Using Metabolite Data to Develop Patient Centric Specification for Amide Impurity in Vildagliptin Tablets
Abstract
:1. Introduction
- Acceptable limit for a specified impurity can be set at the qualification threshold provided no toxicological, immunological, or clinical concerns exist at this level.
- Potent and toxic impurities having immunological, pharmacological, or clinical concerns: Acceptance criteria based solely on ICH Q3A(R2) and Q3B(R2) qualification threshold are not enough and need further justification.
2. Methods
3. Results and Discussion
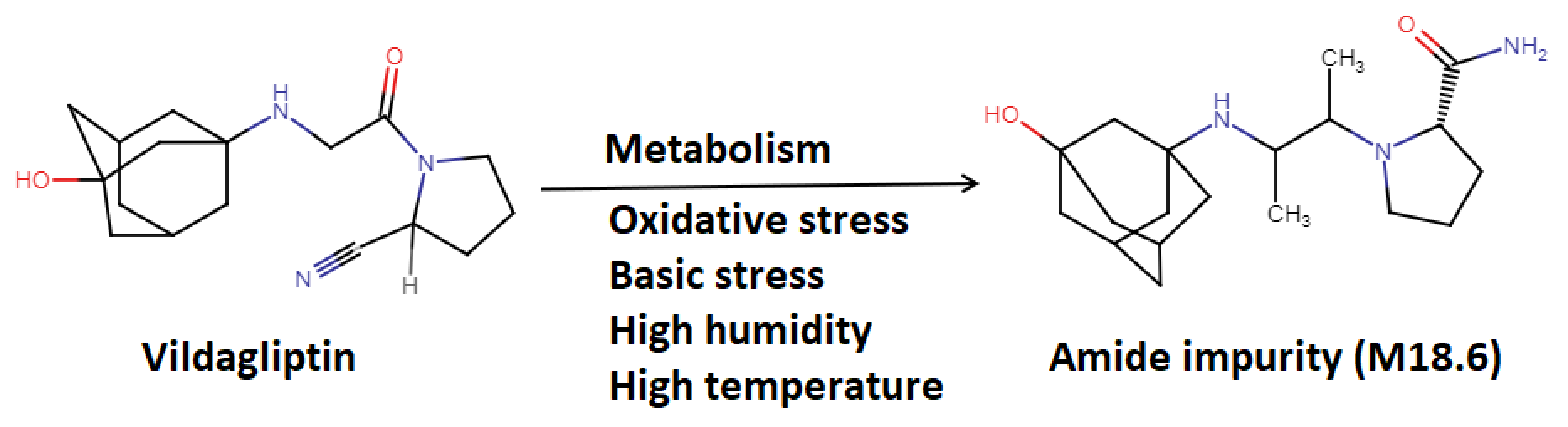
3.1. Vildagliptin Metabolism and Degradation
- M14.9 (hydroxylation at the adamantyl ring)
- M16.7 (hydroxylation at the pyrrolidine ring)
- M17.4 (hydroxylation at the pyrrolidine ring of M20.7)
- M18.6 (amide metabolite resulting from hydrolysis of the cyano moiety)
- M14.2 (amide metabolite)
- M17.7 (a monohydroxy-acid metabolite resulting from the ring opening of the pyrrolidine ring)
- (2S,2S′)-1,1′[[3-hydroxytricyclo[3.3.1.1.3,7]dec-1-yl)imino]bis(1-oxo2,1,-ethanediyl]bis(2-pyrrolidinecarbonitrile) impurity (Dimer impurity) of formula (VI);
- aminoadamantane-3-ol impurity of formula (IV);
- Adamantane-1,3-diol impurity (Di-hydroxyl impurity) of formula (VII)
- deshydroxy impurity of formula (VIII)
- amide impurity of formula (IX)
- impurity E ((2S)-1-[2-[(3-hydroxyadamantan-1-yl)imino]acetyl]pyrrolidine-2-carbonitrile)
- impurity F ((8aS)-3-hydroxy-octahydropyrrolo[1,2-a]piperazine-1,4-dione)
3.2. Estimating Safe Level of Amide Impurity (Impurity B)
4. The Maximum Theoretical Concentration (MTC) versus Maximum Observed Concentration (MOC)
- MOC < MTC: Circulating level is below the to be qualified level. In this case further information (such as in vitro metabolism studies, in vivo PK studies, assessment of coverage across species or urinary excretion) would be required to support the qualification of the impurity. If these studies fail to provide evidence of metabolite formation or interspecies coverage, then GLP toxicity impurity qualification studies would be required.
- MOC ≥ MTC: Circulatory level is above or equal to the level to be qualified, the circulating level can be used to qualify an impurity.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Conference on Harmonization (ICH)—Impurities in New Drug Substances Q3A(R2). 2006. Available online: https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf (accessed on 10 November 2021).
- International Conference on Harmonization (ICH)—Impurities in New Drug Products Q3B(R2). 2006. Available online: https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf (accessed on 10 November 2021).
- Weidolf, L.; Andersson, T.; Bercu, J.; Brink, A.; Glowienke, S.; Harvey, J.; Hayes, M.; Jacques, P.; Lu, C.; Manevski, N.; et al. Qualification of impurities based on metabolite data. Reg. Tox. Pharmacol. 2020, 110, 104524. [Google Scholar] [CrossRef] [PubMed]
- FDA MAPP 5017.2 Rev. 1. Manual of Policy and Procedures, Guidance for Assessors; “Establishing Impurity Acceptance Criteria as Part of Specifications for NDAs, ANDAs, and BLAs Based on Clinical Relevance”. 2020. Available online: https://www.fda.gov/media/124859/download (accessed on 10 November 2021).
- Sharp, S.S. Establishing Clinically Relevant Drug Product Specifications: FDA Perspectives. Presentation at AAPS Annual Meeting. 2012. Available online: https://www.fda.gov/media/85355/download (accessed on 10 November 2021).
- Bercu, J.; Berlam, S.C.; Berridge, J.; Cherney, B.; Cowley, D.; Laughton, H.W.; McLoughlin, D.; McMahon, M.; Moore, C.M.V.; Murti, C.; et al. Establishing patient centric specifications for drug substance and drug product impurities. J. Pharm. Inn. 2019, 14, 76–89. [Google Scholar] [CrossRef] [Green Version]
- International Conference on Harmonization (ICH)—Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances, Q6A. 1999. Available online: https://database.ich.org/sites/default/files/Q6A%20Guideline.pdf (accessed on 10 November 2021).
- International Conference on Harmonization (ICH)—Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R1). 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m7r1-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit_en.pdf (accessed on 10 November 2021).
- Australian Public Assessment Report for Vildagliptin. Proprietary Product Name: Galvus, Xiliarx. Sponsor: Novartis Pharmaceuticals Australia Pty Ltd. 2010. Available online: https://www.tga.gov.au/sites/default/files/auspar-galvus.pdf (accessed on 12 July 2020).
- Australian Public Assessment Report for Vildagliptin/Metformin Hydrochloride. Proprietary Product Name: Galvumet/Sobrea. Sponsor: Novartis Pharmaceuticals Australia Pty Ltd. 2011. Available online: https://www.tga.gov.au/auspar/auspar-vildagliptin-metformin-hydrochloride-0 (accessed on 10 November 2021).
- FDA. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 10 November 2021).
- Summary of Product Characteristics (SmPC). Galvus 50 mg Tablets. 2018. Available online: https://www.medicines.org.uk/EMC/medicine/20734/SPC/Galvus+50+mg+Tablets/ (accessed on 10 November 2021).
- He, H.; Tran, P.; Yin, H.; Smith, H.; Flood, D.; Kramp, R.; Filipeck, R.; Fischer, V.; Howard, D. Disposition of Vildagliptin, a Novel Dipeptidyl Peptidase 4 Inhibitor, in Rats and Dogs. Drug Metabol. Disp. 2009, 37, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Tran, P.; Yin, H.; Smith, H.; Batard, Y.; Wang, L.; Finolf, H.; Gu, H.; Manglod, J.B.; Fischer, V.; et al. Absorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metab. Dispos. 2009, 37, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.H.; Serra, D.; Wang, Y.; Campestrini, J.; Riviere, G.J.; Deacon, C.F.; Schwartz, H.S.; Nielsen, J.C.; Saylan, M.L. Pharmacokintics and Pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin. Pharmacokin. 2007, 46, 577–788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Devineni, S.R.; Singh, G.; Kadirappaa, A.; Dubey, S.K.; Kumar, P. Identification, isolation and characterization of potential process-related impurity and its degradation product in vildagliptin. J. Pharm. Biomed. Anal. 2015, 119, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Thippannachar, M.V.; Sudhir, N.; Vrajlal, P.; Subhash, I.S.; Bhagirath, B.M.; Rajesh, T.; Chaturlal, M.A. A Process for the Preparation of Vildagliptin and Its Intermediate Thereof. WO Patent 2013179300 A3, 28 August 2014. [Google Scholar]
- Arar, S.; Al-Qudah, E.; Alzweiri, M.; Sweidan, K. New forced degradation products of vildagliptin: Identification and structural elucidation using LC-MS, with proposed formation mechanisms. J. Liq. Chromat. Rel. Technol. 2020, 43, 633–644. [Google Scholar] [CrossRef]
- Tianjiao, H.; Guanghui, L.; Hongguo, L.; Yingtao, T.; Ning, X.; Jing, Z.; Tianjiao, H.G. Vildagliptin Tablet and Preparation Method Thereof. China Patent CN105193752B, 30 March 2018. [Google Scholar]
- EMEA—Vildagliptin Scientific Discussion. 2007. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/eucreas-epar-scientific-discussion_en.pdf (accessed on 10 November 2021).
- Busch, S.J.; Hoffmann, P.; Sahota, P.; Johnson, R.; Kothny, W.; Meyer, F.; Foley, J.E. Studies in rodents with the dipeptidyl peptidase-4 inhibitor vildagliptin to evaluate possible drug-induced pancreatic histological changes that are predictive of pancreatitis and cancer development in man. Diabetes Obes. Metab. 2013, 15, 72–76. [Google Scholar] [CrossRef] [PubMed]
| Maximum Daily Dose (Amount of Drug Substance Administered per Day) | Threshold (Expressed Either as a Percentage of the Drug Substance or as Total Daily Intake of the Degradation Product) |
|---|---|
| Reporting thresholds | |
| ≤1 g | 0.1% |
| >1 g | 0.05% |
| Identification Thresholds | |
| <1 mg | 1.0% or 5 µg TDI, whichever is lower |
| 1 mg–10 mg | 0.5% or 20 µg TDI, whichever is lower |
| >10 mg–2 g | 0.2% or 2 mg TDI, whichever is lower |
| >2 g | 0.10% |
| Qualification Thresholds | |
| <10 mg | 1.0% or 50 µg TDI, whichever is lower |
| 10 mg–100 mg | 0.5% or 200 µg TDI, whichever is lower |
| >100 mg–2 g | 0.2% or 3 mg TDI, whichever is lower |
| >2 g | 0.15% |
| Metabolites | Rat | Dog | Human |
|---|---|---|---|
 | √ | √ | √ |
 | √ | √ | √ |
 | √ | √ | √ |
 | - | √ | - |
 | - | √ | √ |
 | √ | ||
 | √ | - | - |
 | √ | - | - |
 | √ | √ | |
 | √ | √ | |
 | √ |
| Parameter | Values | |
|---|---|---|
| Scenario 1 | Scenario II | |
| Maximum Dose (mg) | 20 | 20 |
| Average dose of impurity (mg) | 0.135 | 0.3 |
| Average dose of impurity (μg) | 135 * | 300 # |
| % of dose excreted in urine | 1.5 | 1.5 |
| MTA of impurity (μg) | 135 | 300 |
| MOA excreted in urine (μg) | 300 | 300 |
| MOA/MTA | 2.2 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoo, N.A.; Untoo, S.; Rahman, Z. Using Metabolite Data to Develop Patient Centric Specification for Amide Impurity in Vildagliptin Tablets. Sci. Pharm. 2022, 90, 1. https://doi.org/10.3390/scipharm90010001
Charoo NA, Untoo S, Rahman Z. Using Metabolite Data to Develop Patient Centric Specification for Amide Impurity in Vildagliptin Tablets. Scientia Pharmaceutica. 2022; 90(1):1. https://doi.org/10.3390/scipharm90010001
Chicago/Turabian StyleCharoo, Naseem Ahmad, Syeed Untoo, and Ziyaur Rahman. 2022. "Using Metabolite Data to Develop Patient Centric Specification for Amide Impurity in Vildagliptin Tablets" Scientia Pharmaceutica 90, no. 1: 1. https://doi.org/10.3390/scipharm90010001
APA StyleCharoo, N. A., Untoo, S., & Rahman, Z. (2022). Using Metabolite Data to Develop Patient Centric Specification for Amide Impurity in Vildagliptin Tablets. Scientia Pharmaceutica, 90(1), 1. https://doi.org/10.3390/scipharm90010001






