Effectiveness of Platelet-Rich Plasma in the Treatment of Androgenic Alopecia Compared to Placebo and Topical Minoxidil: A Systematic Review
Abstract
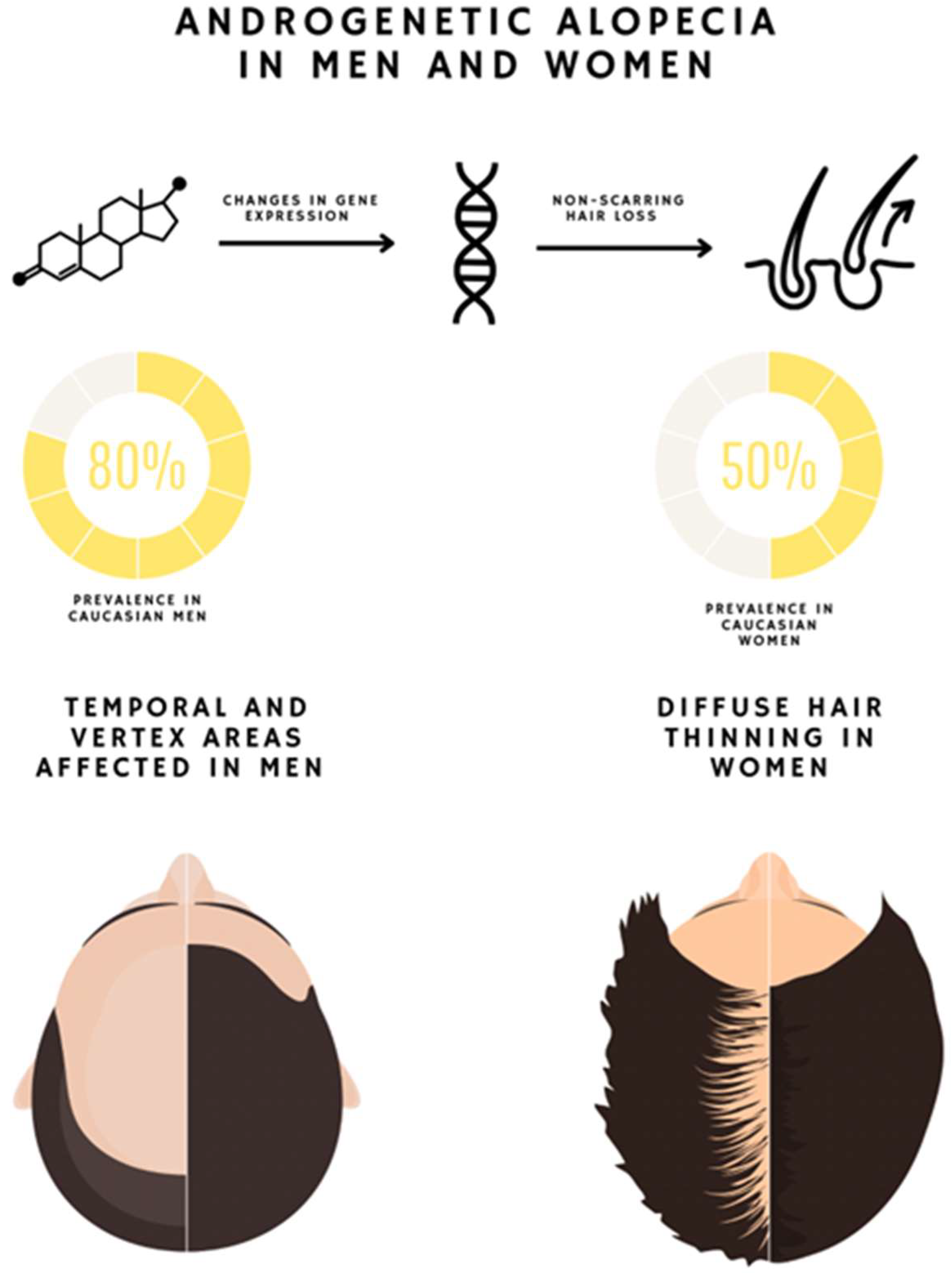
1. Introduction
2. Materials and Methods
2.1. PICOS
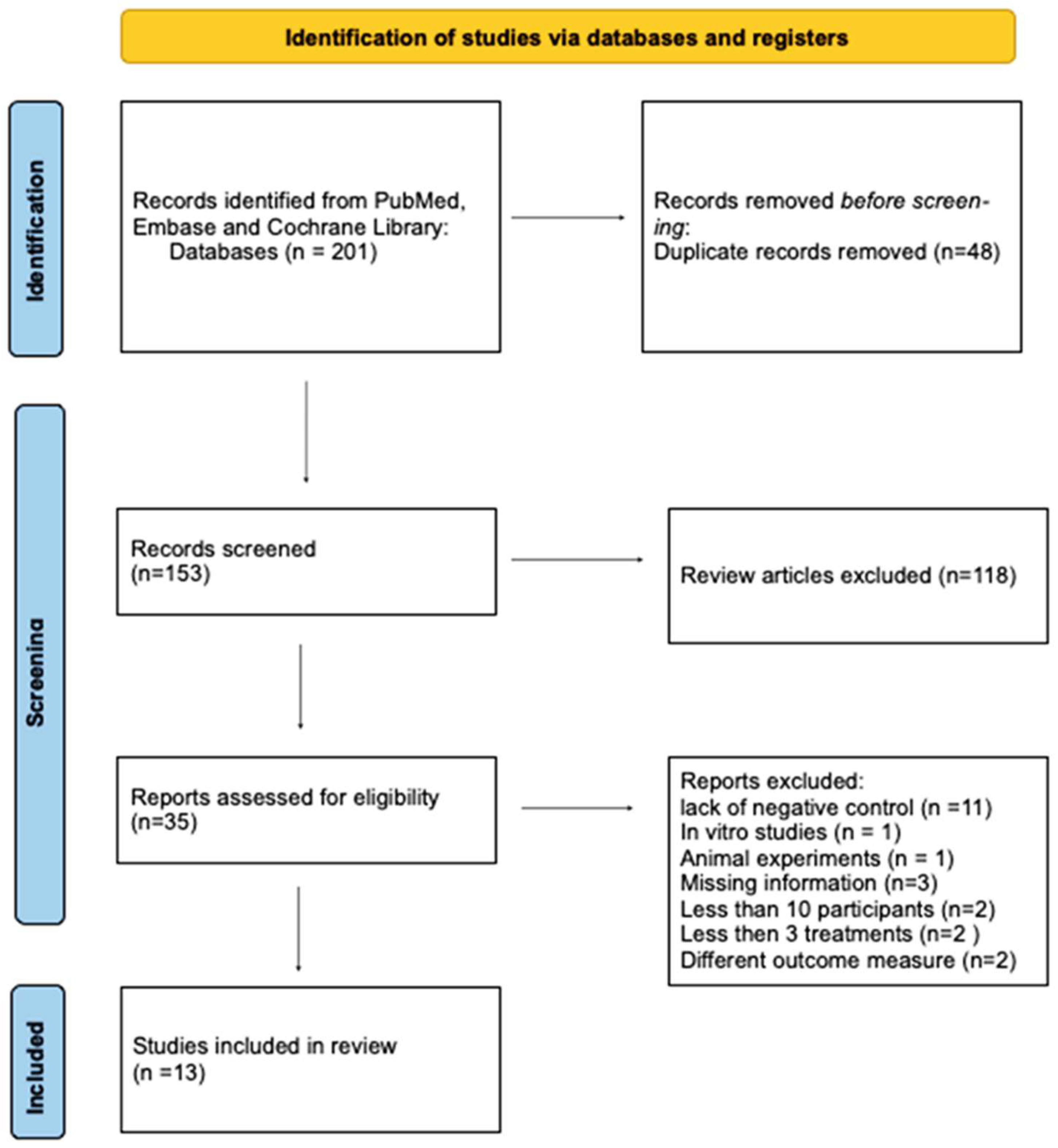
2.2. Study Selection
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, Y.; Blanco, A.; Tosti, A. Androgenetic Alopecia: An Update of Treatment Options. Drugs 2016, 76, 1349–1364. [Google Scholar] [CrossRef]
- Ho, C.H.; Sood, T.; Zito, P.M. Androgenetic Alopecia; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Miranda, B.H.; Charlesworth, M.R.; Tobin, D.J.; Sharpe, D.T.; Randall, V.A. Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life. FASEB J. 2018, 32, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Paolino, G.; Di Nicola, M.R.; Vollono, L. Investigating the Safety and Efficacy of Platelet-Rich Plasma (PRP) Treatment for Female Androgenetic Alopecia: Review of the Literature. Medicina 2021, 57, 311. [Google Scholar] [CrossRef] [PubMed]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- McElwee, K.J.; Shapiro, J.S. Promising Therapies for Treating and/or Preventing Androgenic Alopecia. Skin Therapy Lett 2012, 17, 1–4. [Google Scholar]
- Lee, S.; Lee, Y.B.; Choe, S.J.; Lee, W.-S. Adverse Sexual Effects of Treatment with Finasteride or Dutasteride for Male Androgenetic Alopecia: A Systematic Review and Meta-analysis. Acta Derm. Venereol. 2019, 99, 12–17. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef] [PubMed]
- Egger, A.; Tomic-Canic, M.; Tosti, A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4 Repair Replace Regen Reprogram 2020. p. 8. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32968692 (accessed on 3 April 2022).
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen Med. 2013, 8, 645–658. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Zhou, R.; He, Y.; Wu, Z.; Chen, Y. A narrative review of the research progress and clinical application of platelet-rich plasma. Ann. Palliat. Med. 2021, 10, 4823–4829. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Therapy Lett. 2019, 24, 1–6. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31584784 (accessed on 25 May 2022).
- Ortega-Mejia, H.; Estrugo-Devesa, A.; Saka-Herrán, C.; Ayuso-Montero, R.; López-López, J.; Velasco-Ortega, E. Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review. Materials 2020, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Devel. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, E.; Ribas, J.; Kaushik, G.; Leijten, J.; Khademhosseini, A. Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr. Stem. Cell Rep. 2016, 2, 33–42. [Google Scholar] [CrossRef]
- Takikawa, M.; Nakamura, S.; Nakamura, S.; Ishirara, M.; Kishimoto, S.; Sasaki, K.; Yanagibayashi, S.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol. Surg 2011, 37, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Ho, A.; Sukhdeo, K.; Yin, L.; Sicco, K.L. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1298–1303. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem. Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Cervelli, V.; Garcovich, S.; Bielli, A.; Cervelli, G.; Curcio, B.C.; Scioli, M.G.; Orlandi, A.; Gentile, P. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed. Res. Int. 2014, 2014, 760709. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol. Surg 2016, 42, 491–497. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Mechanical and Controlled PRP Injections in Patients Affected by Androgenetic Alopecia. J. Vis. Exp. 2018, e56406. [Google Scholar] [CrossRef]
- Dubin, D.P.; Lin, M.J.; Leight, H.M.; Farberg, A.S.; Torbeck, R.L.; Burton, W.B.; Khorasani, H. The effect of platelet-rich plasma on female androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1294–1297. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Montalvão, S.A.; Cancela, R.B.; Silva, F.A.; Urban, A.; Huber, S.C.; Júnior, J.L.R.; Lana, J.F.S.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019, 80, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Siah, T.W.; Guo, H.; Chu, T.; Santos, L.; Nakamura, H.; Leung, G.; Shapiro, J.; McElwee, K.J. Growth factor concentrations in platelet-rich plasma for androgenetic alopecia: An intra-subject, randomized, blinded, placebo-controlled, pilot study. Exp. Dermatol. 2020, 29, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.A.; Osman, M.A.R. The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: A randomized placebo-controlled study. J. Cosmet. Dermatol. 2018, 17, 47–53. [Google Scholar] [CrossRef]
- Sultana, B.; Paul, H. Efficacy and safety of platelet rich plasma therapy in male androgenetic alopecia. J. Pak. Assoc. Dermatol. 2020, 3, 375–381. [Google Scholar]
- Pakhomova, E.E.; Smirnova, I.O. Comparative Evaluation of the Clinical Efficacy of PRP-Therapy, Minoxidil, and Their Combination with Immunohistochemical Study of the Dynamics of Cell Proliferation in the Treatment of Men with Androgenetic Alopecia. Int. J. Mol. Sci. 2020, 21, 6516. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, V.; Rai, T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: A randomized, double-blind placebo control trial. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 150–157. [Google Scholar] [CrossRef]
- Cruciani, M.; Masiello, F.; Pati, I.; Marano, G.; Pupella, S.; De Angelis, V. Platelet-rich plasma for the treatment of alopecia: A systematic review and meta-analysis. Blood Transfus. 2021. [Google Scholar] [CrossRef]

| Author | Number of Patients and Gender | Outcome Measure | Treatment Protocol | Evaluation of the Effect Compared to Control Group | Age Range (years) | TrichoScan Difference in Density (%) |
|---|---|---|---|---|---|---|
| Takikawa et al., 2011 [16] | 26 (16 men, 10 women) | Hair density, hair cross section, | 5 sessions with 2–3-week intervals | Positive | 28–59 | 1.9% (control) 13.4% (PRP) 15.8% (PRP/DP) |
| Shapiro et al., 2020 [17] | 35 (18 men, 17 women) | Hair density | 3 monthly treatment sessions with evaluation after 3 months after the final treatment | No difference between study group and control group Positive effect compared to the baseline | 18–58 | 10.7% (control) 13.3% (PRP) |
| Gentile et al., 2015 [18] | 20 (20 men) | Residual hair count and hair density based on computerized trichogram | 3 sessions with 30-day intervals, final evaluation after 2 years | Positive | 19–63 | 2.4% (control) 28.6% (PRP) |
| Cervelli et al., 2014 [19] | 10 (10 men) | Hair density, anagen/telogen ratio | 3 sessions with evaluation after 14 weeks, 6 months and 12 months | Positive | 22–60 | −1.8% (control) 14.8% (PRP) |
| Alves and Grimalt, 2016 [20] | 22 (11 men, 11 women) | Hair density, mean anagen hairs | 3 sessions with 30-day intervals | Positive | 21–62 | 1.3% (control) 7.7% (PRP) |
| Gentile et al., 2018 [21] | 23 (18 men, 5 women) | Hair density | 3 sessions with 30-day intervals, final evaluation 3 months after the last injection | Positive | 21–70 | 1% (control) 31% (PRP) |
| Dubin et al., 2020 [22] | 30 (30 women) | Hair density | 3 sessions, with 1-month intervals, and final evaluation after 6 months | Positive | 27–85 | −4.5% (control) 12.8% (PRP) |
| Rodrigues et al., 2019 [23] | 26 (26 men) | Hair density | 4 sessions, with 15-day intervals and final evaluation 3 months after the last treatment | Positive | 18–50 | No accurate data available |
| Siah et al., 2020 [24] | 10 (1 man, 9 women) | Hair density | 5 sessions, with 2-week intervals and final evaluation 2 months after the last intervention | Positive | 20–55 | 1% (control) 12.8% (PRP) |
| Tawfik and Osman, 2018 [25] | 30 (30 women) | Hair density | 4 sessions, with 1-week intervals and final evaluation 6 months after the last treatment | Positive | 20–45 | 24% (control) 104.9% (PRP) |
| Sultana and Paul, 2020 [26] | 54 (54 men) | Hair density | 3 sessions with 4 weeks intervals | Positive | 18–50 | 13.2% (minoxidil 5%) 24% (PRP) |
| Pakhomova and Smirnova, 2020 [27] | 69 (69 men) | Hair density | 4 procedures with 1 month intervals | Positive | 18–53 | 15.8% (minoxidil 5%) 11.7% (PRP) 32% (PRP + minoxidil 5%) |
| Singh et al., 2020 [28] | 80 (80 men) | Hair density, patient self-assessment, photographies | 3 sessions with 1 month intervals and final evaluation after 2 months | Positive | 18–60 | −1.2% (Control) 54.9% (PRP) 36.7% (Minoxidil 5%) 67.1% (PRP + minoxidil 5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiecka, J.M.; Dalewski, B.; Pałka, Ł. Effectiveness of Platelet-Rich Plasma in the Treatment of Androgenic Alopecia Compared to Placebo and Topical Minoxidil: A Systematic Review. Sci. Pharm. 2023, 91, 4. https://doi.org/10.3390/scipharm91010004
Borowiecka JM, Dalewski B, Pałka Ł. Effectiveness of Platelet-Rich Plasma in the Treatment of Androgenic Alopecia Compared to Placebo and Topical Minoxidil: A Systematic Review. Scientia Pharmaceutica. 2023; 91(1):4. https://doi.org/10.3390/scipharm91010004
Chicago/Turabian StyleBorowiecka, Julia Maria, Bartosz Dalewski, and Łukasz Pałka. 2023. "Effectiveness of Platelet-Rich Plasma in the Treatment of Androgenic Alopecia Compared to Placebo and Topical Minoxidil: A Systematic Review" Scientia Pharmaceutica 91, no. 1: 4. https://doi.org/10.3390/scipharm91010004
APA StyleBorowiecka, J. M., Dalewski, B., & Pałka, Ł. (2023). Effectiveness of Platelet-Rich Plasma in the Treatment of Androgenic Alopecia Compared to Placebo and Topical Minoxidil: A Systematic Review. Scientia Pharmaceutica, 91(1), 4. https://doi.org/10.3390/scipharm91010004






