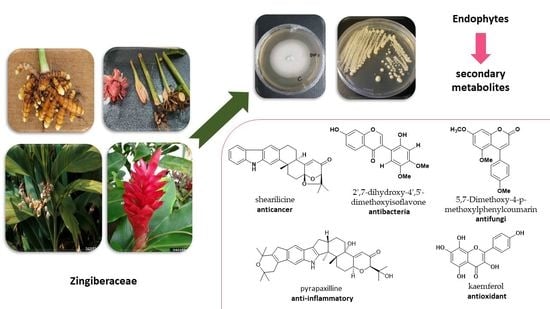
Secondary Metabolites of Endophytes Associated with the Zingiberaceae Family and Their Pharmacological Activities
Abstract
1. Introduction
2. Botany
3. Natural Product Diversity
3.1. Volatile Constituents
3.2. Polyketides
3.3. Nonribosomal Peptides
3.4. Aromatic Compounds
3.5. Alkaloids
3.6. Indole Diterpenoids
4. Pharmacological Activities
4.1. Antimicrobial Activity
4.2. Anticancer Activity
4.3. Antioxidant Activity
4.4. Anti-Inflammatory Activity
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| DMF | Dimethylformamide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ECD | Electronic Circular Dichroism |
| GC–MS | Gas Chromatography–Mass Spectroscopy |
| IC50 | Inhibitory Concentration 50 |
| IZ | Inhibition Zones |
| LPS-induced NO | Lipopolysaccharide-Induced Nitric Oxide |
| MBC | Minimum Bactericidal Concentration |
| MFC | Minimum Fungicidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MMC | Minimum Microbicidal Concentration |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| MTT | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium |
| NMR | Nuclear Magnetic Resonance |
| PCR | Polymerase Chain Reaction |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| ROS | Reactive Oxygen Species |
| SC50 | Scavenging Concentration 50 |
| TCPTP | T-Cell Protein Tyrosine Phosphatase |
| TDDFT | Time-Dependent Density-Functional Theory |
| VCD | Vibrational Circular Dichroism |
References
- López, E.I.C.; Balcázar, M.F.H.; Mendoza, J.M.R.; Ortiz, A.D.R.; Melo, M.T.O.; Parrales, R.S.; Delgado, T.H. Antimicrobial activity of essential oil of Zingiber officinale Roscoe (Zingiberaceae). Am. J. Plant Sci. 2017, 8, 1511–1524. [Google Scholar] [CrossRef]
- Ewon, K.; Bhagya, A.S. A review on golden species of Zingiberaceae family around the world: Genus Curcuma. Afr. J. Agric. Res. 2019, 14, 519–531. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar]
- Preetha, T.S.; Hemanthakumar, A.S.; Krishnan, P.N. A comprehensive review of Kaempferia galanga L. (Zingiberaceae): A high sought medicinal plant in Tropical Asia. J. Med. Plants Stud. 2016, 4, 270–276. [Google Scholar]
- Devi, N.B.; Singh, P.K.; Das, A.K. Ethnomedicinal utilization of Zingiberaceae in the valley districts of Manipur. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 21–23. [Google Scholar]
- Furmuly, A.M.; Azemi, N. A Review on Golden Species of Zingiberaceae Family Genus Curcuma. Sci. Proc. Ser. 2020, 2, 133–138. [Google Scholar] [CrossRef]
- Akinola, A.A.; Ahmad, S.; Maziah, M. Total antioxidant capacity, flavonoid, phenolic acid and polyphenol content in ten selected species of Zingiberaceae rhizomes. Afr. J. Tradit. Complementary Altern. Med. 2014, 11, 7–13. [Google Scholar] [CrossRef]
- Kumar, K.M.P.; Asish, G.R.; Sabu, M.; Balachandran, I. Significance of gingers (Zingiberaceae) in Indian system of medicine-Ayurveda: An overview. Anc. Sci. Life 2013, 32, 253. [Google Scholar] [CrossRef]
- Wohlmuth, H. Phytochemistry and Pharmacology of Plants from the Ginger family, Zingiberaceae. Ph.D. Thesis, Southern Cross University, East Lismore, Australia, 2008. [Google Scholar]
- Victório, C.P. Therapeutic value of the genus Alpinia, Zingiberaceae. Rev. Bras. Farmacogn. 2011, 21, 194–201. [Google Scholar] [CrossRef]
- Zahara, M.; Hasanah, M.; Zalianda, R. Identification of Zingiberaceae as medicinal plants in Gunung Cut Village, Aceh Barat Daya, Indonesia. J. Trop. Hortic. 2018, 1, 24–28. [Google Scholar] [CrossRef]
- Anisha, C.; Radhakrishnan, E.K. Metabolite analysis of endophytic fungi from cultivars of Zingiber officinale Rosc. identifies myriad of bioactive compounds including tyrosol. 3 Biotech 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Krishnapura, P.R.; Belur, P.D. Isolation and screening of endophytes from the rhizomes of some Zingiberaceae plants for L-asparaginase production. Prep. Biochem. Biotechnol. 2016, 46, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Sasaki, T.; Shibuya, F.; Yasuda, Y.; Koseki, T.; Supratman, U. Isolation of a phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from the mangrove, Rhizhopora mucronata. Nat. Prod. Commun. 2013, 8, 1934578X1300801220. [Google Scholar] [CrossRef]
- Supratman, U.; Hirai, N.; Sato, S.; Watanabe, K.; Malik, A.; Annas, S.; Harneti, D.; Maharani, R.; Koseki, T.; Shiono, Y. New naphthoquinone derivatives from Fusarium napiforme of a mangrove plant. Nat. Prod. Res. 2021, 35, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Supratman, U.; Suzuki, T.; Nakamura, T.; Yokoyama, Y.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Koseki, T.; Shiono, Y. New metabolites produced by endophyte Clonostachys rosea B5−2. Nat. Prod. Res. 2021, 35, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Azhari, A.; Supratman, U. The Chemistry and Pharmacology of Fungal Genus Periconia: A Review. Sci. Pharm. 2021, 89, 34. [Google Scholar] [CrossRef]
- Taechowisan, T.; Lu, C.; Shen, Y.; Lumyong, S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 2005, 151, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Taechowisan, T.; Wanbanjob, A.; Tuntiwachwuttikul, P.; Taylor, W.C. Identification of Streptomyces sp. Tc022, an endophyte in Alpinia galanga, and the isolation of actinomycin D. Ann. Microbiol. 2006, 56, 113–117. [Google Scholar] [CrossRef]
- Taechowisan, T.; Chuaychot, N.; Chanaphat, S.; Wanbanjob, A.; Shen, Y. Biological activity of chemical constituents isolated from Streptomyces sp. Tc052, an endophyte in Alpinia galanga. Int. J. Pharm. 2008, 4, 95–101. [Google Scholar] [CrossRef]
- Taechowisan, T.; Chaisaeng, S.; Phutdhawong, W.S. Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07: An endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agric. Immunol. 2017, 28, 1330–1346. [Google Scholar] [CrossRef]
- Taechowisan, T.; Samsawat, T.; Puckdee, W.; Phutdhawong, W.S. Cytotoxicity and antibacterial activities of crude extract of Streptomyces sp. W08, an endophyte of Amomum krervanh Pierre. J. Appl. Pharm. Sci. 2021, 11, 134–138. [Google Scholar]
- Suebrasri, T.; Somteds, A.; Harada, H.; Kanokmedhakul, S.; Jogloy, S.; Ekprasert, J.; Lumyong, S.; Boonlue, S. Novel endophytic fungi with fungicidal metabolites suppress sclerotium disease. Rhizosphere 2020, 16, 100250. [Google Scholar] [CrossRef]
- Hammerschmidt, L.; Ola, A.; Mueller, W.E.; Lin, W.; Mandi, A.; Kurtan, T.; Proksch, P.; Aly, A.H. Two new metabolites from the endophytic fungus Xylaria sp. isolated from the medicinal plant Curcuma xanthorrhiza. Tetrahedron Lett. 2015, 56, 1193–1197. [Google Scholar] [CrossRef]
- Alshaibani, M.M.; Jalil, J.; Sidik, N.M.; Edrada-Ebel, R.; Zin, N.M. Isolation and characterization of cyclo-(tryptophanyl-prolyl) and chloramphenicol from Streptomyces sp. SUK 25 with antimethicillin-resistant Staphylococcus aureus activity. Drug Des. Dev. Ther. 2016, 10, 1817. [Google Scholar]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2021, 35, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ariantari, N.P.; Ancheeva, E.; Wang, C.; Mándi, A.; Knedel, T.O.; Kurtán, T.; Chaidir, C.; Müller, W.E.; Kassack, M.U.; Janiak, C.; et al. Indole diterpenoids from an endophytic Penicillium sp. J. Nat. Prod. 2019, 82, 1412–1423. [Google Scholar] [CrossRef]
- Taechowisan, T.; Chanaphat, S.; Ruensamran, W.; Phutdhawong, W.S. Antibacterial activity of new flavonoids from Streptomyces sp. BT01; an endophyte in Boesenbergia rotunda (L.) Mansf. J. Appl. Pharm. Sci. 2014, 4, 8–13. [Google Scholar]
- Taechowisan, T.; Chanaphat, S.; Ruensamran, W.; Phutdhawong, W.S. Antifungal activity of 3-methylcarbazoles from Streptomyces sp. LJK109; an endophyte in Alpinia galangal. J. Appl. Pharm. Sci. 2012, 2, 124–128. [Google Scholar]
- Pansanit, A.; Pripdeevech, P. Antibacterial secondary metabolites from an endophytic fungus, Arthrinium sp. MFLUCC16-1053 isolated from Zingiber cassumunar. Mycology 2018, 9, 264–272. [Google Scholar] [CrossRef]
- Vinayarani, G.; Prakash, H.S. Fungal endophytes of turmeric (Curcuma longa L.) and their biocontrol potential against pathogens Pythium aphanidermatum and Rhizoctonia solani. World J. Microbiol. Biotechnol. 2018, 34, 1–17. [Google Scholar] [CrossRef]
- Das, M.; Prakash, H.S.; Nalini, M.S. Antibacterial metabolites from Bipolaris specifera, an endophytic fungus from the endemic medicinal plant, Zingiber nimmonii (J. Graham) Dalzell. 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Krishnakumar, P.; Varghese, M.; Joe, M.G.; Rajagopal, A.; Varghese, L. Identification and bioactivities of endophytic fungi from Lagenandra toxicaria Dalz. and Kaempferia rotunda L. J. Appl. Biol. Biotechnol. 2021, 9, 1–2. [Google Scholar]
- Anisha, C.; Sachidanandan, P.; Radhakrishnan, E.K. Endophytic Paraconiothyrium sp. from Zingiber officinale Rosc. displays broad-spectrum antimicrobial activity by production of danthron. Curr. Microbiol. 2018, 75, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Abbate, S.; Burgi, L.F.; Castiglioni, E.; Lebon, F.; Longhi, G.; Toscano, E.; Caccamese, S. Assessment of configurational and conformational properties of naringenin by vibrational circular dichroism. Chirality Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2009, 21, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Seephonkai, P.; Kongsaeree, P.; Prabpai, S.; Isaka, M.; Thebtaranonth, Y. Transformation of an irregularly bridged epidithiodiketopiperazine to trichodermamide A. Org. Lett. 2006, 8, 3073–3075. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.; Singh, P.P.; Singh, S.K.; Singh, P.K.; Pandey, K.D. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocatal. Agric. Biotechnol. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Aswathy, A.J.; Jasim, B.; Jyothis, M.; Radhakrishnan, E.K. Identification of two strains of Paenibacillus sp. as indole 3 acetic acid-producing rhizome-associated endophytic bacteria from Curcuma longa. 3 Biotech 2013, 3, 219–224. [Google Scholar] [CrossRef]
- Jasim, B.; Joseph, A.A.; John, C.J.; Mathew, J.; Radhakrishnan, E.K. Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech 2014, 4, 197–204. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Ye, X.; Wei, B.; Emam, M.; Zhang, H.; Wang, H. The structural diversity of marine microbial secondary metabolites based on co-culture strategy: 2009–2019. Mar. Drugs 2020, 18, 449. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, X.; Liu, H.; Liu, Y.; Li, Y.; Yu, X.; Zhao, K.; Gu, Y.; Xu, K.; Chen, C.; et al. Endophytes isolated from ginger rhizome exhibit growth promoting potential for Zea mays. Arch. Agron. Soil Sci. 2018, 64, 1302–1314. [Google Scholar] [CrossRef]
- Hartanto, A.; Lutfia, A.; Munir, E. Identification of a Potential IAA-Producing Fungus Isolated from Alpinia sp. Rhizome in Hutan Sibayak, North Sumatera. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1351, p. 012024. [Google Scholar]
- Smith, A.B.; Cui, H. Total synthesis of (−)-21-isopentenylpaxilline. Org. Lett. 2003, 5, 587–590. [Google Scholar] [CrossRef]
- Springer, J.P.; Clardy, J.; Wells, J.M.; Cole, R.J.; Kirksey, J.W. The structure of paxilline, a tremorgenic metabolite of Penicillium paxilli Bainier. Tetrahedron Lett. 1975, 16, 2531–2534. [Google Scholar] [CrossRef]
- Nozawa, K.; Nakajima, S.; Kawai, K.I.; Udagawa, S.I. Isolation and structures of indoloditerpenes, possible biosynthetic intermediates to the tremorgenic mycotoxin, paxilline, from Emericella striata. J. Chem. Soc. Perkin Trans. 1988, 1, 2607–2610. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.P.; Liu, S.F.; Yin, C.Y.; Tang, D.Y.; Li, Y.H.; Zhang, L.X. 7-Methoxy-13-dehydroxypaxilline: New indole diterpenoid from an endophytic fungus Penicillium sp. Nb 19. Nat. Prod. Res. 2022, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Gessner, G.; Groth, I.; Lange, C.; Christner, A.; Bruhn, T.; Deng, Z.; Li, X.; Heinemann, S.H.; Grabley, S.; et al. Shearinines D–K, new indole triterpenoids from an endophytic Penicillium sp. (strain HKI0459) with blocking activity on large-conductance calcium-activated potassium channels. Tetrahedron 2007, 63, 435–444. [Google Scholar] [CrossRef]
- Gao, N.; Shang, Z.C.; Yu, P.; Luo, J.; Jian, K.L.; Kong, L.Y.; Yang, M.H. Alkaloids from the endophytic fungus Penicillium brefeldianum and their cytotoxic activities. Chin. Chem. Lett. 2017, 28, 1194–1199. [Google Scholar] [CrossRef]
- El-Gendy, M.M.; El-Bondkly, A.M. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J. Ind. Microbiol. Biotechnol. 2010, 37, 831–841. [Google Scholar] [CrossRef]
- El-Lakany, A.M.; Aboul-Ela, M.A.; Abdul-Ghani, M.M.; Mekky, H. Chemical constituents and biological activities of Cichorium intybus L. Nat. Prod. Sci. 2004, 10, 69–73. [Google Scholar]
- Hu, X.Y.; Meng, L.H.; Li, X.; Yang, S.Q.; Li, X.M.; Wang, B.G. Three new indole diterpenoids from the sea-anemone-derived fungus Penicillium sp. AS-79. Mar. Drugs 2017, 15, 137. [Google Scholar] [CrossRef]
- Zhou, L.M.; Kong, F.D.; Fan, P.; Ma, Q.Y.; Xie, Q.Y.; Li, J.H.; Zheng, H.Z.; Zheng, Z.H.; Yuan, J.Z.; Dai, H.F.; et al. Indole-diterpenoids with protein tyrosine phosphatase inhibitory activities from the marine-derived fungus Penicillium sp. KFD28. J. Nat. Prod. 2019, 82, 2638–2644. [Google Scholar] [CrossRef]
- Taechowisan, T.; Chuaychot, N.; Chanaphat, S.; Wanbanjob, A.; Shen, Y. Cytoprotective activity of chemical constituents isolated from Streptomyces sp. Int. J. Biol. Chem. 2009, 3, 11–17. [Google Scholar] [CrossRef]
- Chan, S.C.; Chang, Y.S.; Wang, J.P.; Chen, S.C.; Kuo, S.C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 1998, 64, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Ikeda, Y.; Iinuma, H.; Kushida, N.; Kunisada, T.; Simizu, S.; Umezawa, K. Isolation of a novel paxilline analog pyrapaxilline from fungus that inhibits LPS-induced NO production. J. Antibiot. 2014, 67, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Salasiah, M.; Meekiong, K. Preliminary anatomical study on leaf surfaces of bornean Zingiberaceae (tribe Alpinieae) from north east sarawak. Malays. Appl. Biol. 2018, 47, 289–293. [Google Scholar]
- Pedersen, L.B. Phylogenetic analysis of the subfamily Alpinioideae (Zingiberaceae), particularly Etlingera Giseke, based on nuclear and plastid DNA. Plant Syst. Evol. 2004, 245, 239–258. [Google Scholar] [CrossRef]
- Gevú, K.V.; Da Cunha, M.; Barros, C.F.; Pereira, S.M.; Lima, H.R.P. Structural analysis of subterranean organs in Zingiberaceae. Plant Syst. Evol. 2014, 300, 1089–1098. [Google Scholar] [CrossRef]
- Zhao, H.; Xiao, M.H.; Zhong, Y.; Wang, Y.Q. Leaf epidermal micromorphology of Zingiber (Zingiberaceae) from China and its systematic significance. PhytoKeys 2022, 190, 131. [Google Scholar] [CrossRef]
- Eswani, N.; Abd Kudus, K.; Nazre, M.; Noor, A.A.; Ali, M. Medicinal plant diversity and vegetation analysis of logged over hill forest of Tekai Tembeling Forest Reserve, Jerantut, Pahang. J. Agric. Sci. 2010, 2, 189. [Google Scholar] [CrossRef]
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S. Diversity, traditional uses and conservation status of Zingiberaceae in Udorn Thani Province, Thailand. Biodiversitas J. Biol. Divers. 2021, 22, 3083–3097. [Google Scholar] [CrossRef]
- Larsen, K.; Lock, J.M.; Maas, H.; Maas, P.J.M. Zingiberaceae. In Flowering Plants· Monocotyledons; Springer: Berlin/Heidelberg, Germany, 1998; pp. 474–495. [Google Scholar]
- Newman, M.; Lhuillier, A.; Poulsen, A.D. Checklist of the Zingiberaceae of Malesia. Blumea. Suppl. 2004, 16, 1–166. [Google Scholar]
- Kuehny, J.S.; Sarmiento, M.; Paz, M.P.; Branch, P.C. Effect of light intensity, photoperiod and plant growth retardants on production of Zingiberaceae as pot plants. Acta Hortic. 2005, 683, 145. [Google Scholar] [CrossRef]
- Thomas, S.; Britto, S.J.; Mani, B. A comprehensive account on the genus Hedychium J. konig (Zingiberaceae) in south India. Plant Sci. Today 2022, 9, 12–18. [Google Scholar] [CrossRef]
- Basak, S.; Sarma, G.C.; Rangan, L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J. Ethnopharmacol. 2010, 132, 286–296. [Google Scholar]
- Navia, Z.I.; Audira, D.; Afifah, N.; Turnip, K.; NURAINI, N.; Suwardi, A.B. Ethnobotanical investigation of spice and condiment plants used by the Taming tribe in Aceh, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 4467–4473. [Google Scholar] [CrossRef]
- Rachkeeree, A.; Kantadoung, K.; Suksathan, R.; Puangpradab, R.; Page, P.A.; Sommano, S.R. Nutritional compositions and phytochemical properties of the edible flowers from selected Zingiberaceae found in Thailand. Front. Nutr. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ghataury, S.K.; Sarathe, A.; Dubey, G.; Parkhe, G. Curcuma angustifolia Roxb, (Zingiberaceae): Ethnobotany, phytochemistry and pharmacology: A review. J. Pharmacogn. Phytochem. 2019, 8, 1535–1540. [Google Scholar]
- Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; UNIDO: Trieste, Italy, 2008. [Google Scholar]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Mamusa, M.; Resta, C.; Sofroniou, C.; Baglioni, P. Encapsulation of volatile compounds in liquid media: Fragrances, flavors, and essential oils in commercial formulations. Adv. Colloid Interface Sci. 2021, 298, 102544. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Abate, L.; Bachheti, A.; Bachheti, R.K.; Husen, A.; Getachew, M.; Pandey, D.P. Potential role of forest-based plants in essential oil production: An approach to cosmetic and personal health care applications. In Non-Timber Forest Products; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Okigbo, R.N.; Anuagasi, C.L.; Amadi, J.E. Advances in selected medicinal and aromatic plants indigenous to Africa. J. Med. Plants Res. 2009, 3, 86–95. [Google Scholar]
- Paibon, W.; Yinmoi, C.A.; Tembab, N.; Boonlue, W.; Jampachaisri, K.; Nuengchamnong, N.; Waranuch, N.; Ingkaninan, K. Comparison and evaluation of volatile oils from three different extraction methods for some Thai fragrant flowers. Int. J. Cosmet. Sci. 2011, 33, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Plaszkó, T.; Szűcs, Z.; Kállai, Z.; Csoma, H.; Vasas, G.; Gonda, S. Volatile organic compounds (VOCs) of endophytic fungi growing on extracts of the host, horseradish (Armoracia rusticana). Metabolites 2020, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, F.; Zeng, J.; Zhan, J. Type III polyketide synthases in natural product biosynthesis. IUBMB Life 2012, 64, 285–295. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Kennedy, J.; Park, C.; Kendrew, S.; Auclair, K.; Vederas, J. Aspects of the biosynthesis of non-aromatic fungal polyketides by iterative polyketide synthases. Antonie Van Leeuwenhoek 2000, 78, 287–295. [Google Scholar] [CrossRef]
- Wang, H.; Fewer, D.P.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef]
- Miyanaga, A. Structure and function of polyketide biosynthetic enzymes: Various strategies for production of structurally diverse polyketides. Biosci. Biotechnol. Biochem. 2017, 81, 2227–2236. [Google Scholar] [CrossRef]
- Bhattarai, K.; Kabir, M.E.; Bastola, R.; Baral, B. Fungal natural products galaxy: Biochemistry and molecular genetics toward blockbuster drugs discovery. Adv. Genet. 2021, 107, 193–284. [Google Scholar] [PubMed]
- Sun, D.J.; Zhu, L.J.; Zhao, Y.Q.; Zhen, Y.Q.; Zhang, L.; Lin, C.C.; Chen, L.X. Diarylheptanoid: A privileged structure in drug discovery. Fitoterapia 2020, 142, 104490. [Google Scholar] [CrossRef] [PubMed]
- Dell, M.; Dunbar, K.L.; Hertweck, C. Ribosome-independent peptide biosynthesis: The challenge of a unifying nomenclature. Nat. Prod. Rep. 2022, 39, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.H.; Vickery, C.R.; Burkart, M.D. Explorations of catalytic domains in nonribosomal peptide synthetase enzymology. Nat. Prod. Rep. 2012, 29, 1074–1098. [Google Scholar] [CrossRef] [PubMed]
- Little, R.F.; Hertweck, C. Chain release mechanisms in polyketide and nonribosomal peptide biosynthesis. Nat. Prod. Rep. 2022, 39, 163–205. [Google Scholar] [CrossRef] [PubMed]
- Finking, R.; Marahiel, M.A. Biosynthesis of Nonribosomal Peptides. Annu. Rev. Microbiol. 2004, 58, 453–488. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhu, L.; Zeng, D.; Long, W.; Zhu, S.M. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J. Food Drug Anal. 2016, 24, 602–609. [Google Scholar] [CrossRef]
- Samarth, R.M.; Samarth, M.; Matsumoto, Y. Medicinally important aromatic plants with radioprotective activity. Future Sci. OA 2017, 3, FSO247. [Google Scholar] [CrossRef]
- Kumar, Y.; Prakash, O.; Tripathi, H.; Tandon, S.; Gupta, M.M.; Rahman, L.U.; Khan, F. AromaDb: A database of medicinal and aromatic plant’s aroma molecules with phytochemistry and therapeutic potentials. Front. Plant Sci. 2018, 9, 1081. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. IJPB 2017, 3, 1–9. [Google Scholar]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735. [Google Scholar] [CrossRef] [PubMed]
- Aniszewski, T. Alkaloids-Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Yang, L.; Stöckigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Wang, B.G. Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Med. 2016, 82, 832–842. [Google Scholar] [CrossRef]
- Corsello, M.A.; Kim, J.; Garg, N.K. Indole diterpenoid natural products as the inspiration for new synthetic methods and strategies. Chem. Sci. 2017, 8, 5836–5844. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar]
- Agafonova, M.N.; Kazakova, R.R.; Lubina, A.P.; Zeldi, M.I.; Nikitina, E.V.; Balakin, K.V.; Shtyrlin, Y.G. Antibacterial activity profile of miramistin in in vitro and in vivo models. Microb. Pathog. 2020, 142, 104072. [Google Scholar] [CrossRef]
- Thushara, R.M.; Hemshekhar, M.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Girish, K.S. Differential action of phytochemicals on platelet apoptosis: A biological overview. Curr. Med. Chem. 2013, 20, 1018–1027. [Google Scholar] [PubMed]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile: An anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar] [PubMed]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
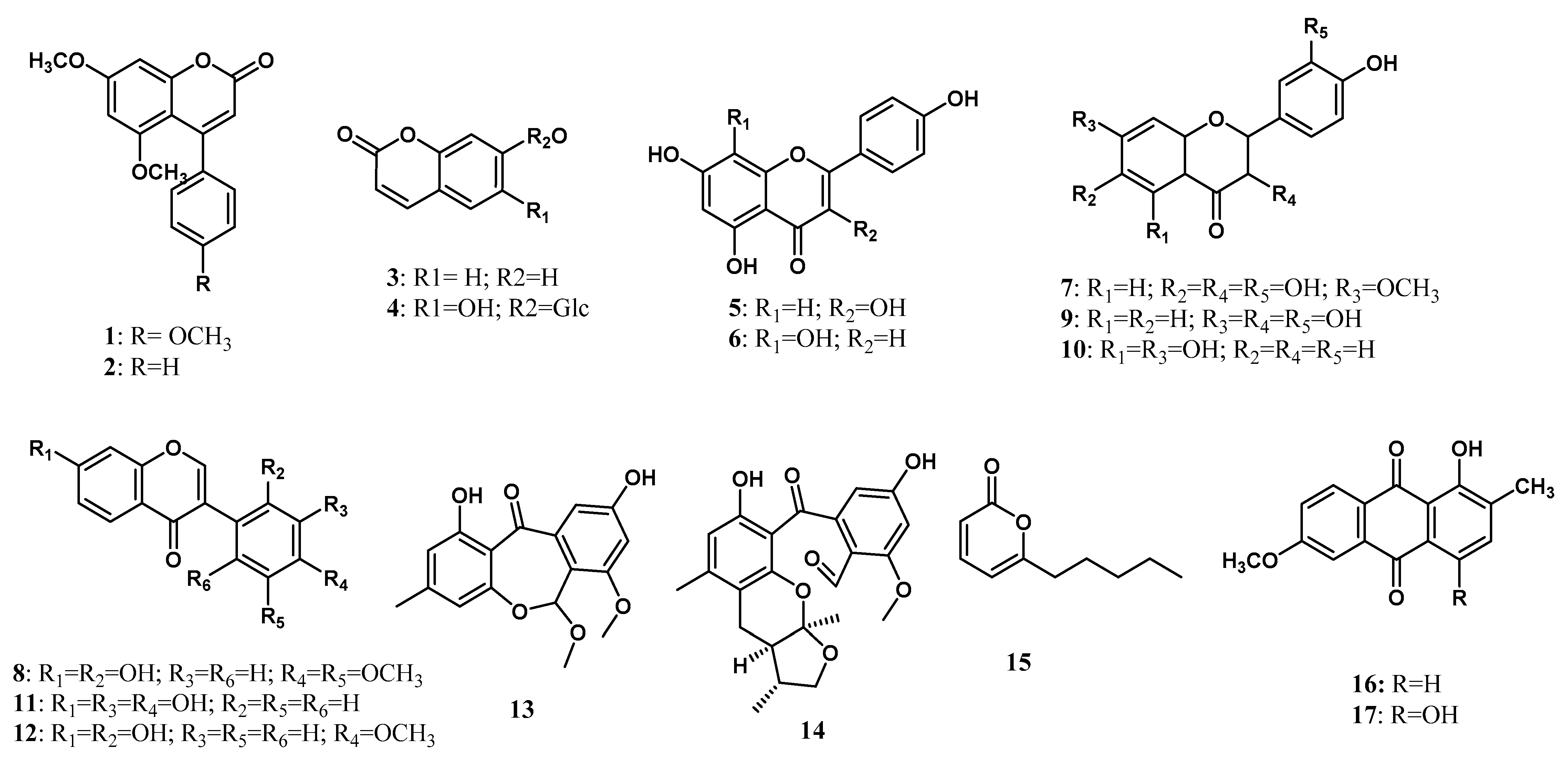

| Type | Name of Compound | Strain | Host Origin | Part of Host Origin | Reference |
|---|---|---|---|---|---|
| Polyketide | 5,7-Dimethoxy-4-p-methoxylphenylcoumarin (1) | Streptomyces aureofaciens CMUAc130 | Z. officinale Rosc. | Root | [18] |
| 5,7-Dimethoxy-4-phenylcoumarin (2) | S. aureofaciens CMUAc130 | Z. officinale Rosc. | Root | [18] | |
| Umbelliferon (3) | Streptomyces sp. Tc052 | Alpinia galanga | Root | [19] | |
| Chicorii (4) | Streptomyces sp. Tc052 | A. galanga | Root | [19] | |
| Kaempferol (5) | Streptomyces sp. Tc052 | A. galanga | Root | [19] | |
| Isoscutellarin (6) | Streptomyces sp. Tc052 | A. galanga | Root | [19] | |
| 7-methoxy-3, 3′,4′,6-tetrahydroxyflavone (7) | Streptomyces sp. BT01 | Boesenbergia rotunda (L.) Mansf. | Root | [28] | |
| 2′,7-dihydroxy-4′,5′-dimethoxyisoflavone (8) | Streptomyces sp. BT01 | B. rotunda (L.) Mansf. | Root | [28] | |
| 3,3′,4′,7-tetrahydroxyflavone (fisetin) (9) | Streptomyces sp. BT01 | B. rotunda (L.) Mansf. | Root | [28] | |
| 4′,5,7-trihydroxyflavanone (naringenin) (10) | Streptomyces sp. BT01 | B. rotunda (L.) Mansf. | Root | [28] | |
| 3′-hydroxydaidzein (11) | Streptomyces sp. BT01 | B. rotunda (L.) Mansf. | Root | [28] | |
| xenognosin B (12) | Streptomyces sp. BT01 | B. rotunda (L.) Mansf. | Root | [28] | |
| arugosin J (13) | Xylaria sp. | Curcuma xanthorrhiza | Leaves | [24] | |
| xylarugosin (14) | Xylaria sp. | C. xanthorrhiza | Leaves | [24] | |
| 6-n-pentyl-2H-pyran-2-one (15) | Trichoderma erinaceum ST-KKU2 | Z. officinale | Root | [23] | |
| 1-hydroxy-2-methyl-6-methoxyanthraquinone (16) | Streptomyces sp. W08 | Amomum krervanh Pierre | Root | [22] | |
| 6-methoxy-2-methylquinizarin (17) | Streptomyces sp. W08 | A. krervanh Pierre | Root | [22] | |
| Nonribosomal Peptides | actinomycin D (18) | Streptomyces sp. Tc022 | A. galanga | Root | [19] |
| brevianamide F (19) | Streptomyces omiyaensis NBRC 11449T | Zingiber spectabile | Root | [25] | |
| 2,2-dichloro-N-[(1r, 2r)-2-hydroxy- 1-(hydroxymethyl)-2-(4-nitrophenyl) ethyl]-acetamide (20) | S. omiyaensis NBRC 11449T | Z. spectabile | Root | [25] | |
| pretrichodermamide G (21) | Trichoderma harzianum | Z. officinale | Leaves | [26] | |
| pretrichodermamide A (22) | T. harzianum | Z. officinale | Leaves | [26] | |
| Aromatic Compound | Vanillin (23) | Streptomyces aureofaciens CMUAc130 | Z. officinale Rosc. | Root | [18] |
| 3-methoxy-4-hydroxytoluene (24) | S. aureofaciens CMUAc130 | Z. officinale Rosc. | Root | [18] | |
| Resacetophenone (25) | Xylaria sp. | C. xanthorrhiza | Leaves | [24] | |
| 3′-hydroxy-5-methoxy-3,4-methylenedioxybiphenyl (26) | Streptomyces sp. BO-07 | B. rotunda (L.) Mansf A | Root | [21] | |
| 3′- hydroxy-5,5′-dimethoxy-3,4-methylenedioxybiphenyl (27) | Streptomyces sp. BO-07 | B. rotunda (L.) Mansf A | Root | [21] | |
| Alkaloid | 3-methylcarbazole (28) | Streptomyces sp. LJK109 | A. galanga (L.) Willd. | Root | [29] |
| 1-methoxy-3- methylcarbazole (29) | Streptomyces sp. LJK109 | A. galanga (L.) Willd. | Root | [29] | |
| Indole acetic acid (30) | Bacillus subtilis CL1, Bacillus sp. CL3, Burkholderia thailandensis CL4, Agrobacterium tumefaciens CL5, Klebsiella sp. CL6, Bacillus cereus CL7, Pseudomonas putida CL9, Pseudomonas fluorescens CLI2 and Azotobacter chroococcum CL13 | Curcuma longa L. | Rhizome | [37] | |
| Paenibacillus favisporus Paenibacillus sp. | C. longa L. | Rhizome | [38] | ||
| Pseudomonas sp. | Z. officinale | Rhizome | [39] | ||
| Pseudomonas, Pantoea agglomerans, Aeromonas, Serratia, Enterobacter asburiae, and Rhizobium. | Z. officinale Roscoe | Root, stems, tubers, and Leaves | [40] | ||
| Ochrobactrum, Agrobacterium, Acinetobacter, Stenotrophomonas, Serratia and Bacillus | Z. officinale Roscoe | [41] | |||
| Bacillus cereus (ECL1), Bacillus thuringiensis (ECL2), Bacillus sp. (ECL3), Bacillus pumilis (ECL4), Pseudomonas putida (ECL5), and Clavibacter michiganensis (ECL6) | C. longa L. | [37] | |||
| T. harzianum T. asperellum T. atroviride | C. longa L. | [31] | |||
| Aspergillus flavus | Alpinia sp. | [42] | |||
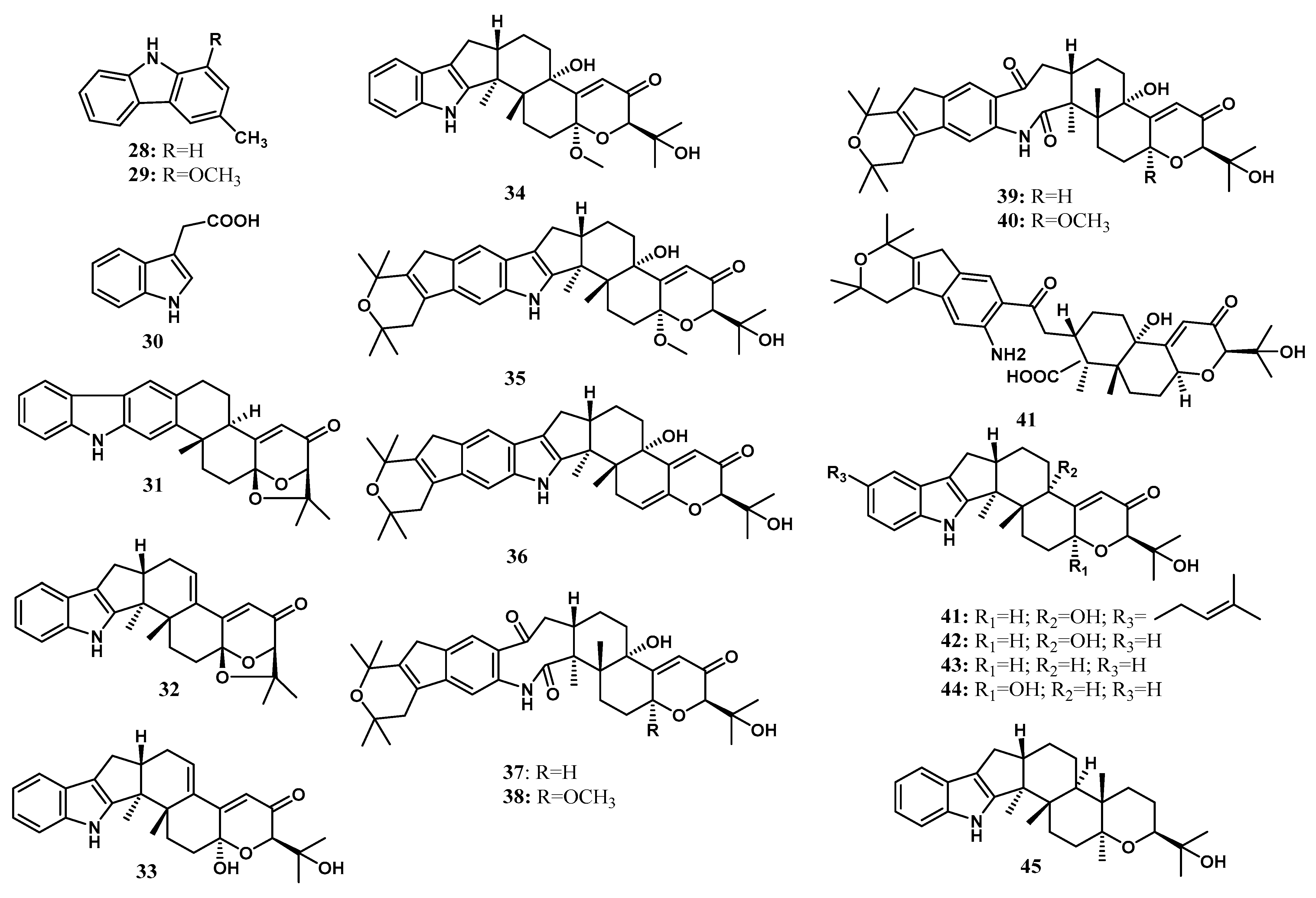
| Indole Diterpenoids | Shearilicine (31) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] |
| Paspalinine-13-ene (32) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 7-Hydroxypaxilline-13-ene (33) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 7-Methoxypaxilline (34) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Shearinine N (35) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Shearinine O (36) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Shearinine P (37) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 7-Methoxyshearinine P (38) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Shearinine Q (39) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Emindole SB (40) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 21-isopentenylpaxilline (41) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Paxilline (42) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Dehydroxypaxilline (43) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 7-hydroxy-13- dehydroxypaxilline (44) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Paspaline (45) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
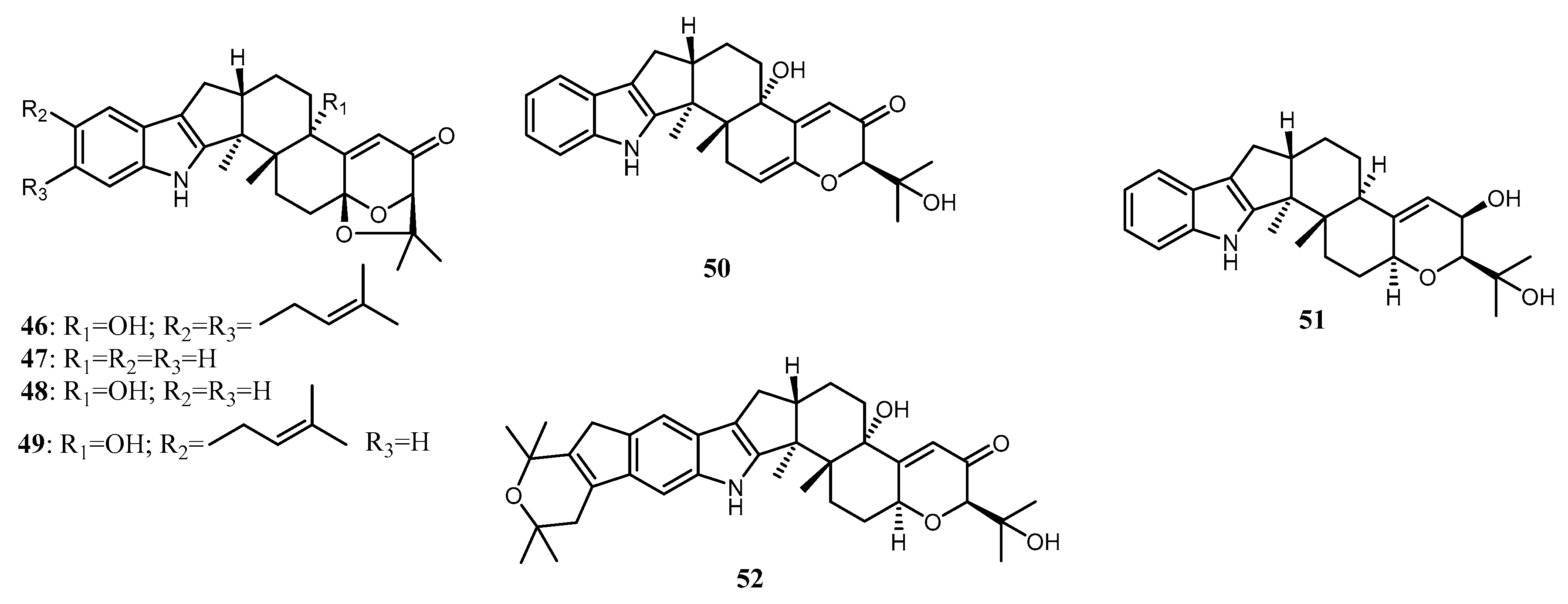
| shearinine F (46) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Paspalicine (47) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Paspalinine (48) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| paspalitrem A (49) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 6,7-dehydropaxilline (50) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| 10β-hydroxy-13-desoxypaxilline (51) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] | |
| Pyrapaxilline (52) | Penicillium sp. (strain ZO-R1-1) | Z. officinale | Root | [23] |
| No. | Compound | Molecular Formula | Fungal Species | Host | Reference |
|---|---|---|---|---|---|
| Terpenoids | |||||
| 1. | α fencho-camphorone | C9H14O | Arthrinium sp. MFLUCC16-1053 | Zingiber cassumunar | [30] |
| 2. | α-muurolol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 3. | α-sinensal | C15H22O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 4. | β-bisabolenol | C15H24O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 5. | β-chenopodiol | C15H26O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 6. | β-cyclocitral | C10H16O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 7. | β-isocomene | C15H24 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 8. | γ-curcumene | C15H24 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 9. | 3-p-menthone | C10H18O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 10. | 11,12-dihydroxy-valencene | C18H30O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 11. | 13-manool oxide | C20H34O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 12. | 2Z,6E-farnesol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 13. | 3E-cembrene A | C20H32 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 14. | 5E,9E-farnesyl acetone | C18H30O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 15. | 7-α-hydroxy manool | C20H34O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 16. | 7-epi-α-selinene | C15H24 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 17. | abienol | C20H34O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 18. | allocedrol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 19. | amorpha-4,9-dien-2-ol | C15H24O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 20. | bicyclogermacrene | C15H24 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 21. | Bornyl acetate | C12H20O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 22. | caryophyllenyl alcohol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 23. | cembrene | C20H32 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 24. | cis-cadin-4-en-7-ol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 25. | cis-vertocitral C | C9H14O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 26. | curzerenone | C15H18O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 27. | Dehydro-aromadendrene | C15H22 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 28. | E,E-geranyl linalool | C20H34O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 29. | E-iso-γ-bisabolene | C15H24 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 30. | E-phytol acatate | C22H42O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 31. | epi-α-bisabolol acetate | C17H28O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 32. | E-β-santalol acetate | C17H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 33. | himachalene epoxide | C15H24O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 34. | laurenan-2-one | C20H32O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 35. | Linalool acetate | C12H20O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 36. | Linalool isobutanoate | C14H24O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 37. | longiborneol | C15H26O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 38. | occidol | C15H22O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 39. | ophiobolin A | C25H36O4 | Trichoderma harzianum TharDOB-31 | C. longa L. | [31] |
| 40. | oplopanone | C15H26O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 41. | phyllocladanol | C20H34O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 42. | Punctaporin B | C15H24O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 43. | Sapindoside A | C41H66O12 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 44. | Sclareol | C20H36O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 45. | Z,E-geranyl linalool | C20H34O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 46. | Z,Z-farnesyl acetone | C18H30O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| Steroids | |||||
| 47. | Calicoferol D | C28H42O2 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 48. | Ergosta-4, 6, 8(14), 22-tetraen-3-one (ergone) | C28H40O | GF6 (Gliocladiopsis) | Z. officinale | [12] |
| Alkaloids | |||||
| 49. | Diethanolamine | C4H11NO2 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 50. | Harmalol | C12H12N2O | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 51. | N-Aminopyrrolidine | C4H10N2 | GFM12 (Uncultured fungus clone/Cerrena sp.) | Z. officinale | [12] |
| Aromatics | |||||
| 52. | 2-Amino-3-methoxy-benzoic acid Antifungal | C8H9NO3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 53. | 2-Isopropyl-3-Methoxycinnamic acid | C13H16O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 54. | 3-Nonaprenyl-4-hydroxybenzoic acid | C52H78O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 55. | 4-hydroxy-stilbene | C14H12O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 56. | Benzene acetaldehyde | C8H8O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 57. | Benzenacetic acid | C6H8O | GFV1 (Fungal sp.) | Z. officinale | [12] |
| 58. | Benzeneethanol, 4-hydroxy-(tyrosol) | C8H10O | GFV1 (Fungal sp.) GFM12 (Uncultured fungus clone/Cerrena sp.) | Z. officinale | [12] |
| 59. | Benzenemethanol, 2-(2-aminopropoxy)-3-methyl- | C11H16O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 60. | E-cinnamyl alcohol | C9H10O | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 61. | E-isoeugenyl benzyl ether | C17H18O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 62. | E-methyl isoprenyl cinnamate | C20H32 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 63. | Ellagic acid | C14H6O8 | A. flavus | Kaempferia rotunda | [33] |
| 64. | Ferulic acid | C10H10O4 | A. flavus | K. rotunda | [33] |
| 65. | Hydrocinnamyl acetate | C11H14O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 66. | Isoamyl benzoate | C12H16O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 67. | p-Coumaric acid | C9H8O3 | A. flavus | K. rotunda | [33] |
| 68. | phenethyl cinnamate | C17H16O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 69. | Syringic acid | C9H10O5 | A. flavus | K. rotunda | [33] |
| 70. | Vanillic acid | C8H8O4 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 71. | Z-cinnamyl acetate | C11H12O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 72. | Z-isoeugenyl phenyl acetate | C18H18O3 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 73. | Z-methyl isoprenyl cinnamate | C20H32 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| Flavonoids | |||||
| 74. | Kaempferol | C15H10O6 | A. flavus | K. rotunda | [33] |
| 75. | Myricetin | C15H10O8 | A. flavus | K. rotunda | [33] |
| Ketones | |||||
| 76. | Bicyclo[3.2.0]heptan-2-one, 6-hydroxy-5-methyl-6-vinyl | C10H14O2 | B. specifera | Zingiber nimmonii (J. Graham) Dalzell | [32] |
| 77. | trans-methyl dihydrojasmonate | C13H22O3 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| Lactone | |||||
| 78. | Dehydromevalonic lactone | C6H8O | GFV1 (Fungal sp.) | Z. officinale | [12] |
| Quinones | |||||
| 79. | 1,4-Naphthoquinone, 6-acetyl-2,5-dihydroxy | C12H8O5 | B. specifera | Z. nimmonii (J. Graham) Dalzell | [32] |
| 80. | catalpalactone | C15H14O4 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 81. | catalponone | C15H16O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| Anthraquinone | |||||
| 82. | Danthron (1,8-dihydroxyanthraquinone) | C14H8O4 | Paraconiotyrium sp. | Z. officinale Rosc. | [34] |
| Macrolides | |||||
| 83. | Dirithromycin Antimicrobial | C42H78N2O14 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| Sugars | |||||
| 84. | Acarbose | C24H40O14 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 85. | Topiramate | C12H21NO8S | T. harzianum TharDOB-31 | C. longa L. | [31] |
| Esters | |||||
| 86. | (Z)-4-Hexenoic acid, 2-acetyl-2-methyl-, ethyl ester | C11H18O3 | B. specifera | Z. nimmonii (J. Graham) Dalzell | [32] |
| 87. | Adipic acid divinyl ester | C10H14O4 | B. specifera | Z. nimmonii (J. Graham) Dalzell | [32] |
| 88. | Butanoic acid, 2-acetyl-3-methyl-, methyl ester | C8H14O | B. specifera | Z. nimmonii (J. Graham) Dalzell | [32] |
| 89. | Citronellyl formate | C11H20O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 90. | Decanedioic acid, 3,7-dimethyl-,dimethyl ester | C12H22O4 | B. specifera | Z. nimmonii (J. Graham) Dalzell | [32] |
| 91. | Dihydro citronellol acetate | C12H22O3 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| 92. | ethyl octadecanoate | C20H40O2 | Arthrinium sp. MFLUCC16-1053 | Z. cassumunar | [30] |
| Organic Acids | |||||
| 93. | 2-Methyl-2E-hexenoic acid | C7H12O2 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 94. | 2-Oxo-3-methylvaleric acid | C6H10O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 95. | 2,3-Dihydroxy stearic acid | C18H36O4 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 96. | 3-Hydroxy-tridecanoic acid | C13H26O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 97. | 3-Methyl-tetradecanedioic acid | C15H28O4 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 98. | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (methyl linoleate) | C16H32O2 | GFV1 (Fungal sp.) | Z. officinale | [12] |
| 99. | 9,12-Octadecadienoic acid (Z,Z) (linoleic acid) | C18H32O | GFV1 (Fungal sp.), GFM12 (Pseudolagarobasidium sp.), GFM11 (Uncultured fungus clone/Cerrena sp.) | Z. officinale | [12] |
| 100. | 9R-hydroxy-10E-octadecenoic acid | C18H34O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 101. | 12-Hydroxy-10-octadecynoic acid | C18H32O3 | T. harzianum TharDOB-31 | C. longa L. | [31] |
| 102. | Hexadecanoic acid (palmitic acid) | C16H32O2 | GFV1 (Fungal sp.), GFM10 (Uncultured fungus clone) | Z. officinale | [12] |
| 103. | n-Hexadecanoic acid | C16H32O2 | GFV1 (Fungal sp.), GFM12 (Uncultured fungus clone/Cerrena sp.), GFM11 (Pseudolagarobasidium sp.), GFA1 (Trichothecium sp.), GFA2 (Uncultured fungus clone), GFM10 (Uncultured fungus clone) | Z. officinale | [12] |
| 104. | Oleic acid | C18H34O2 | GFV1 (Fungal sp.), GFM12 (Uncultured fungus clone/Cerrena sp.) | Z. officinale | [12] |
| 105. | palmitic acid methyl ester | C17H34O2 | GFV1 (Fungal sp.), GFM10 (Uncultured fungus clone) | Z. officinale | [12] |
| 106. | Tetradecanoic acid (myristic acid) | C14H28O2 | GFV1 (Fungal sp.) | Z. officinale | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurjannah, L.; Azhari, A.; Supratman, U. Secondary Metabolites of Endophytes Associated with the Zingiberaceae Family and Their Pharmacological Activities. Sci. Pharm. 2023, 91, 3. https://doi.org/10.3390/scipharm91010003
Nurjannah L, Azhari A, Supratman U. Secondary Metabolites of Endophytes Associated with the Zingiberaceae Family and Their Pharmacological Activities. Scientia Pharmaceutica. 2023; 91(1):3. https://doi.org/10.3390/scipharm91010003
Chicago/Turabian StyleNurjannah, Laita, Azmi Azhari, and Unang Supratman. 2023. "Secondary Metabolites of Endophytes Associated with the Zingiberaceae Family and Their Pharmacological Activities" Scientia Pharmaceutica 91, no. 1: 3. https://doi.org/10.3390/scipharm91010003
APA StyleNurjannah, L., Azhari, A., & Supratman, U. (2023). Secondary Metabolites of Endophytes Associated with the Zingiberaceae Family and Their Pharmacological Activities. Scientia Pharmaceutica, 91(1), 3. https://doi.org/10.3390/scipharm91010003