Pharmacological Activity and Phytochemical Profile of Acacia Heartwood Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Acacia Heartwood
2.3. Determination of Phytochemical Profile
2.4. Determination of Antioxidant Capacity
2.5. Determination of Toxicity toward Artemia salina Leach
2.6. Data Analysis
3. Results
3.1. Yield of Methanolic Extracts and Their Phytochemical Profile
3.2. Pharmacological Activity of Acacia Heartwood Methanolic Extracts
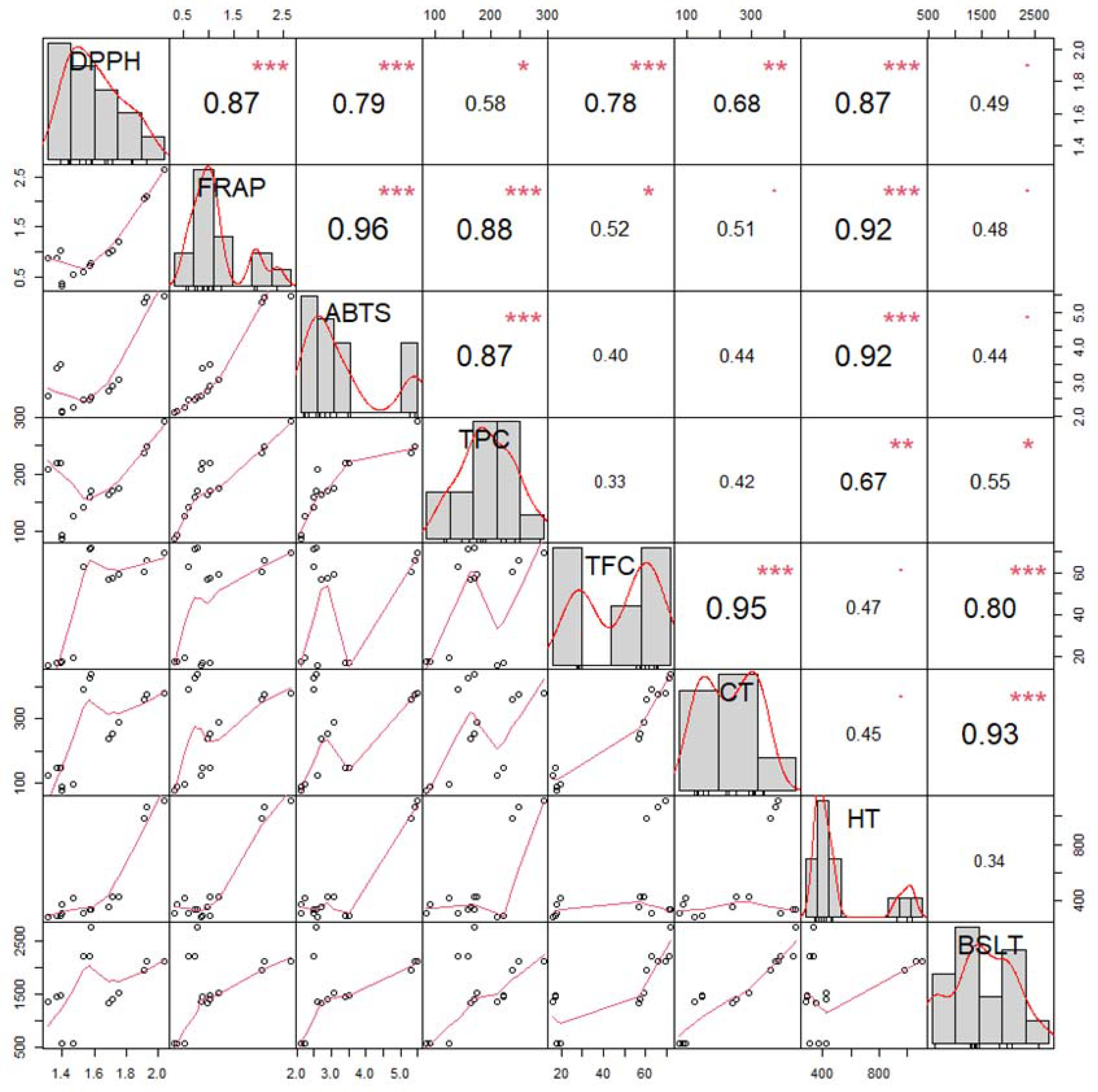
3.3. Multivariate Analysis and Extract Selection
3.4. Bioactive Compounds in Both Selected Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- World Health Organization. Global Cancer Observatory: Globocan 2018; International Agency for Research on Cancer (IARC): Lyon, France, 2018; Volume 256. [Google Scholar]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ashour, A.; Amen, Y.; Ohnuki, K.; Fujimoto, N.; Shimizu, K. Antioxidant and anti-lipase compounds isolated from heartwood of Yakushima native cedar (Cryptomeria japonica). J. Wood Chem. Technol. 2019, 39, 305–312. [Google Scholar] [CrossRef]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant activity of extracts from acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef] [PubMed]
- Singhatong, S.; Leelarungrayub, D.; Chaiyasut, C. Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. heartwood extract. J. Med. Plants Res. 2010, 4, 947–953. [Google Scholar] [CrossRef]
- Wetwitayaklung, P.; Phaechamud, T.; Keokitichai, S. The antioxidant activity of caesalpinia sappan l. heartwood in various ages. Naresuan Univ. J. 2005, 13, 43–52. [Google Scholar]
- Deepak, H.B.; Chandrasekaran, C.V.; Dethe, S.; Mundkinajeddu, D.; Pandre, M.K.; Balachandran, J.; Agarwal, A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 2012, 8, 116–123. [Google Scholar] [CrossRef]
- Mihara, R.; Barry, K.M.; Mohammed, C.L.; Mitsunaga, T. Comparison of antifungal and antioxidant activities of Acacia mangium and A. auriculiformis heartwood extracts. J. Chem. Ecol. 2005, 31, 789–804. [Google Scholar] [CrossRef]
- Ghate, N.; Hazra, B.; Sarkar, R.; Mandal, N. Heartwood extract of Acacia catechu induces apoptosis in human breast carcinoma by altering bax/bcl-2 ratio. Pharmacogn. Mag. 2014, 10, 27–33. [Google Scholar] [CrossRef]
- Afsar, T.; Khan, M.R.; Razak, S.; Ullah, S.; Mirza, B. Antipyretic, anti-inflammatory and analgesic activity of Acacia hydaspica R. Parker and its phytochemical analysis. BMC Complement. Altern. Med. 2015, 15, 136. [Google Scholar] [CrossRef]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Hichem, H. Ben Antioxidant and anti-acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef] [PubMed]
- Sathya, A.; Siddhuraju, P. Protective effect of bark and empty pod extracts from Acacia auriculiformis against paracetamol intoxicated liver injury and alloxan induced type II diabetes. Food Chem. Toxicol. 2013, 56, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Acacia Mangium Willd.: Ecology, Silviculture and Productivity; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2011. [Google Scholar]
- Hendrarti, R.L.; Nurrohmah, S.H.; Susilawati, S.; Budi, S. Budidaya Acacia Auriculiformis Untuk Kayu Energi, 1st ed.; Naiem, M., Mahfudz Prabawa, S.B., Eds.; IPB Press: Bogor, Indonesia, 2014. [Google Scholar]
- Nirsatmanto, A.; Sunarti, S. Sugarcane (Saccharum spp.): Breeding and genomics. In Advances in Plant Breeding Strategies: Industrial and Food Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 6, pp. 3–28. ISBN 9783030232658. [Google Scholar]
- Lorenzo, P.; González, L.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Sunardi, S.; Sulistijorini; Setyawati, T. Invasion of acacia decurrens willd. After eruption of Mount Merapi, Indonesia. Biotropia 2017, 24, 35–46. [Google Scholar] [CrossRef]
- Okoli, B.; Jummai, A.; Molefe, N.; Ledwaba, I.; Modis, S. South African Invasive Tree: Studies of the Chemical and Biological Profiles of Acacia decurrens (Wild). Int. J. Chem. 2018, 10, 60. [Google Scholar] [CrossRef][Green Version]
- Hendrik, A.C.; Kusmana, C. Muhdin Stand and site characteristics of kabesak (Acacia leucophloea) in Timor Island, East Nusa Tenggara, Indonesia. J. Pendidik. Kehutan. Wallacea 2019, 8, 147–157. [Google Scholar] [CrossRef]
- Badan Pusat Statistik. Statistik Produksi Kehutanan 2017; Badan Pusat Statistik (BPS): Jakarta, Indonesia, 2017; ISBN 2580-1740.
- Badan Pusat Statistik. Statistik Produksi Kehutanan 2018; Badan Pusat Statistik (BPS): Jakarta, Indonesia, 2018; ISBN 2580-1740.
- Batubara, I.; Komariah, K.; Sandrawati, A.; Nurcholis, W. Genotype selection for phytochemical content and pharmacological activities in ethanol extracts of fifteen types of Orthosiphon aristatus (Blume) Miq. leaves using chemometric analysis. Sci. Rep. 2020, 10, 20945. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-throughput micro-plate HCl-vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Netzel, M.E.; Tinggi, U.; Osborne, S.A.; Fletcher, M.T.; Sultanbawa, Y. Antioxidant rich extracts of terminalia ferdinandiana inhibit the growth of foodborne bacteria. Foods 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evidence-Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Kadifkova Panovska, T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Barry, K.M.; Mihara, R.; Davies, N.W.; Mitsunaga, T.; Mohammed, C.L. Polyphenols in Acacia mangium and Acacia auriculiformis heartwood with reference to heart rot susceptibility. J. Wood Sci. 2005, 51, 615–621. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Sjöholm, R.E.; Holmbom, B.R. Bioactive phenolic substances in important tree species. Part 3: Knots and stemwood of Acacia crassicarpa and A. mangium. Holzforschung 2005, 59, 94–101. [Google Scholar] [CrossRef]
- Okoli, B.J.; Modise, J.S. New Pharmacophore from the Stem Bark Fractions of Acacia decurrens (Willd), an Invasive South Africa Tree. J. Appl. Chem. 2017, 2017, 815278. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Gopalakrishnan, V.K.; Balachandran, I. Phenolic Compounds and Antioxidant Properties of Selected Acacia species. J. Biol. Act. Prod. Nat. 2014, 4, 316–324. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Wang, Y.; Wu, D.; Xu, M. Phenolic extracts from Acacia mangium bark and their antioxidant activities. Molecules 2010, 15, 3567–3577. [Google Scholar] [CrossRef]
- Ismayati, M.; Nakagawa-izumi, A.; Ohi, H. Structural elucidation of condensed tannin from the bark waste of Acacia crassicarpa plantation wood in Indonesia. J. Wood Sci. 2017, 63, 350–359. [Google Scholar] [CrossRef][Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Tiku, A.K.; Singh, G.; Vyas, D.; Bhardwaj, A. Antioxidant activity and protective effect against plasmid DNA strand scission of leaf, bark, and heartwood extracts from Acacia catechu. J. Food Sci. 2011, 76, C959–C964. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Krishnaraju, A.V.; Rao, T.V.N.; Sundararaju, S.; Vanisree, M.; Tsay, H.-S.; Subbaraju, G. V Assessment of bioactivity of some Sudanese medicinal plants using brine shrimp (Artemia salina) lethality assay. J. Chem. Pharm. Res. 2012, 4, 5145–5148. [Google Scholar]
- Ramli, S.; Harada, K.I.; Ruangrungsi, N. Antioxidant, antimicrobial and cytotoxicity activities of Acacia farnesiana (L.) Willd. Leaves ethanolic extract. Pharmacogn. J. 2011, 3, 50–58. [Google Scholar] [CrossRef]
- Calzada, F.; Ramirez-Santos, J.; Valdes, M.; Garcia-Hernandez, N.; Pina-Jiménez, E.; Ordoñez-Razo, R.M. Evaluation of Acute Oral Toxicity, Brine Shrimp Lethality, and Antilymphoma Activity of Geranylgeraniol and Annona macroprophyllata Leaf Extracts. Rev. Bras. Farmacogn. 2020, 30, 301–304. [Google Scholar] [CrossRef]
- Senhaji, S.; Lamchouri, F.; Toufik, H. Phytochemical Content, Antibacterial and Antioxidant Potential of Endemic Plant Anabasis aretioïdes Coss. & Moq. (Chenopodiaceae). Biomed. Res. Int. 2020, 2020, 6152932. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Joseph, J.M.; Manian, S. Antioxidant and free radical scavenging activities of Indian acacias: Acacia leucophloea (Roxb.) willd., Acacia ferruginea dc., Acacia dealbata link. and Acacia pennata (L.) willd. Int. J. Food Prop. 2013, 16, 1717–1729. [Google Scholar] [CrossRef]
- Eddebbagh, M.; Messaoudi, M.; Abourriche, A.; Berrada, M.; Attaleb, M.; Benbacer, L.; Bennamara, A. Correlation of the Cytotoxic and Antioxidant Activities of Moroccan Pomegranate (Punica Granatum) with Phenolic and Flavonoid Contents. J. Pharm. Pharmacol. 2016, 4, 511–519. [Google Scholar] [CrossRef][Green Version]
- Tuy-On, T.; Itharat, A.; Maki, P.; Thongdeeying, P.; Pipatrattanaseree, W.; Ooraikul, B. In Vitro Cytotoxic Activity against Breast, Cervical, and Ovarian Cancer Cells and Flavonoid Content of Plant Ingredients Used in a Selected Thai Traditional Cancer Remedy: Correlation and Hierarchical Cluster Analysis. Evidence-based Complement. Altern. Med. 2020, 2020, 8884529. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Kawano, C.; Maeda-Murayama, A.; Nakamura, A.; Koike-Miki, A.; Yukihira, D.; Hayakawa, E.; Ishii, T.; Tachibana, H.; Wariishi, H.; et al. A Chemometrics-driven Strategy for the Bioactivity Evaluation of Complex Multicomponent Systems and the Effective Selection of Bioactivity-predictive Chemical Combinations. Sci. Rep. 2017, 7, 6–10. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Püls, S.; Barghouti, T.; Staudinger, A.; Melchart, D. Radix Polygalae—Yuanzhi. In Chromatographic Fingerprint Analysis of Herbal Medicines; Wagner, H., Püls, S., Barghouti, T., Staudinger, A., Melchart, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume V, pp. 103–115. ISBN 978-3-319-67061-4. [Google Scholar]
- Cano, P.; Echavarren, A.; Prados, P.; Fariña, F. Polycyclic Hydroxyquinones. 13.1 A Novel Synthesis of Islandicin and Digitopurpone. J. Org. Chem. 1983, 48, 5373–5376. [Google Scholar] [CrossRef]
- Eom, T.; Kim, E.; Kim, J.S. In vitro antioxidant, antiinflammation, and anticancer activities and anthraquinone content from rumex crispus root extract and fractions. Antioxidants 2020, 9, 726. [Google Scholar] [CrossRef]

| Extracts | Extraction Yield (%) | TPC (mg GAE/g DE) | TFC (mg QE/g DE) | CT (mg CE/g DE) | HT (mg TAE/g DE) |
|---|---|---|---|---|---|
| A. mangium | 7.658 | 156.46 ± 13.98 b | 68.45 ± 5.05 c | 417.38 ± 25.40 e | 324.78 ± 13.21 a,b |
| A. auriculiformis | 8.591 | 170.06 ± 5.57 b | 57.73 ± 1.27 b | 260.47 ± 26.68 c | 398.35 ± 41.93 b |
| A. leucophloea | 3.882 | 216.86 ± 6.44 c | 16.88 ± 0.57 a | 138.85 ± 12.45 b | 284.17 ± 7.19 a |
| A. crassicarpa | 3.010 | 259.09 ± 29.16 d | 65.24 ± 4.44 c | 370.99 ± 11.25 d | 1055.77 ± 65.96 c |
| A. decurrens | 3.596 | 101.16 ± 21.08 a | 18.48 ± 1.04 a | 87.02 ± 10.24 a | 366.20 ± 55.00 a,b |
| Extracts | LC50 (μg·mL−1) | Antioxidant Capacity (mmol TE/g DE) | ||
|---|---|---|---|---|
| DPPH | ABTS | FRAP | ||
| A. mangium | 2390.40 ± 326.71 d | 1.56 ± 0.02 c | 2.50 ± 0.04 c,d | 0.70 ± 0.08 b,c |
| A. auriculiformis | 1411.54 ± 97.67 b | 1.72 ± 0.03 b | 2.89 ± 0.17 b,c | 1.08 ± 0.12 b |
| A. leucophloea | 1418.21 ± 62.65 b | 1.36 ± 0.04 d | 3.16 ± 0.49 b | 0.92 ± 0.09 b |
| A. crassicarpa | 2054.09 ± 92.74 c | 1.96 ± 0.07 a | 5.40 ± 0.10 a | 2.28 ± 0.32 a |
| A. decurrens | 566.10 ± 5.83 a | 1.42 ± 0.04 d | 2.16 ± 0.07 d | 0.40 ± 0.11 c |
| Compound Name | Class of Compound | m/z | Abundance (%) | |
|---|---|---|---|---|
| A | C | |||
| 3-(3,4-Dihydroxybenzyl)-7-hydroxychroman-4-one | Flavonoid | 287.09 | 1.62 | - |
| 5,7,2’,5’-Tetrahydroxyflavone | Flavonoid | 287.05 | 24.95 | - |
| 5,7,3′,5′-Tetrahydroxyflavanone | Flavonoid | 289.07 | 16.70 | 12.47 |
| Genistein | Flavonoid | 273.07 | 16.65 | - |
| 3-Hydroxy-7-methoxy baicalein | Flavonoid | 301.07 | - | 6.79 |
| Digitopurpone | Anthraquinone | 271.06 | - | 5.18 |
| Onjixanthone II | Xanthonoid | 305.06 | - | 7.38 |
| Quercetin | Flavonoid | 303.04 | - | 10.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prayogo, Y.H.; Syafii, W.; Sari, R.K.; Batubara, I.; Danu. Pharmacological Activity and Phytochemical Profile of Acacia Heartwood Extracts. Sci. Pharm. 2021, 89, 37. https://doi.org/10.3390/scipharm89030037
Prayogo YH, Syafii W, Sari RK, Batubara I, Danu. Pharmacological Activity and Phytochemical Profile of Acacia Heartwood Extracts. Scientia Pharmaceutica. 2021; 89(3):37. https://doi.org/10.3390/scipharm89030037
Chicago/Turabian StylePrayogo, Yanico Hadi, Wasrin Syafii, Rita Kartika Sari, Irmanida Batubara, and Danu. 2021. "Pharmacological Activity and Phytochemical Profile of Acacia Heartwood Extracts" Scientia Pharmaceutica 89, no. 3: 37. https://doi.org/10.3390/scipharm89030037
APA StylePrayogo, Y. H., Syafii, W., Sari, R. K., Batubara, I., & Danu. (2021). Pharmacological Activity and Phytochemical Profile of Acacia Heartwood Extracts. Scientia Pharmaceutica, 89(3), 37. https://doi.org/10.3390/scipharm89030037






