Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Culture
2.3. Cell Viability Analysis by MTT Assay
2.4. Apoptosis Analysis by Flow Cytometry
2.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot Analysis
2.7. Scratch Assay
2.8. Statistical Analysis
3. Results
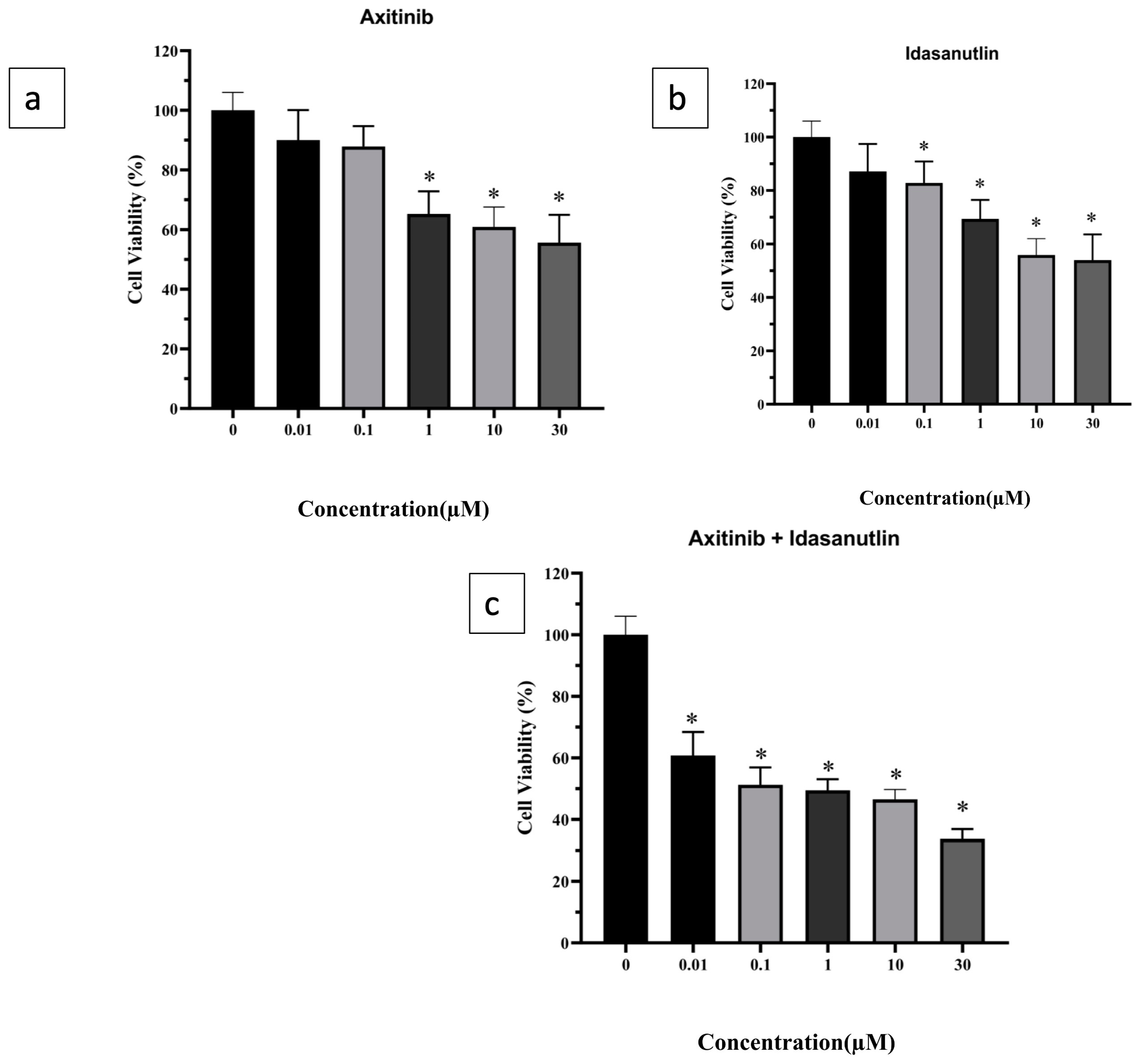
3.1. Evaluation of Cytotoxicity of Axitinib and Idasanutlin on Human Breast Cancer Cell-Line
3.2. Inhibitory Effect of Axitinib and Idasanutlin on Cell Migration
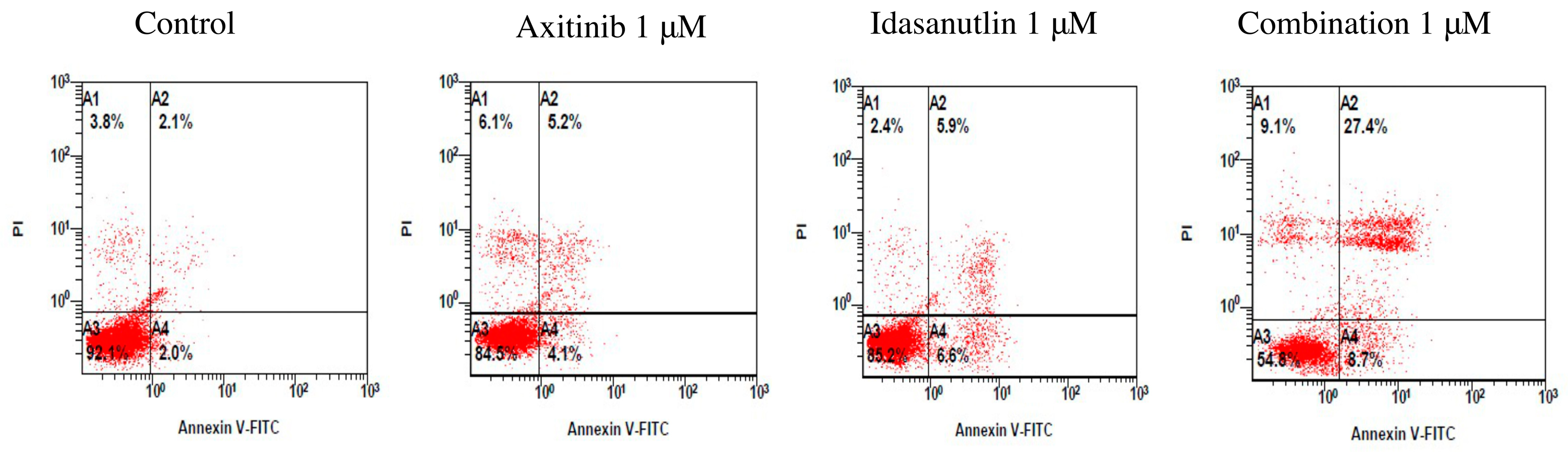
3.3. Evaluation of Cell Apoptosis Induced by Axitinib and Idasanutlin
3.4. Effect of Axitinib and Idasanutlin on Gene Expression of Apoptosis Markers
3.5. Effect of Axitinib and Idasanutlin on Gene Expression of VEGF, TGF-β, MMP9, and MMP3
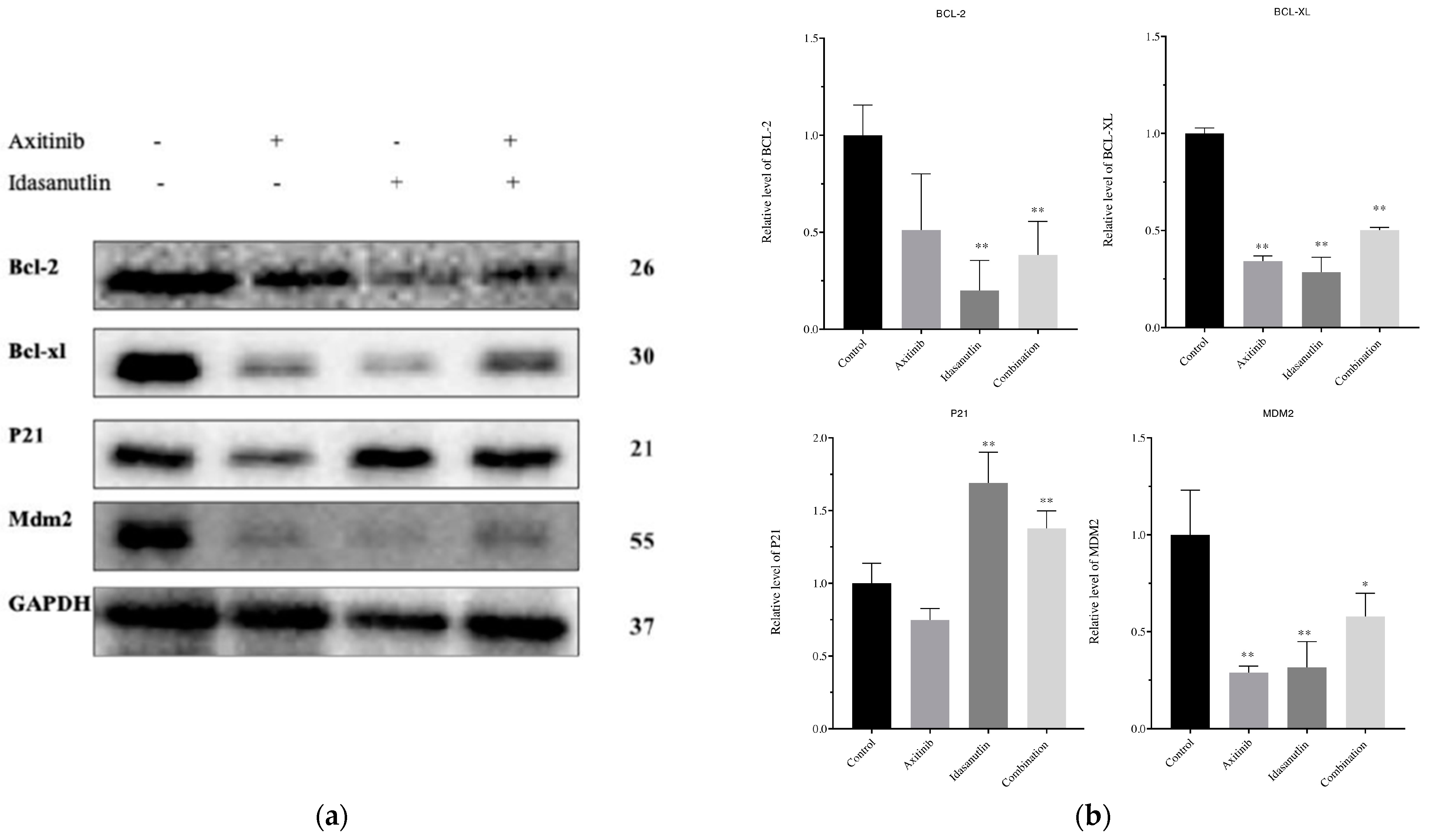
3.6. Effect of Axitinib and Idasanutlin on BCL-2, BCL-XL, p21, and MDM2 Protein Expression Levels in MCF-7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl 1), 9S–16S. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Loman, N.; Johannsson, O.; Bendahl, P.; Borg, Å.; Fernö, M.; Olsson, H. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 83, 310–319. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting p53 for the treatment of cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Lehmann, C.; Friess, T.; Birzele, F.; Kiialainen, A.; Dangl, M. Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models. J. Hematol. Oncol. 2016, 9, 50. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 29, pp. 15–18. [Google Scholar]
- Soleimani, M.; Nappi, L.; Kollmannsberger, C. Avelumab and axitinib combination therapy for the treatment of advanced renal cell carcinoma. Futur. Oncol. 2020, 16, 3021–3034. [Google Scholar] [CrossRef]
- Bellesoeur, A.; Carton, E.; Alexandre, J.; Goldwasser, F.; Huillard, O. Axitinib in the treatment of renal cell carcinoma: Design, development, and place in therapy. Drug Des. Devel. Ther. 2017, 11, 2801–2811. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534. [Google Scholar]
- Boere, I.A.; Hamberg, P.; Sleijfer, S. It takes two to tango: Combinations of conventional cytotoxics with compounds targeting the vascular endothelial growth factor-vascular endothelial growth factor receptor pathway in patients with solid malignancies. Cancer Sci. 2010, 101, 7–15. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet. Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rosen, L.S.; Vokes, E.E.; Kies, M.S.; Forastiere, A.A.; Worden, F.P.; Kane, M.A.; Sherman, E.; Kim, S.; Bycott, P. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: Results from a phase II study. J. Clin. Oncol. 2008, 26, 4708. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Larson, T.; Ou, S.-H.I.; Limentani, S.; Sandler, A.; Vokes, E.; Kim, S.; Liau, K.; Bycott, P.; Olszanski, A.J. Efficacy and safety of axitinib in patients with advanced non–small-cell lung cancer: Results from a phase II study. J. Clin. Oncol. 2009, 27, 3836–3841. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Canu, B.; Fioravanti, A.; Orlandi, P.; Di Desidero, T.; Alì, G.; Fontanini, G.; Di Paolo, A.; Del Tacca, M.; Danesi, R.; Bocci, G. Irinotecan synergistically enhances the antiproliferative and proapoptotic effects of axitinib in vitro and improves its anticancer activity in vivo. Neoplasia 2011, 13, 217-IN3. [Google Scholar] [CrossRef] [PubMed]
- Rössler, J.; Monnet, Y.; Farace, F.; Opolon, P.; Daudigeos-Dubus, E.; Bourredjem, A.; Vassal, G.; Geoerger, B. The selective VEGFR1-3 inhibitor axitinib (AG-013736) shows antitumor activity in human neuroblastoma xenografts. Int. J. cancer 2011, 128, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, Z.; Liu, J.-J.; Jiang, N.; Zhang, J.; Ross, T.M.; Chu, X.-J.; Bartkovitz, D.; Podlaski, F.; Janson, C. Discovery of RG7388, a potent and selective p53–MDM2 inhibitor in clinical development. J. Med. Chem. 2013, 56, 5979–5983. [Google Scholar] [CrossRef] [PubMed]
- Tovar, C.; Graves, B.; Packman, K.; Filipovic, Z.; Xia, B.H.M.; Tardell, C.; Garrido, R.; Lee, E.; Kolinsky, K.; To, K.-H. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013, 73, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, L.; Bate-Eya, L.T.; Alles, L.K.; Lee, J.Y.; Koopmans, B.; Jonus, H.C.; Schubert, N.A.; Schild, L.; Lelieveld, D.; Egan, D.A. High-throughput screening identifies idasanutlin as a resensitizing drug for venetoclax-resistant neuroblastoma cells. Mol. Cancer Ther. 2021, 20, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Coto-Llerena, M.; Gallon, J.; Taha-Mehlitz, S.; Kancherla, V.; Konantz, M.; Srivatsa, S.; Montazeri, H.; Panebianco, F.; De Menna, M. GATA3 and MDM2 are synthetic lethal in estrogen receptor-positive breast cancers. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stehle, F.; Schulz, K.; Fahldieck, C.; Kalich, J.; Lichtenfels, R.; Riemann, D.; Seliger, B. Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J. Biol. Chem. 2013, 288, 16334–16347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-R.; Wang, H.; Hui, N.; Zhang, P. Enhanced antitumor effect of axitinib synergistic interaction with AG490 via VEGFR2/JAK2/STAT3 signaling mediated epithelial-mesenchymal transition in cervical cancer in vitro. Asian Biomed. 2013, 7, 39–49. [Google Scholar]
- Shamloo, B.; Usluer, S. p21 in cancer research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Hall, S.J.; Sehgal, I.; Wang, J.; Timme, T.L.; Yang, G.; Connell-Crowley, L.; Elledge, S.J.; Zhang, W.-W.; Harper, J.W. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995, 55, 5151–5155. [Google Scholar] [PubMed]
- Peng, N.X.; Liu, C.X.; Wang, X.S.; Zhang, Z.J.; Liao, S.C. Combination of axitinib and dasatinib for anti-cancer activities in two prostate cancer cell lines. Bangladesh J. Pharmacol. 2016, 11, 130–137. [Google Scholar] [CrossRef]
- Zanjirband, M.; Edmondson, R.J.; Lunec, J. Pre-clinical efficacy and synergistic potential of the MDM2-p53 antagonists, Nutlin-3 and RG7388, as single agents and in combined treatment with cisplatin in ovarian cancer. Oncotarget 2016, 7, 40115. [Google Scholar] [CrossRef]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar] [CrossRef]
- Tawfik, K.; Kimler, B.F.; Davis, M.K.; Fan, F.; Tawfik, O. Prognostic significance of Bcl-2 in invasive mammary carcinomas: A comparative clinicopathologic study between “triple-negative” and non–“triple-negative” tumors. Hum. Pathol. 2012, 43, 23–30. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Lakoma, A.; Chen, Z.; Tao, L.; Scorsone, K.A.; Schild, L.; Aviles-Padilla, K.; Nikzad, R.; Zhang, Y.; Chakraborty, R. p53 nongenotoxic activation and mTORC1 inhibition lead to effective combination for neuroblastoma therapy. Clin. Cancer Res. 2017, 23, 6629–6639. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, J.; Rong, F. Combination of metformin and RG7388 enhances inhibition of growth and induction of apoptosis of ovarian cancer cells through the PI3K/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2020, 533, 665–671. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Song, J.; Wu, H.; Yang, M.; Lu, L.; Weng, X.; Liu, L.; Nie, G. MDM2 inhibitor RG7388 potently inhibits tumors by activating p53 pathway in nasopharyngeal carcinoma. Cancer Biol. Ther. 2019, 20, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Al-Odaini, A.A.; Fils-Aimé, N.; Villatoro, M.A.; Guo, J.; Arakelian, A.; Rabbani, S.A.; Ali, S.; Lebrun, J.J. Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013, 15, 3246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef]



| Gene | Primer |
|---|---|
| P53 | Forward Sequence CCCCTCCTGGCCCCTGTAATCTTC Reverse Sequence GCAGCGCCTCACAACCTCGGTCAT |
| P21 | Forward Sequence GTTCCTTGTGGAGCCGGAGC Reverse Sequence GGTACAAGACAGTGACAGGTC |
| BAX | Forward Sequence GTTTCATCCAGGATCGAGCAG Reverse Sequence CATCTTCTTCCAGATGGTGA |
| BCL2 | Forward Sequence CCTGTGGATGACTGAGTACC Reverse Sequence GAGACAGCCAGGAGAAATCA |
| GAPDH | Forward Sequence GTCTCCTCTGACTTCAACAGCG Reverse Sequence ACCACCCTGTTGCTGTAGCCAA |
| MMP3 | Forward Sequence TATGAAGGAGAGGCTGATATAATG Reverse Sequence TGTGAGTGAGTGATAGAGTGG |
| MMP9 | Forward Sequence GCCACTACTGTGCCTTTGAGTC Reverse Sequence CCCTCAGAGAATCGCCAGTACT |
| VEGF | Forward Sequence TTGCCTTGCTGCTCTACCTCCA Reverse Sequence GATGGCAGTAGCTGCGCTGATA |
| Protein | Molecular Weight | Company | Dilution Ratio |
|---|---|---|---|
| P21 | 21 kDa | Cell Signaling Technology, USA | 1:1000 |
| BCL2 | 26 kDa | Cell Signaling Technology, USA | 1:1000 |
| BCL-XL | 30 kDa | Cell Signaling Technology, USA | 1:1000 |
| MDM2 | 55 kDa | Sigma-Aldrich. (USA) | 1:1000 |
| GAPDH | 37 kDa | Cell Signaling Technology, USA | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaseem, A.M.; Alhazzani, K.; Alanazi, A.Z.; Alqarni, Y.; Algahtani, M.M.; Alhamed, A.S.; Alasiri, G.; Alotaibi, F.T.; Jawaid, T.; Aldali, J.A. Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment. Sci. Pharm. 2023, 91, 12. https://doi.org/10.3390/scipharm91010012
Alaseem AM, Alhazzani K, Alanazi AZ, Alqarni Y, Algahtani MM, Alhamed AS, Alasiri G, Alotaibi FT, Jawaid T, Aldali JA. Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment. Scientia Pharmaceutica. 2023; 91(1):12. https://doi.org/10.3390/scipharm91010012
Chicago/Turabian StyleAlaseem, Ali M., Khalid Alhazzani, Ahmed Zuwaiel Alanazi, Yasser Alqarni, Mohammad M. Algahtani, Abdullah S. Alhamed, Glowi Alasiri, Fahad T. Alotaibi, Talha Jawaid, and Jehad A. Aldali. 2023. "Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment" Scientia Pharmaceutica 91, no. 1: 12. https://doi.org/10.3390/scipharm91010012
APA StyleAlaseem, A. M., Alhazzani, K., Alanazi, A. Z., Alqarni, Y., Algahtani, M. M., Alhamed, A. S., Alasiri, G., Alotaibi, F. T., Jawaid, T., & Aldali, J. A. (2023). Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment. Scientia Pharmaceutica, 91(1), 12. https://doi.org/10.3390/scipharm91010012






