were synthesised using the MERMADE 8 equipment through a process of deblocking, coupling, capping, and oxidation; afterward, the cleavage process was applied to the product of the synthesis, and later it was purified by alcohols and hybridised in a 10 mM HEPES solution with a pH of 7.5 and 150 mM NaCl. It was then heated at 80 °C for 1 min, shaken, and incubated for an hour at 37 °C. Finally, the synthesised siRNAs were prepared in a Turbofect solution with 5% glucose for later administration according to the manufacturer’s instructions (Thermofisher, Waltham, MA, USA).
2.7. siRNA Administration
The siRNA was administered by intrajugular injection one week after the administration of cadmium chloride. Using the Turboefect transfection vehicle (ThermoFisher Scientific, Waltham, MA, USA), 5 mL of a solution with a concentration of 0.109 mg/mL of the siRNAs were administered in a single administration at a dose of 1.6 mg/kg. The expression was evaluated one week after the administration of siRNA.
2.8. Statistical Analysis
Values are expressed as the media ± SEM (Standard Error of the Mean), and the statistical analysis was helpful in determining ANOVA proofs from a via, followed by the Tukey post-hoc test.
3. Results
The administration of cadmium chloride produces a noteworthy decrease in rat weights (Table 1) without changes in the heart rate or systolic or diastolic blood pressure (Table 2, Table 3 and Table 4) in comparison with the control group.

Table 1.
Weight of Rats threatened with cadmium chloride.

Table 2.
Systolic pressure of Rats threatened with cadmium chloride.

Table 3.
Diastolic pressure of rats threatened with cadmium chloride.

Table 4.
Heart rate of rats threatened with cadmium chloride.
The respiratory mechanics analysis showed that the area under the curve decreased significantly after cadmium chloride administration; however, this was reversed by siRNA administration (Figure 1). On the other hand, no changes are observed in breathing frequency (Figure 2).
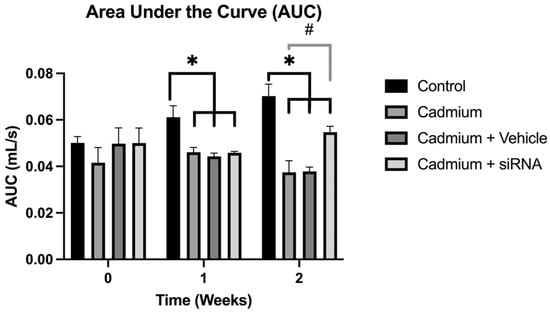
Figure 1.
Areas under the curve for the different groups are shown at weeks 0, 1, and 2. Values are expressed as mean ± standard error of the mean (SEM), n = 6 rats. Significant differences: * p < 0.05 vs. control, and # p < 0.05 vs. cadmium. One-way ANOVA was used for the comparisons between the different groups.
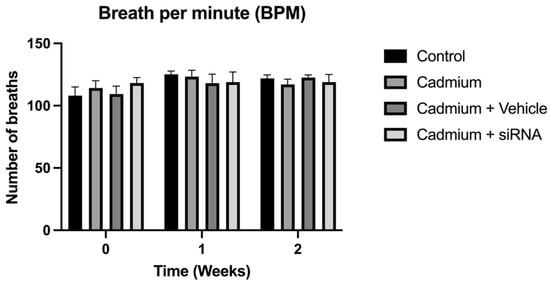
Figure 2.
Breath per minute for the different groups. Values are expressed as mean ± standard error of the mean (SEM), n = 6 rats. Significant differences: One-way ANOVA was used for the comparisons between the different groups.
Cadmium chloride administration produced a reduction in lung weight, which was to a lesser extent in the right lung after the administration of siRNA (Figure 3). In addition, a significant increase in PAD4 was observed, which was also reduced after the administration of siRNA (Figure 4).
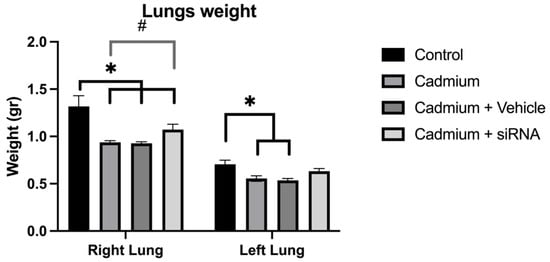
Figure 3.
Lung weights for the different groups. Values are expressed as mean ± standard error of the mean (SEM), n = 6 rats. Significant differences: * p < 0.05 vs. control, and # p < 0.05 vs. cadmium. One-way ANOVA was used for the comparisons between the different groups.
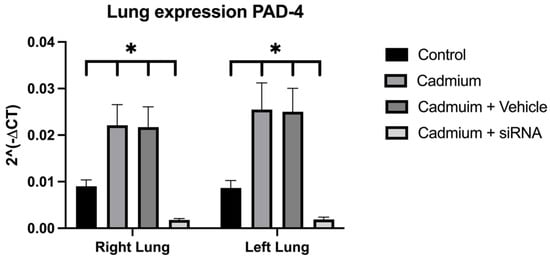
Figure 4.
PAD4 lung expression for the different groups. Values are expressed as mean ± standard error of the mean (SEM)), n = 6 rats. Significant differences: * p < 0.05 vs. control. One-way ANOVA was used for the comparisons between the different groups.
The siRNA design showed that the rat-human transcripts had a 29-nucleotide similarity, which indicates equality in the sequences of both rats and humans. This equality in the region motivated the selection of this area to perform the sequences. (Figure 5). Regions susceptible to silencing were also identified at the gene coordinates NM_012387.3 of NCBI, which encodes human PAD4 for the nucleotides 1118–1147 (Table 5). These regions belong to a region CDS and present loop and stem loop structures in the secondary structure in the different variants of the mRNA from both rats and humans, which suggests susceptibility to silencing by siRNA (Figure 6). We found only three important proteins associated with the generation of off-targets (Table 6 and Table 7).

Figure 5.
Blast comparison of the different messenger RNA sequences in humans and rats and their various variants. Asterisks indicate regions where the sequences are identical; this area was selected for siRNA design.

Table 5.
siRNA sequences.
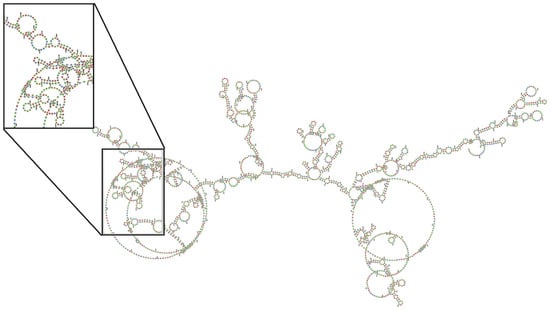
Figure 6.
The image displays the secondary structure of the PAD4 messenger RNA as determined by RNAfold software.

Table 6.
Evaluation of off-targets of the siRNA 1.

Table 7.
Evaluation of off-targets of the siRNA 2.
4. Discussion
Cadmium has been implicated as one of the components present in cigarettes that may be responsible for causing lung damage in smokers [26], but there is little information regarding the mechanisms involved in this damage. Our results show that PAD4 may be part of one of the signalling pathways involved in this damage, likely due to an increase in the formation of NETs.
There was a decrease in the weight of the rats supplemented with cadmium, which is concordant with previous studies carried out by other research groups that used cadmium to induce lung inflammation through intraperitoneal administration of cadmium chloride [27,28].
This decrease may be due to reduced food consumption, as has been previously reported [29]. In addition, an increase in the amount of reactive oxygen species has been observed, which is related to reduced protein synthesis, lipid peroxidation with an increase in malondialdehyde, and a decrease in glutathione peroxidase, as well as weight loss in rats treated with cadmium [30]. These mechanisms can be summarised as an increase in pro-inflammatory substances with a decrease in anti-inflammatory mechanisms, which activate other pathways such as the apoptosis pathway. In addition to changes in the expression of transcription factors related to cell growth and differentiation, which could also be part of the mechanism of the decrease in the weight of rats [31], it is important to note that this decrease in rat weight was not reversed by the administration of our siRNA. These findings may suggest that weight loss has pathophysiological mechanisms other than PAD4 expression, which could explain our results.
No statistically significant differences were found in systolic blood pressure, diastolic pressure, or heart rate. The relationship between the administration or ingestion of cadmium, its accumulation and concentration, renal damage, and increased blood pressure is a controversial topic in the literature [32,33]. Although some studies have reported this relationship, others, like ours, have not found a significant increase in blood pressure levels in the groups treated with cadmium [34,35]. Furthermore, the duration of exposure of our groups to cadmium was relatively short compared to other studies that have shown such a relationship, which could also explain the lack of statistically significant changes in blood pressure in our study. On the other hand, our rats were kept on a normal diet, in contrast to some other studies where, to demonstrate changes in pressure, they were kept on a high-sodium diet.
There was no difference in heart rate, which is concordant with previous studies where no changes have been demonstrated with respect to this parameter [36]. A significant decrease in lung weight was observed, which is an important marker in the evaluation of toxicity [33]. This reduction in lung weight may be linked to decreased protein synthesis, in addition to altered metabolism and physiological changes [37]. However, the administration of our siRNA partially diminished this weight reduction, mainly in the right lung, which could be explained by differences in the bioavailability of the siRNA between lungs as well as a greater surface area and size of the lung. Further studies would be needed to fully understand this effect.
The partial recovery of lung weight can be explained by the decrease in PAD4 expression that is achieved after the administration of siRNA. It has been reported that lung damage associated with exposure to some toxic substances, such as cadmium, may be associated with an increase in the number of NETs activated by an increase in the expression of PAD4 [10]. In our study, we obtained similar results that could corroborate that the IP cadmium administration model causes an increase in the expression of PAD4 at the lung level in the Cadmium and Cadmium + vehicle groups, which is partially reversed in the group where the siRNA was administered. SiRNA administration may be associated with less damage and less activation of NET formation, as has been seen in previous studies where PAD4 inhibitors have been administered [38,39,40].
Regarding the other respiratory parameters, a significant improvement in AUC was observed in the groups treated with cardio, cadmium + vehicle, and cadmium + siRNA compared to the control group. However, when comparing the cadmium + siRNA group with the group treated with it, it is seen that although it does not reach the values of the control group, there is a significant recovery of this parameter, which suggests less damage in the respiratory pathways. This could be related to the decreased expression of PAD4 in the group treated with our siRNA.
The off-targets that we found were dynein axonemal assembly factor 9 (DNAAF9), interleukin 9 receptor (IL9R), and arylsulfatase B (ARSB). DNAAF9 is a protein that is involved in the assembly and function of the ciliary and flagellar motility apparatus [41], while interleukin 9 (IL-9) is a cytokine that binds to its receptor, and together they are involved in several immunological processes. IL9R is mainly expressed in lymphoid cells, such as T cells and mast cells [42], and arylsulfatase B proteins are enzymes that play a crucial role in sulphate metabolism, especially in the degradation of glycosaminoglycans such as dermatan sulphate and heparan sulphate [43]. However, due to the values of E, we cannot exclude the probability of the side effects associated with these proteins.
In summary, the effect of cadmium administration in our model caused an increase in the expression of PAD4, which is related to the formation of NETs and lung damage in COPD. However, our siRNA targeting PAD4 reduced the expression of PAD4 and partially improved the parameters measured in our assay. These findings suggest a possible relationship between cadmium and increased PAD4 expression in the pathogenesis of COPD and the use of siRNA as a potential therapeutic strategy to attenuate the harmful effects of cadmium in the respiratory system. More research is still required to fully understand the mechanisms involved, as well as further studies that corroborate the clinical relevance of these findings.
The clinical relevance of our study lies in its potential for the development of more effective and targeted interventions for patients with COPD, especially those whose condition is exacerbated by environmental factors such as cadmium exposure from cigarette use. Given the emerging role of PAD4 in the pathogenesis of COPD, our findings suggest that siRNA therapy could be a promising strategy, although further studies are still needed to establish its safety and efficacy in clinical settings.
Future work should therefore focus on longitudinal studies and more complex models to validate these results and explore how PAD4 modulation may influence long-term COPD progression, as well as its role in other lung diseases such as COVID. It would also be interesting to test our siRNAs for other types of lung disease [44].
5. Conclusions
In conclusion, our study provides significant evidence that siRNA targeting PAD4 may be an effective therapeutic approach to mitigate lung damage in the rat cadmium-induced COPD model. These results are an important step toward understanding COPD at the molecular level, and they offer a promising direction for future research and therapeutic applications.
Supplementary Materials
It can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92010012/s1.
Author Contributions
Conceptualization, S.A.O.-O. and S.V.; Methodology, S.E.C.-B., S.A.O.-O., C.M.B.-N., V.M.S.-S. and S.V.; Formal Analysis, S.E.C.-B., S.A.O.-O., C.M.B.-N., R.R.-N. and S.V.; Investigation, V.G.G.-R., S.E.C.-B., S.A.O.-O., C.M.B.-N., V.M.S.-S. and S.V.; Writing—Original Draft Preparation, V.G.G.-R., S.A.O.-O., V.M.S.-S., R.R.-N., F.H., E.H., A.A.-M. and S.V.; Writing—Review and Editing, V.G.G.-R., S.A.O.-O., F.H., E.H., A.A.-M. and S.V.; Funding Acquisition, S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research protocol was funded by grants from the Secretaria de Investigación y Posgrado del Instituto Politécnico Nacional: Proyectos Insignia IPN-2015 and SIP-IPN: 20231313 and 20240060; Consejo Nacional de Humanidades, Ciencias y Tecnologías: Master fellowship 958823.
Institutional Review Board Statement
The experimental procedures were conducted following the regulations proved by our Institutional Committee for the Care and Use of Laboratory Animals (reference: ESM-CICUAL-01/18-12-2019) and the Official Mexican Standard NOM-062-ZOO1999 Technical specifications for the production, care, and use of laboratory animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the relevant data found in the study are available in the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Tamondong-Lachica, D.R.; Skolnik, N.; Hurst, J.R.; Marchetti, N.; Rabe, A.P.J.; Montes de Oca, M.; Celli, B.R. GOLD 2023 Update: Implications for Clinical Practice. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (COPD) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.E.; Loretan, C.G.; Wang, T.W.; Jamal, A.; Homa, D.M. Tobacco Product Use Among Adults—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous Compounds in Tobacco Smoke. Int. J. Environ. Res. Public. Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.D. Cadmium in Sludges Used as Fertilizer. In Cadmium in the Environment; Birkhäuser: Basel, Switzerland, 1986; pp. 55–65. [Google Scholar]
- Beton, D.C.; Andrews, G.S.; Davies, H.J.; Howells, L.; Smith, G.F. Acute Cadmium Fume Poisoning: Five Cases with One Death from Renal Necrosis. Occup. Environ. Med. 1966, 23, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, J.R.; Finckh, E.S. Fatal Cadmium Fume Pneumonitis. Med. J. Aust. 1976, 2, 151. [Google Scholar] [CrossRef]
- Johansson, A.; Curstedt, T.; Robertson, B.; Camner, P. Lung Morphology and Phospholipids after Experimental Inhalation of Soluble Cadmium, Copper, and Cobalt. Environ. Res. 1984, 34, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Karg, E.; Kreyling, W.G.; Lentner, B.; Schulz, H.; Ziesenis, A.; Schramel, P.; Heyder, J. Fate and Toxic Effects of Inhaled Ultrafine Cadmium Oxide Particles in the Rat Lung. Inhal. Toxicol. 2004, 16, 83–92. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Han, Z.; Wang, J.; Zhang, X.; Wang, Y.; Liu, Q.; Yang, Z. Neutrophil Extracellular Traps Promote Cadmium Chloride-Induced Lung Injury in Mice. Environ. Pollut. 2019, 254, 113021. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Uddin, M.; Watz, H.; Malmgren, A.; Pedersen, F. NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma. Front. Immunol. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.E.; Harding, H.W.J.; Llewellyn-Smith, I.J. The Origin of Citrulline-Containing Proteins in the Hair Follicle and the Chemical Nature of Trichohyalin, an Intracellular Precursor. Biochim. Biophys. Acta (BBA)-Protein Struct. 1977, 495, 159–175. [Google Scholar] [CrossRef]
- Sugawara, K.; Oikawa, Y.; Ouchi, T. Identification and Properties of Peptidylarginine Deiminase from Rabbit Skeletal Muscle1. J. Biochem. 1982, 91, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, M.; Sugawara, K. Properties of Peptidylarginine Deiminase from the Epidermis of Newborn Rats1. J. Biochem. 1981, 89, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Akiyama, K.; Hikichi, K.; Ohtsuka, R.; Okuyama, A.; Senshu, T. Combined Biochemical and Immunochemical Comparison of Peptidylarginine Deiminases Present in Various Tissues. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1988, 966, 375–383. [Google Scholar] [CrossRef]
- Takahara, H.; Tsuchida, M.; Kusubata, M.; Akutsu, K.; Tagami, S.; Sugawara, K. Peptidylarginine Deiminase of the Mouse. Distribution, Properties, and Immunocytochemical Localization. J. Biol. Chem. 1989, 264, 13361–13368. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Zendman, A.J.W.; van Venrooij, W.J.; Pruijn, G.J.M. PAD, a Growing Family of Citrullinating Enzymes: Genes, Features and Involvement in Disease. BioEssays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Kilsgård, O.; Andersson, P.; Malmsten, M.; Nordin, S.L.; Linge, H.M.; Eliasson, M.; Sörenson, E.; Erjefält, J.S.; Bylund, J.; Olin, A.I.; et al. Peptidylarginine Deiminases Present in the Airways during Tobacco Smoking and Inflammation Can Citrullinate the Host Defense Peptide LL-37, Resulting in Altered Activities. Am. J. Respir. Cell Mol. Biol. 2012, 46, 240–248. [Google Scholar] [CrossRef]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil Extracellular Traps Are Associated with Inflammation in Chronic Airway Disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef]
- NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 5 March 2020).
- Berkson, J. Tests of Significance Considered as Evidence*. Int. J. Epidemiol. 2003, 32, 687–691. [Google Scholar] [CrossRef]
- Chow, S.-C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research, 3rd ed.; Chow, S.-C., Shao, J., Wang, H., Lokhnygina, Y., Eds.; Chapman & Hall/CRC Biostatistics Series; Chapman and Hall/CRC: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2017; ISBN 9781315183084. [Google Scholar]
- Wu, W. A Novel Approach for Evaluating the Efficiency of SiRNAs on Protein Levels in Cultured Cells. Nucleic Acids Res. 2004, 32, e17. [Google Scholar] [CrossRef]
- Ahmed, F.; Raghava, G.P.S. Designing of Highly Effective Complementary and Mismatch SiRNAs for Silencing a Gene. PLoS ONE 2011, 6, e23443. [Google Scholar] [CrossRef]
- Lv, B.-B.; Yang, C.-L.; Tan, Z.-X.; Zheng, L.; Li, M.-D.; Jiang, Y.-L.; Liu, L.; Tang, M.-M.; Hua, D.-X.; Yang, J.; et al. Association between Cadmium Exposure and Pulmonary Function Reduction: Potential Mediating Role of Telomere Attrition in Chronic Obstructive Pulmonary Disease Patients. Ecotoxicol. Environ. Saf. 2023, 251, 114548. [Google Scholar] [CrossRef]
- Kirschvink, N.; Vincke, G.; Fiévez, L.; Onclinx, C.; Wirth, D.; Belleflamme, M.; Louis, R.; Cataldo, D.; Peck, M.J.; Gustin, P. Repeated Cadmium Nebulizations Induce Pulmonary MMP-2 and MMP-9 Production and Enphysema in Rats. Toxicology 2005, 211, 36–48. [Google Scholar] [CrossRef]
- Kotsonis, F.N.; Klaassen, C.D. Toxicity and Distribution of Cadmium Administered to Rats at Sublethal Doses. Toxicol. Appl. Pharmacol. 1977, 41, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kozŀowska, K.; Brzozowska, A.; Sulkowska, J.; Roszkowski, W. The Effect of Cadmium on Iron Metabolism in Rats. Nutr. Res. 1993, 13, 1163–1172. [Google Scholar] [CrossRef]
- Rencuzogullari, N.; Erdogan, S. Oral Administration of Lycopene Reverses Cadmium-Suppressed Body Weight Loss and Lipid Peroxidation in Rats. Biol. Trace Elem. Res. 2007, 118, 175–183. [Google Scholar] [CrossRef]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An Overview of Molecular Mechanisms in Cadmium Toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Ohanian, E.V.; Iwai, J. Effects of Cadmium Ingestion in Rats with Opposite Genetic Predisposition to Hypertension. Environ. Health Perspect. 1979, 28, 261–266. [Google Scholar] [CrossRef]
- Balaraman, R.; Gulati, O.D.; Bhatt, J.D.; Rathod, S.P.; Hemavathi, K.G. Cadmium-Induced Hypertension in Rats. Pharmacology 1989, 38, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, K. Cadmium and Hypertension. Lancet 1977, 309, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.M.; Erlanger, M.; Perry, E.F. Hypertension Following Chronic, Very Low Dose Cadmium Feeding. Exp. Biol. Med. 1977, 156, 173–176. [Google Scholar] [CrossRef]
- Ozturk, I.M.; Buyukakilli, B.; Balli, E.; Cimen, B.; Gunes, S.; Erdogan, S. Determination of Acute and Chronic Effects of Cadmium on the Cardiovascular System of Rats. Toxicol. Mech. Methods 2009, 19, 308–317. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S. Curcumin Protection against Cadmium Chloride-Induced Biochemical Alterations in Lungs of Swiss Albino Mice. Asian J. Pharm. Clin. Res. 2020, 13, 103–107. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 Activity Is Sufficient to Disrupt Mouse and Human NET Formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, C.; Deng, H.; Strnad, J.; Bernabei, L.; Vogl, D.T.; Burke, J.J.; Nefedova, Y. A Novel Peptidylarginine Deiminase 4 (PAD4) Inhibitor BMS-P5 Blocks Formation of Neutrophil Extracellular Traps and Delays Progression of Multiple Myeloma. Mol. Cancer Ther. 2020, 19, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.; Zhou, Z.; Liu, F.; Shen, Y.; You, Q.; Lu, S.; Wu, J. The Role of Protein Arginine Deiminase 4-Dependent Neutrophil Extracellular Traps Formation in Ulcerative Colitis. Front. Immunol. 2023, 14, 1144976. [Google Scholar] [CrossRef]
- Braschi, B.; Omran, H.; Witman, G.B.; Pazour, G.J.; Pfister, K.K.; Bruford, E.A.; King, S.M. Consensus Nomenclature for Dyneins and Associated Assembly Factors. J. Cell Biol. 2022, 221, e202109014. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. A Brief History of IL-9. J. Immunol. 2011, 186, 3283–3288. [Google Scholar] [CrossRef]
- Fluharty, A.L.; Stevens, R.L.; Sanders, D.L.; Kihara, H. Arylsulfatase B Deficiency in Maroteaux-Lamy Syndrome Cultured Fibroblasts. Biochem. Biophys. Res. Commun. 1974, 59, 455–461. [Google Scholar] [CrossRef]
- Arisan, E.D.; Uysal-Onganer, P.; Lange, S. Putative Roles for Peptidylarginine Deiminases in COVID-19. Int. J. Mol. Sci. 2020, 21, 4662. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).