Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PD Extract
2.2. UPLC-QTOF/MS Analysis of PD Extract
2.3. Experimental Animals
2.4. BPH Induction and Drug Administration
2.5. Determination of DHT and Testosterone
2.6. Histological Examination
2.7. Proliferative Cell Nuclear Antigen (PCNA) Staining
2.8. Western Blot
2.9. Quantitative Real-Time PCR (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. Analysis of PD
3.2. PD Extract Reduces Prostate Weight in BPH Rats
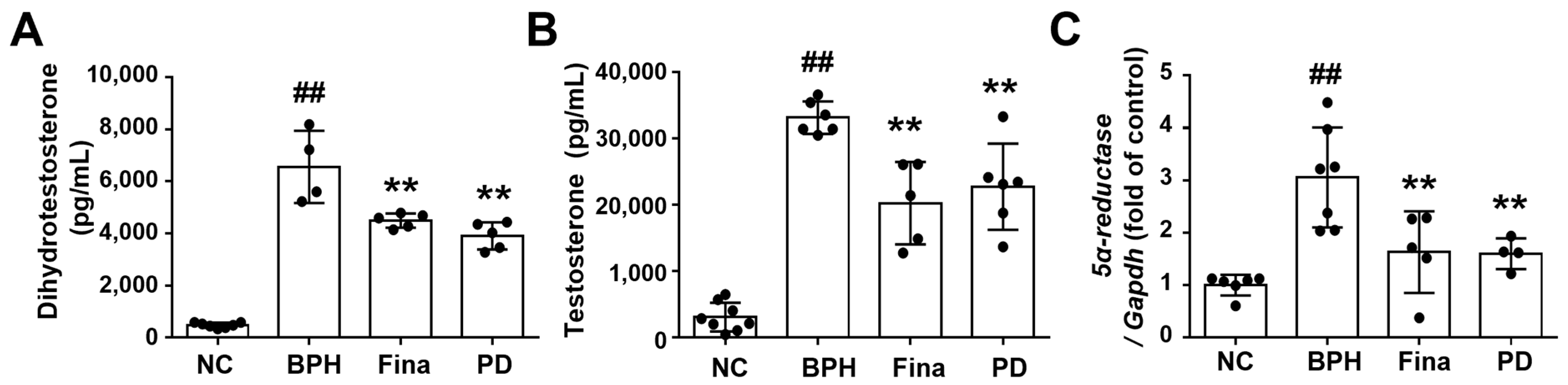
3.3. PD Extract Decreases Androgen and 5α-Reductase Levels in BPH Rats
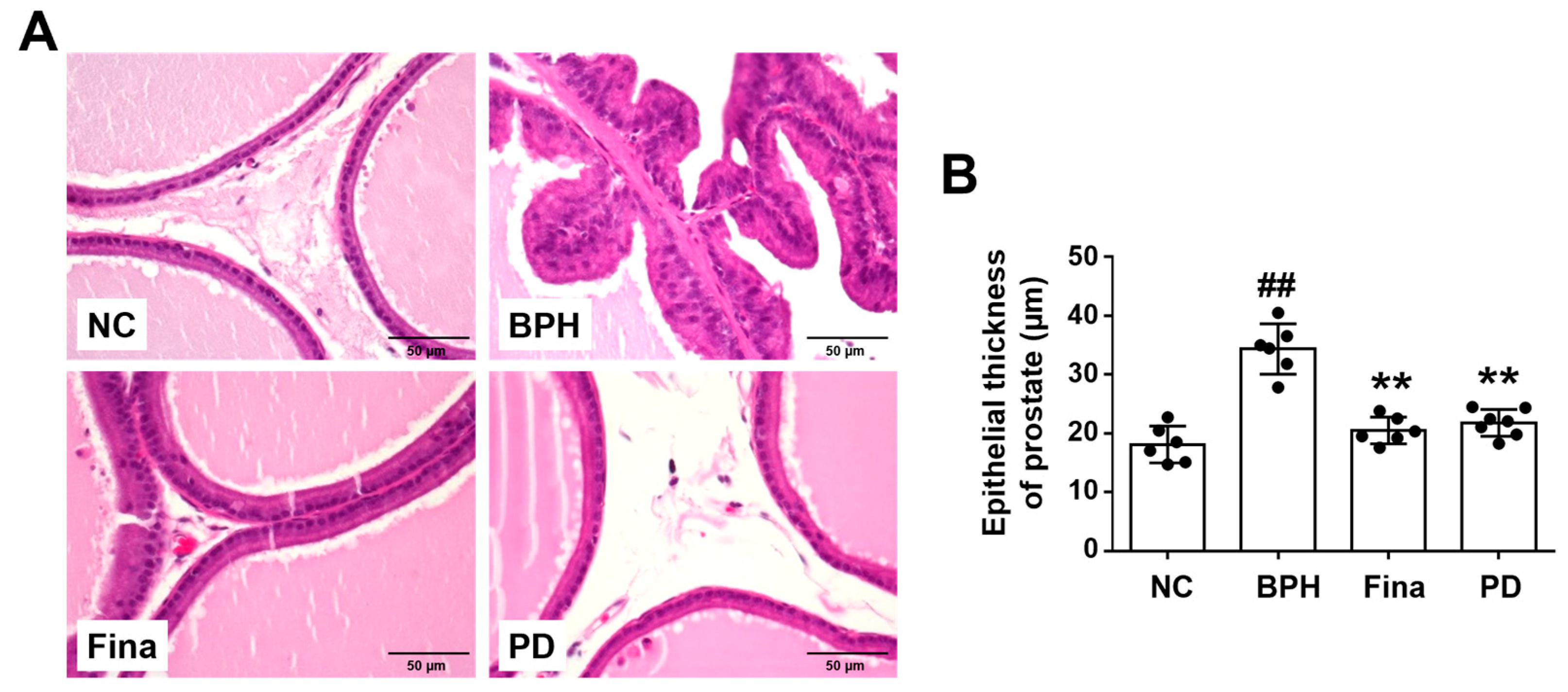
3.4. PD Extract Decreases the Thickness of Glandular Epithelium in BPH Rats
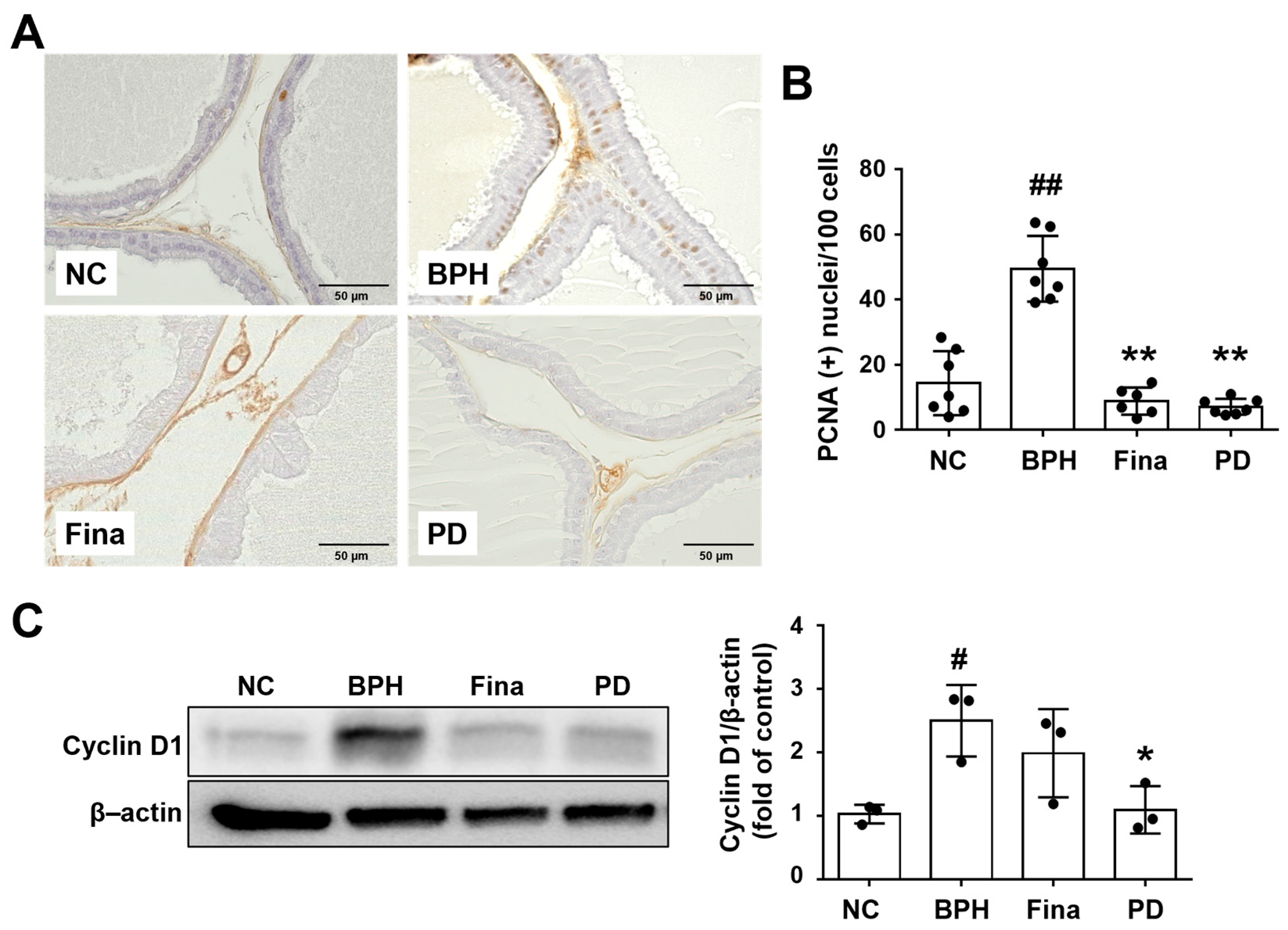
3.5. PD Extract Ameliorates Testosterone-Induced Glandular Epithelial Cell Proliferation
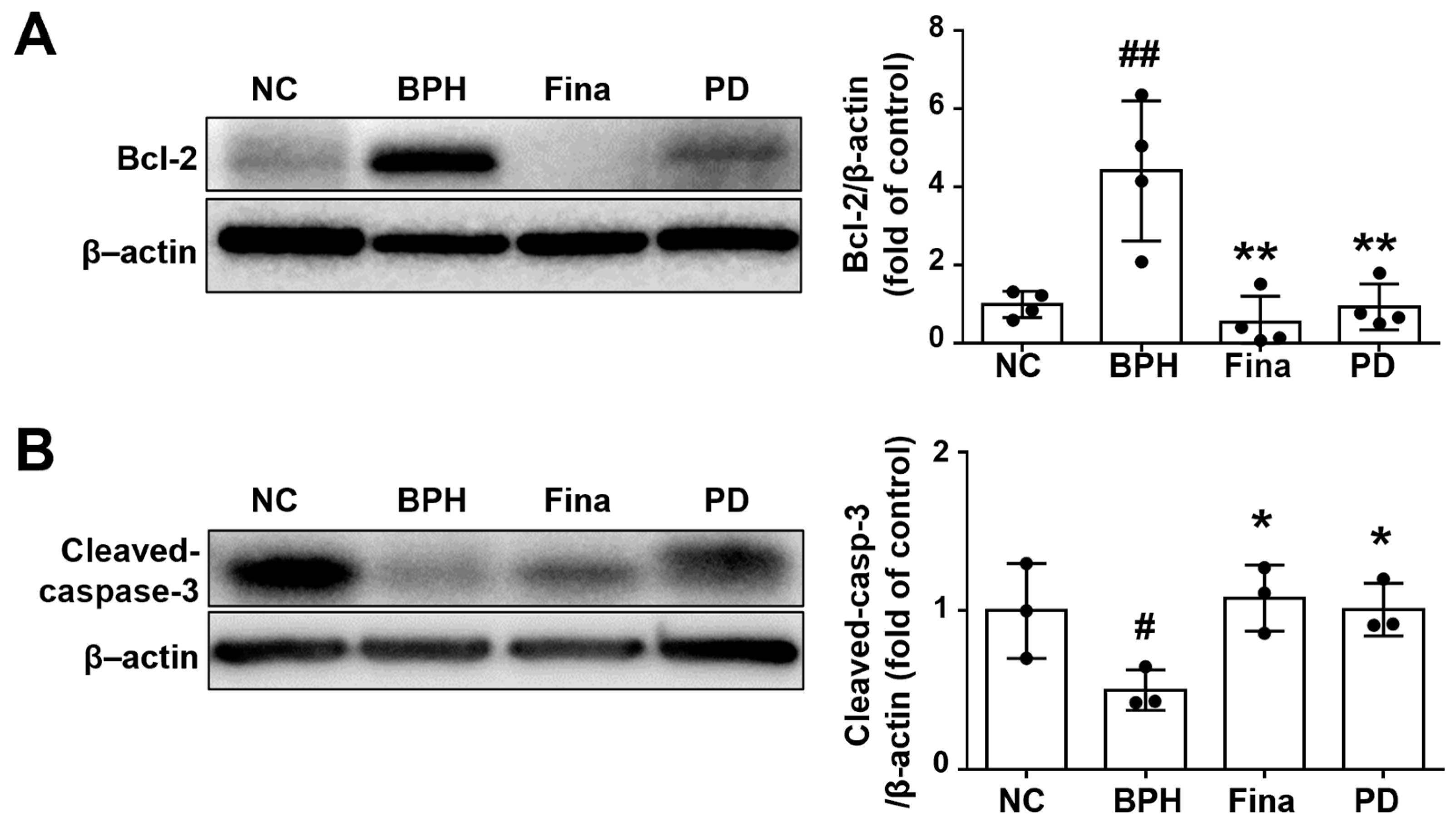
3.6. PD Extract Activates Apoptosis of Prostatic Tissue in BPH Rats
3.7. PD Extract Inhibits the Testosterone-Triggered Induction of Growth Factors and Akt Activation
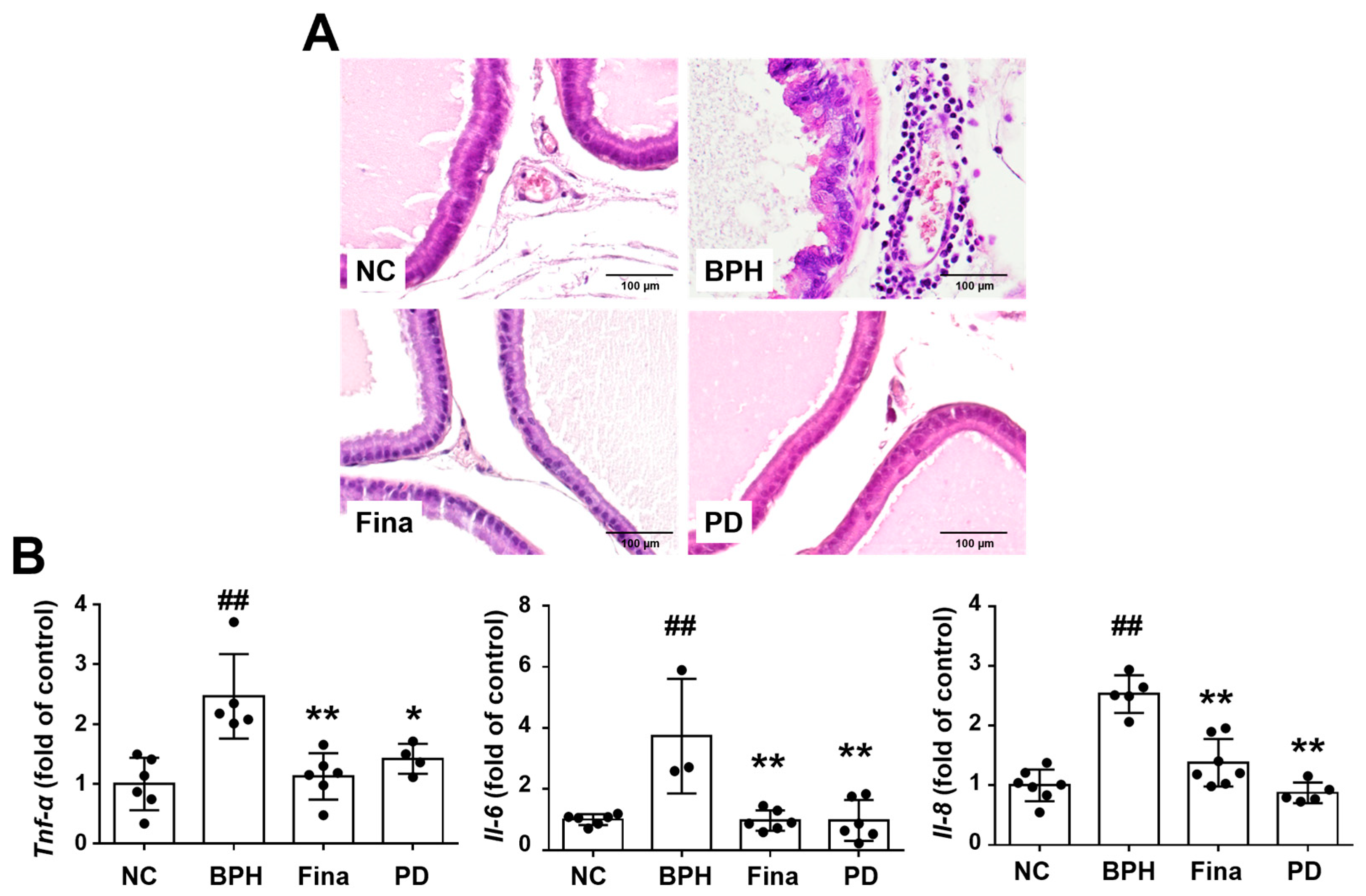
3.8. PD Extract Suppresses Inflammatory Cytokines in BPH Rats
3.9. PD Extract Does Not Affect Liver Function or Histology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20 (Suppl. S3), S11–S18. [Google Scholar] [CrossRef]
- Foo, K.T. Pathophysiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 152–157. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Xu, X. Prevalence, Burden, and Treatment of Lower Urinary Tract Symptoms in Men Aged 50 and Older: A Systematic Review of the Literature. SAGE Open Nurs. 2018, 4, 2377960818811773. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Smock, S.L.; Castleberry, T.A.; Owen, T.A. Molecular cloning and functional characterization of the canine androgen receptor. Mol. Cell Biochem. 2001, 226, 129–140. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Carson, C., 3rd; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Naber, K.G.; Weidner, W. Chronic prostatitis-an infectious disease? J. Antimicrob. Chemother. 2000, 46, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Larson, J.A.; Andriole, G.L. Management of Benign Prostatic Hyperplasia. Annu. Rev. Med. 2016, 67, 137–151. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Nangia, A.K. 5alpha-Reductase Inhibitors (5ARIs) and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 59–61. [Google Scholar] [CrossRef]
- Tarter, T.H.; Vaughan, E.D., Jr. Inhibitors of 5alpha-reductase in the treatment of benign prostatic hyperplasia. Curr. Pharm. Des. 2006, 12, 775–783. [Google Scholar] [CrossRef]
- Lowe, F. Alpha-1-adrenoceptor blockade in the treatment of benign prostatic hyperplasia. Prostate Cancer Prostatic. Dis. 1999, 2, 110–119. [Google Scholar] [CrossRef]
- Burrows, G.E.; Tyrl, R.J. Toxic Plants of North America, 2nd ed.; Wiley-Blackwell: Hoboken, HJ, USA, 2013. [Google Scholar]
- Yoon, S.Y. Korean Resources Plant Encyclopedia; Academic Book: Seoul, Republic of Korea, 1995. [Google Scholar]
- Bae, J.J. Phytochemical Constituents of Panicum dichotomiflorum Michaux. Korean J. Pharmacogn. 2017, 48, 285–288. [Google Scholar]
- In, S.J.; Seo, K.H.; Song, N.Y.; Lee, D.S.; Kim, Y.C.; Baek, N.I. Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Arch. Pharm. Res. 2015, 38, 26–34. [Google Scholar] [CrossRef]
- Ahmad, I.; Waheed, A.; Tahir, N.B.; Rais, A.K. Anti-inflammatory constituents from Perovskia atriplicifolia. Pharm. Biol. 2015, 53, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Biedma, A.; Sanchez-Quesada, C.; Beltran, G.; Delgado-Rodriguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Shalini, V.; Jayalekshmi, A.; Helen, A. Mechanism of anti-inflammatory effect of tricin, a flavonoid isolated from Njavara rice bran in LPS induced hPBMCs and carrageenan induced rats. Mol. Immunol. 2015, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kwon, H.J.; Jung, H.J. Tricin, 4’,5,7-trihydroxy-3’,5’-dimethoxyflavone, exhibits potent antiangiogenic activity in vitro. Int. J. Oncol. 2016, 49, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, P.G.; Liang, W.Q.; Hu, Y.J.; Xu, P.; Zhou, J.; Pu, J.B.; Zhang, H.J. Luteolin-4’-O-glucoside and its aglycone, two major flavones of Gnaphalium affine D. Don, resist hyperuricemia and acute gouty arthritis activity in animal models. Phytomedicine 2018, 41, 54–61. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Ali, Z.; Wang, Y.H.; El-Amier, Y.A.; Khan, S.I.; Khan, I.A. Cytotoxic steroidal saponins from Panicum turgidum Forssk. Steroids 2017, 125, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Temraz, A.; Hozaien, H.E.; El-Tantawy, W.H.; El-Gindi, O.D.; Taha, K.F. Cholestane and spirostane-type glycosides from the roots and rhizomes of Panicum repens L. Phytochem. Lett. 2014, 10, 173–178. [Google Scholar] [CrossRef]
- Farag, M.A.; El Fishawy, A.M.; El-Toumy, S.A.; Amer, K.F.; Mansour, A.M.; Taha, H.E. Antihepatotoxic Effect and Metabolite Profiling of Panicum turgidum Extract via UPLC-qTOF-MS. Pharmacogn. Mag. 2016, 12, S446–S453. [Google Scholar] [CrossRef] [PubMed]
- Zakia, A.A.; Qiub, L.; Alib, Z.; Khanb, S.I.; Khanb, I.A. Anti-inflammatory Steroidal Saponins from Panicum turgidum. J. Agric. Basic Sci. 2016, 1, 1–6. [Google Scholar]
- Radha, R.; Vijayalakshmi, P. Hypolipidemic potential of Panicum miliare on selected cardiovascular subjects. Anc. Sci. Life 2007, 26, 29–32. [Google Scholar]
- Antia, B.S.; Okokon, J.E.; Umoh, E.E.; Udobang, J.A. Antidiabetic activity of ethanolic leaf extract of Panicum maximum. Int. J. Drug Dev. Res. 2010, 2, 488–492. [Google Scholar]
- Kanife, U.; Odesanmi, O.; Doherty, V. Phytochemical composition and antifungal properties of leaf, stem and florets of Panicum maximum Jacq. (Poaceae). Int. J. Biol. 2012, 4, 64. [Google Scholar] [CrossRef]
- Ub Wijerathne, C.; Park, H.S.; Jeong, H.Y.; Song, J.W.; Moon, O.S.; Seo, Y.W.; Won, Y.S.; Son, H.Y.; Lim, J.H.; Yeon, S.H.; et al. Quisqualis indica Improves Benign Prostatic Hyperplasia by Regulating Prostate Cell Proliferation and Apoptosis. Biol. Pharm. Bull. 2017, 40, 2125–2133. [Google Scholar] [CrossRef]
- Rho, J.; Seo, C.S.; Park, H.S.; Wijerathne, C.U.; Jeong, H.Y.; Moon, O.S.; Seo, Y.W.; Son, H.Y.; Won, Y.S.; Kwun, H.J. Ulmus macrocarpa Hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J. Ethnopharmacol. 2019, 233, 115–122. [Google Scholar] [CrossRef]
- Park, H.S.; Wijerathne, C.U.B.; Jeong, H.Y.; Seo, C.S.; Ha, H.; Kwun, H.J. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J. Ethnopharmacol. 2018, 216, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, B.; Zielinski, H.; Dabrowski, M. Role of apoptosis and chronic prostatitis in the pathogenesis of benign prostatic hyperplasia. Pol. Merkur. Lek. 2004, 17, 307–310. [Google Scholar]
- Reynolds, A.R.; Kyprianou, N. Growth factor signalling in prostatic growth: Significance in tumour development and therapeutic targeting. Br. J. Pharmacol. 2006, 147 (Suppl. S2), S144–S152. [Google Scholar] [CrossRef] [PubMed]
- Gershtein, E.S.; Scherbakov, A.M.; Shatskaya, V.A.; Kushlinsky, N.E.; Krasil’nikov, M.A. Phosphatidylinositol 3-kinase/AKT signalling pathway components in human breast cancer: Clinicopathological correlations. Anticancer Res. 2007, 27, 1777–1782. [Google Scholar] [PubMed]
- Sillman, S.J.; Lee, S.T.; Claborn, J.; Boruch, J.; Harris, S.P. Fall panicum (Panicum dichotomiflorum) toxicosis in three juvenile goats. J. Vet. Diagn. Investig. 2019, 31, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Divers, T.J.; Freckleton, M.L.; McKenzie, H.C.; Mitchell, E.; Cullen, J.M.; McDonough, S.P. Fall panicum (Panicum dichotomiflorum) hepatotoxicosis in horses and sheep. J. Vet. Intern. Med. 2006, 20, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- Wurzel, R.; Ray, P.; Major-Walker, K.; Shannon, J.; Rittmaster, R. The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men with benign prostatic hyperplasia. Prostate Cancer Prostatic. Dis. 2007, 10, 149–154. [Google Scholar] [CrossRef]
- Lee, G.; Shin, J.; Choi, H.; Jo, A.; Pan, S.; Bae, D.; Lee, Y.; Choi, C. Cynanchum wilfordii Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating 5alpha-Reductase and Androgen Receptor Activities in a Rat Model. Nutrients 2017, 9, 1070. [Google Scholar] [CrossRef]
- Park, H.S.; Seo, C.S.; Wijerathne, C.U.; Jeong, H.Y.; Moon, O.S.; Seo, Y.W.; Won, Y.S.; Son, H.Y.; Lim, J.H.; Kwun, H.J. Effect of Veratrum maackii on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats. Biol. Pharm. Bull. 2019, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Vela-Navarrete, R.; Escribano-Burgos, M.; Farre, A.L.; Garcia-Cardoso, J.; Manzarbeitia, F.; Carrasco, C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. J. Urol. 2005, 173, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Djavan, B.; Margreiter, M.; Dianat, S.S. An algorithm for medical management in male lower urinary tract symptoms. Curr. Opin. Urol. 2011, 21, 5–12. [Google Scholar] [CrossRef]
- Liu, R.F.; Fu, G.; Li, J.; Yang, Y.F.; Wang, X.G.; Bai, P.D.; Chen, Y.D. Roles of autophagy in androgen-induced benign prostatic hyperplasia in castrated rats. Exp. Ther. Med. 2018, 15, 2703–2710. [Google Scholar] [CrossRef]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Radmayr, C.; Hittmair, A.; Zhang, J.; Thurnher, M.; Bartsch, G.; Klocker, H. Regulation of prostatic growth and function by peptide growth factors. Prostate 1996, 28, 392–405. [Google Scholar] [CrossRef]
- Lucia, M.S.; Lambert, J.R. Growth factors in benign prostatic hyperplasia: Basic science implications. Curr. Urol. Rep. 2008, 9, 272–278. [Google Scholar] [CrossRef]
- Sampson, N.; Zenzmaier, C.; Heitz, M.; Hermann, M.; Plas, E.; Schafer, G.; Klocker, H.; Berger, P. Stromal insulin-like growth factor binding protein 3 (IGFBP3) is elevated in the diseased human prostate and promotes ex vivo fibroblast-to-myofibroblast differentiation. Endocrinology 2013, 154, 2586–2599. [Google Scholar] [CrossRef]
- Descazeaud, A.; Weinbreck, N.; Robert, G.; Vacherot, F.; Abbou, C.C.; Labrousse, F.; Allory, Y.; Rubin, M.A.; de la Taille, A. Transforming growth factor beta-receptor II protein expression in benign prostatic hyperplasia is associated with prostate volume and inflammation. BJU Int. 2011, 108, E23–E28. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.C.; Wang, Y.; Zhang, S.W.; Luo, D.K.; Chang, D.G.; Wu, X.Q.; Tang, M.; He, Z.M. Angiogenesis and regulatory factors in rats with BPH induced by testosterone. Zhonghua Nan Ke Xue 2005, 11, 413–418. [Google Scholar] [PubMed]
- Shah, A.; Shah, A.A.; Nandakumar, K.; Lobo, R. Mechanistic targets for BPH and prostate cancer-a review. Rev. Environ. Health 2020, 36, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.L.; Antonarakis, E.S. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer Metast. Rev. 2014, 33, 581–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macoska, J.A. Chemokines and BPH/LUTS. Differentiation 2011, 82, 253–260. [Google Scholar] [CrossRef]
- McLaren, I.D.; Jerde, T.J.; Bushman, W. Role of interleukins, IGF and stem cells in BPH. Differentiation 2011, 82, 237–243. [Google Scholar] [CrossRef]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Laverny, G.; Gacci, M.; Crescioli, C.; Maggi, M.; Adorini, L. Human Benign Prostatic Hyperplasia Stromal Cells As Inducers and Targets of Chronic Immuno-Mediated Inflammation. J. Immunol. 2009, 182, 4056–4064. [Google Scholar] [CrossRef]
- Schenk, J.M.; Kristal, A.R.; Neuhouser, M.L.; Tangen, C.M.; White, E.; Lin, D.W.; Kratz, M.; Thompson, I.M. Biomarkers of systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2010, 171, 571–582. [Google Scholar] [CrossRef]
- Mechergui, Y.B.; Ben Jemaa, A.; Mezigh, C.; Fraile, B.; Ben Rais, N.; Paniagua, R.; Royuela, M.; Oueslati, R. The Profile of Prostate Epithelial Cytokines and its Impact on Sera Prostate Specific Antigen Levels. Inflammation 2009, 32, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.R.; Li, Q.J.; Han, P.; Li, X.; Zeng, H.; Zhu, Y.C.; Wei, Q. Evaluation of Interleukin-8 in Expressed Prostatic Secretion as a Reliable Biomarker of Inflammation in Benign Prostatic Hyperplasia. Urology 2009, 74, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Yang, L.; Zhao, H.; Yue, C.; Li, J.; Han, L. Expression of IL-6 and TNF-α in benign prostatic hyperplasia combined with histological inflammation. Discuss. Clin. Cases 2016, 3, 11–15. [Google Scholar] [CrossRef]
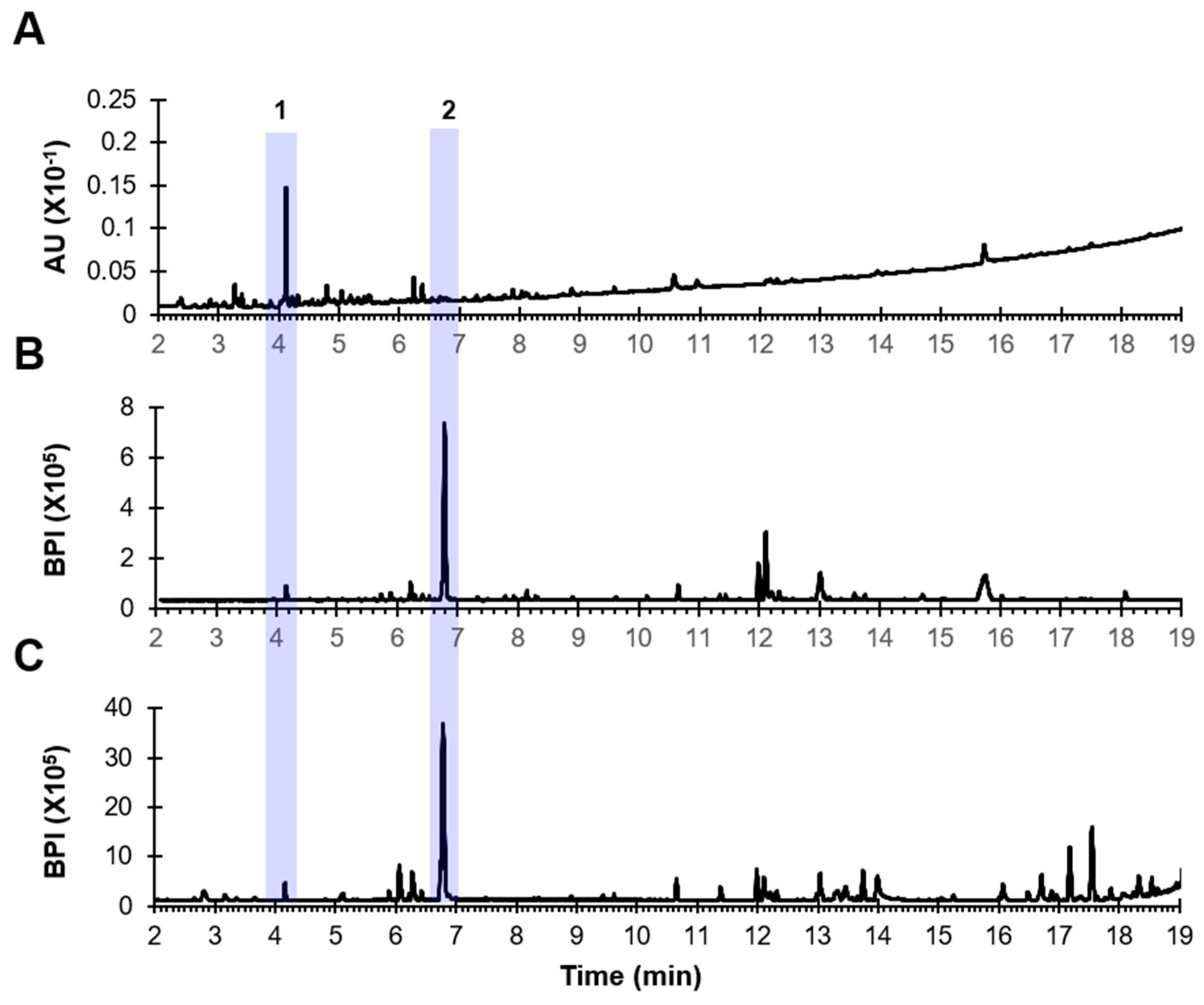


| No. | Identification | Molecular Formula | Rt (min) | Class | Detected Ion | Positive Mode | ||
|---|---|---|---|---|---|---|---|---|
| Calculated Ion | Error (ppm) | Major Fragment Ions | ||||||
| 1 | Tetrahydroxyflavonoid-C-hexosyl-O-deoxyhexoside(Isoorientin-O-rhamnoside isomer) | C27H30O15 | 4.12 | Flavonoid glycoside | 595.1663 [M+H]+ | 595.1669 [M+H]+ | 1.0 | 449, 329, 299 |
| 2 | Dichotomin | C57H94O26 | 6.74 | Steroidal saponin | 1177.6006 [M+H-H2O]+ | 1177.6030 [M+H-H2O]+ | 2.0 | 1031, 869, 723, 577, 415 |
| Group | Treatment | Body Weight (g) | Absolute Prostate Weight (g) | Relative Prostate Weight (%) | Percent Inhibition |
|---|---|---|---|---|---|
| NC | Corn oil/PBS | 424.13 ± 31.17 | 0.82 ± 0.11 | 0.21±0.01 | - |
| BPH | Testosterone/PBS | 405.67 ± 45.66 | 1.54 ± 0.15 ## | 0.36±0.04 ## | - |
| Fina | Testosterone/Finasteride | 402.60 ± 55.19 | 1.07 ± 0.12 ** | 0.26±0.02 ** | 63.98% |
| PD | Testosterone/PD | 429.43 ± 35.33 | 1.33 ± 0.07 ** | 0.32±0.03 * | 30.28% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, E.B.; Hong, E.-J.; Kim, J.-H.; Kim, M.; Ahn, J.; Kwun, H.-J. Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia. Sci. Pharm. 2024, 92, 13. https://doi.org/10.3390/scipharm92010013
Baek EB, Hong E-J, Kim J-H, Kim M, Ahn J, Kwun H-J. Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia. Scientia Pharmaceutica. 2024; 92(1):13. https://doi.org/10.3390/scipharm92010013
Chicago/Turabian StyleBaek, Eun Bok, Eun-Ju Hong, Jung-Hee Kim, Min Kim, Jongmin Ahn, and Hyo-Jung Kwun. 2024. "Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia" Scientia Pharmaceutica 92, no. 1: 13. https://doi.org/10.3390/scipharm92010013
APA StyleBaek, E. B., Hong, E.-J., Kim, J.-H., Kim, M., Ahn, J., & Kwun, H.-J. (2024). Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia. Scientia Pharmaceutica, 92(1), 13. https://doi.org/10.3390/scipharm92010013







