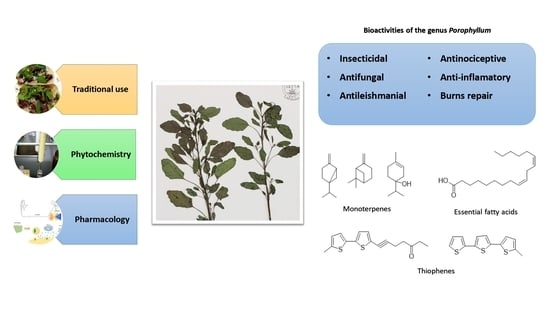
Porophyllum Genus Compounds and Pharmacological Activities: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Constituents Isolated from Plants of the Genus Porophyllum
3.2. Insecticidal Activity
3.3. Antifungal Activity
3.4. Antileishmanial Activity
3.5. Antinociceptive and Anti-Inflammatory Activities
3.6. Burn Repair Activity
3.7. Toxicological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso-Castro, A.J.; Domínguez, F.; Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Carranza-Álvarez, C.; Solano, E.; Isiordia-Espinoza, M.A.; Juárez-Vázquez, M.C.; Zapata-Morales, J.R.; Argueta-Fuertes, M.A.; et al. Use of medicinal plants by health professionals in Mexico. J. Ethnopharmacol. 2017, 198. [Google Scholar] [CrossRef] [PubMed]
- Castro Lara, D.; Bye Boettler, R.A.; Mera Ovando, L.M. Diagnóstico del pápaloquelite en México, 1st ed.; Universidad Autónoma Chapingo: Chapingo, Mexico, 2011; pp. 1–47. ISBN 978-607-12-0199-7. [Google Scholar]
- Moreno-Salazar, S.F.; Robles-Zepeda, R.E.; Johnson, D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia 2008, 79, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.M.; Silva, M.R.; Bonfim, R.R.; Thomazzi, S.M.; Santos, M.R.V.; Quintans-Júnior, L.J.; Bonjardim, L.R.; Araújo, A.A.S. Assessment of antinociceptive and anti- inflammatory activities of Porophyllum ruderale aqueous extract. Braz. J. Pharm. 2011, 21, 486–490. [Google Scholar] [CrossRef]
- Juárez, Z.N.; Hernández, L.R.; Bach, H.; Sánchez-Arreola, E.; Bach, H. Antifungal activity of essential oils extracted from Agastache mexicana ssp. xolocotziana and Porophyllum linaria against post-harvest pathogens. Ind. Crop. Prod. 2015, 74, 178–182. [Google Scholar] [CrossRef]
- García de Alba García, J.E.; Ramírez Hernández, B.C.; Robles Arellano, G.; Zañudo Hernández, J.; Salcedo Rocha, A.L.; García de Alba Verduzco, J.E. Conocimiento y uso de las plantas medicinales en la zona metropolitana de Guadalajara. RAS 2012, 39, 29–44. [Google Scholar] [CrossRef][Green Version]
- Urióstegui-Flores, A. Hierbas medicinales utilizadas en la atención de enfermedades del sistema digestivo en la ciudad de Taxco, Guerrero, México. Rev. Salud Pública 2015, 17, 85–96. [Google Scholar] [CrossRef]
- Strother, J.L. Porophyllum. In Flora of North America, North of Mexico; Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2007; p. 233235. [Google Scholar]
- Milan, P.; Hayashi, A.H.; Appezzato-da-Glória, B. Appezzato-da-Glória, B. Comparative leaf morphology and anatomy of three Asteraceae species. Braz. Arch. Biol. Technol. 2006, 49, 135–144. [Google Scholar] [CrossRef]
- Mattana, C.; Satorres, S.; Alcaráz, L.; Petenatti, E.; del Vitto, L.; Petenatti, M.; Laciar, A. Evaluation of the antibacterial properties of extracts obtained from native Porophyllum lanceolatum in San Luis, Argentina. PhOL 2012, 3, 162–166. [Google Scholar]
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagnosis Photodyn. Ther. 2017, 20, 263–272. [Google Scholar] [CrossRef]
- Estrada, A.S.; Ortiz, E.; Villaseñor, J.L.; Espinosa-García, F.J. The distribution of cultivated species of Porophyllum (Asteraceae) and their wild relatives under climate change. Syst. Biodivers. 2016, 14, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Leporini, N.; Raggi, L.; Zara, K.R.; Young, M.C.M. Influence of mineral nutrition on plant development and chemical composition of volatile oils of Porophyllum ruderale (Jacq.) Cass subspecies. Afr. J. Agric. Res. 2019, 14, 1870–1877. [Google Scholar] [CrossRef]
- Guillet, G.; Bélanger, A.; Arnason, J.T. Volatile monoterpenes in Porophyllum gracile and P. ruderale (Asteraceae): Identification, localization and insecticidal synergism with α-Terthienyl. Phytochemistry 1998, 49, 423–429. [Google Scholar] [CrossRef]
- Hernández-Cruz, J.; Luna-Cruz, A.; Loera-Alvarado, E.; Villanueva-Sánchez, E.; Landero-Valenzuela, N.; Zárate-Nicolás, B.H.; Diego-Nava, F.; Granados-Echegoyen, C.A. Effiiency of the essential oil of Porophyllum linaria (Asteraceae) a Mexican Endemic Plant Against Sitophilus zeamais (Coleoptera: Curculionidae). J. Insect Sci. 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Baren, C.M.; Juárez, B.; Ferraro, G.E.; Coussio, J.D. Phenolic Constituents of Porophyllum obscurum. Biochem. Syst. Ecol. 1994, 22, 543. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Zygadlo, J.A.; Faillaci, S.M.; Ariza Espinar, L. Constituents of the volatile oil of Porophyllum obscurum (Spreng.) D.C. Flavour Fragr. J. 1999, 14, 107–108. [Google Scholar] [CrossRef]
- Postigo, A.; Cardoso, S.P.; Funes, M.; Sortino, M. Mechanistic studies of Candida albicans photodynamic inactivation with Porophyllum obscurum hexanic extract and its isolated thiophenic compounds. Photodiagnosis Photodyn. Ther. 2019, 26, 420–429. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovich, J.; Robinson, H.; King, R.M. A Dithienylacetylene from Porophyllum ruderale. Phytochemistry 1980, 19, 2760. [Google Scholar] [CrossRef]
- Souza, M.C.; Siani, A.C.; Ramos, M.F.S.; Menezes-de-Lima, O.; Henriques, M.G.M.O. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie 2003, 58, 582–586. [Google Scholar]
- Takahashi, H.T.; Novello, C.R.; Ueda-Nakamura, T.; Filho, B.P.; Palazzo de Mello, J.C.; Nakamura, C.V. Thiophene derivatives with antileishmanial activity isolated from aerial parts of Porophyllum ruderale (Jacq.) Cass. Molecules 2011, 16, 3469–3478. [Google Scholar] [CrossRef]
- Fontes, U.R., Jr.; Ramos, C.S.; Serafini, M.R.; Cavalcanti, S.C.H.; Alves, P.B.; Lima, G.M.; Andrade, P.H.S.; Bonjardim, L.R.; Quintans, L.J., Jr.; Araújo, A.A.S. Evaluation of the lethality of Porophyllum ruderale essential oil against Biomphalaria glabrata, Aedes aegypti and Artemia salina. Afr. J. Biotechnol. 2012, 11, 3169–3172. [Google Scholar] [CrossRef]
- Jimenez, M.; Guzman, A.P.; Azuara, E.; Garcia, O.; Mendoza, M.R.; Beristain, C.I. Volatile Compounds and Antioxidative Activity of Porophyllum tagetoides Extracts. Plant Food Hum. Nutr. 2012, 67, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Baruah, R.N.; Dominguez, X. A further dithienyl derivative from Porophyllum scoparia. J. Med. Plant Nat. Prod. Res. 1984, 77, 77–78. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Thymol derivatives from Porophyllum riedelii. Phytochemistry 1983, 22, 1035–1036. [Google Scholar] [CrossRef]
- Chantraine, J.M.; Dominique, L.; Ballivian, C.; Saavedra, G.; Ibañez, R.; Vilaseca, L.A. Insecticidal Activity of Essential Oils on Aedes aegypti Larvae. Phytother. Res. 1998, 12, 350–354. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 715–720. [Google Scholar] [CrossRef]
- Zarrad, K.; Hamouda, A.B.; Chaieb, I.; Laarif, A.; Jemâa, J.M. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. Ind. Crop. Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Sánchez-Torres, L.E.; Nevárez-Moorillón, G.V.; Camacho, A.D.; Nogueda-Torres, B. Aceites esenciales y sus componentes como una alternativa en el control de mosquitos vectores de enfermedades. Biomédica 2017, 37, 224–243. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Amiteye, S. Efficacy of mixing vegetable oils with pirimiphos-methyl against the maize weevil, Sitophilus zeamais Motschulsky in stored maize. J. Stored Prod. Res. 2005, 41, 57–66. [Google Scholar] [CrossRef]
- Bakker, J.; Gommers, F.J.; Nieuwenhuis, I.; Wynberg, H. Photoactivation of the nematicidal compound alpha-terthienyl from roots of marigolds (Tagetes species). A possible singlet oxygen role. J. Biol. Chem. 1979, 25, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Nivsarkar, M.; Cherian, B.; Padh, H. Alpha-Terthienyl: A plant-derived new generation insecticide. Curr. Sci. 2001, 81, 667–672. [Google Scholar]
- Barrera, L.L.; García, L.J. Actividad antifúngica de aceites esenciales y sus componentes sobre el crecimiento de Fusarium sp. aislado de papaya (Carica papaya). Rev. UDO Agrícola 2008, 8, 33–41. [Google Scholar]
- Rondón, M.E.; Delgado, J.; Velasco, J.; Rojas, J.; Rojas, L.B.; Morales, A. Chemical composition and antibacterial activity of the essential oil from aerial parts of Porophyllum ruderale (Jacq.) Cass. collected in Venezuela. Ciência 2008, 16, 5–9. [Google Scholar]
- Croft, S.L.; Sundar, S.; Fairlamb, A. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Minodier, P.; Parola, P. Cutaneous leishmaniasis treatment. Travel Med. Infect. Dis. 2007, 5, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Coutinho, C.E.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment-a challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Días, B.P.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011, 15, e525–e532. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Ioset, J.R. Natural Products for Neglected Diseases: A Review. Curr. Org. Chem. 2008, 12, 643–666. [Google Scholar] [CrossRef]
- Takahashi, H.T.; Britta, E.A.; Longhini, R.; Ueda-Nakamura, T.; Palazzo de Mello, J.C.; Nakamura, C.V. Antileishmanial Activity of 5-Methyl-2,2′:5′,2″-tertiophene isolated from Porophyllum ruderale is related to mitochondrial dysfunction in Leishmania amazonensis. Planta Med. 2013, 79, 330–333. [Google Scholar] [CrossRef]
- Melo, M.S.; Sena, L.C.S.; Barreto, F.J.N.; Bonjardim, L.R.; Almeida, J.R.G.S.; Lima, J.T.; de Sousa, D.P.; Quintans-Júnior, L.J. Antinociceptive effects of citronellal in mice. Pharm. Biol. 2010, 48, 411–416. [Google Scholar] [CrossRef]
- Jácomo, A.C.; de Andrade Velozo, K.; Lotti, R.G.; Neves, L.M.; de Gaspari de Gaspi, F.O.; Esquisatto, M.A.; do Amaral, M.E.; Mendonça, F.A.; dos Santos, G.M. Activity of Porophyllum ruderale leaf extract and 670-nm InGaP laser during burns repair in rats. BMC Complement. Altern. Med. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A. Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Clement, Y.N.; Williams, A.F.; Khan, K.; Bernard, T.; Bhola, S.; Fortuné, M.; Medupe, O.; Nagee, K.; Seaforth, C.E. A gap between acceptance and knowledge of herbal remedies by physicians: The need for educational intervention. BMC Complement. Altern. Med. 2005, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Uniyal, S.K.; Singh, K.N.; Jamwal, P.; Lal, B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2006, 2, 1–8. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Rosa, I.M.L. Biodiversity, traditional medicine and public health: Where do they meet? J. Ethnobiol. Ethnomed. 2007, 3, 1–9. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 31, 1–18. [Google Scholar] [CrossRef]
- Soria, N.; Ramos, P. Uso de plantas medicinales en la atención primaria de salud en Paraguay: Algunas consideraciones para su uso seguro y eficaz. Mem. Inst. Investig. Cienc. Salud 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Soria, N. Las Plantas Medicinales y su aplicación en la Salud Pública. Rev. Salud Publica Parag. 2018, 8, 7–8. [Google Scholar] [CrossRef]
- Gallegos-Zurita, M. Las plantas medicinales: Principal alternativa para el cuidado de la salud, en la población rural de Babahoyo, Ecuador. Anales de la Facultad de Medicina 2016, 77, 327–332. [Google Scholar] [CrossRef]
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2012, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human health—A Structure-Activity Relationship Study. Psyche J. Entomol. 2016, 2, 1–17. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Huang, J.; Hamasaki, T.; Ozoe, F.; Ohta, H.; Enomoto, K.; Kataoka, H.; Sawa, Y.; Hirota, A.; Ozoe, Y. Identification of Critical Structural Determinants Responsible or Octopamine Binding to the α-Adrenergic-like Bombyx mori Octopamine Receptor. Biochemistry 2007, 46, 5896–5903. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Martin, E.; Péguillet, I.; Guihot, A.; Froux, N.; Coré, M.; Lévy, E.; Dusseaux, M.; Meyssonier, V.; Premel, V.; et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef]
- Gold, M.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.; Vogt, T.; Delepine, J.; Winata, E.; Swarbrick, G.; Chua, W.; Yu, Y.; et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed]
- Mniff, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Sabino, C.K.; Fereira-Filho, E.S.; Mendes, M.; da Silva-Filho, J.C.; Ponte, M.; Moura, L.; Oliveira, E.; Quintans-Junior, L.J.; dos Santos, M.; Oliveira, R.; et al. Cardiovascular effects induced by α-terpineol in hypertensive rats. Flavour Fragr. J. 2013, 28, 333–339. [Google Scholar] [CrossRef]
- Quintans-Junior, L.; Oliveira, M.; Santana, M.; Santana, M.; Guimaraes, A.; Siqueira, J.; de Sousa, P.; Almeida, R. α-Terpineol reduces nociceptive behavior in mice. Pharm. Biol. 2011, 49, 583–586. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Zaki, M.Y.; Abbas, N.S. Synthesis, anti-inflammatory and anti-ulcer evaluations of thiazole, thiophene, pyridine and pyran derivatives derived from androstenedione. Steroids 2015, 98, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Alatibi, F.; El-Sayed, N.N.E.; Al-Showiman, S.; Kheder, N.A.; Wadood, A.; Rauf, A.; Bawazeer, S.; Hadda, T.B. Antimicrobial Activity of Some Novel Armed Thiophene Derivatives and Petra/Osiris/Molinspiration (POM) Analyses. Molecules 2016, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Mendonça Junior, F.J.B.; Lima-Neto, R.G.; Oliveira, T.B.; Lima, M.; Pitta, I.; Galdino, S.L.; Cruz, R.M.D.; Neves, R.P. Synthesis and Evaluation of the Antifungal Activity of 2-(Substituted-Amino)-4,5-Dialkyl-Thiophene-3-Carbonitrile Derivatives. Lat. Am. J. Pharm. 2011, 30, 1492–1499. [Google Scholar]
- Romagnoli, R.; Baraldi, P.G.; Salvador, M.K.; Preti, D.; Tabrizi, M.A.; Bassetto, M.; Brancale, A.; Hamel, E.; Castagliuolo, I.; Bortolozzi, R.; et al. Synthesis and Biological Evaluation of 2-(Alkoxycarbonyl)-3-Anilinobenzo[b]thiophenes and Thieno [2,3-b]pyridines as New Potent Anticancer Agents. J. Med. Chem. 2013, 56, 2606–2618. [Google Scholar] [CrossRef]
- Gil, A.; Ghersa, C.M.; Perelman, S. Root thiophenes in Tagetes minuta L. accessions from Argentina: Genetic and Environmental contribution to changes in concentration and composition. Biochem. Syst. Ecol. 2002, 30, 1–13. [Google Scholar] [CrossRef]
- Van Hellemond, J.J.; Opperdoes, F.R.; Tielens, A.G. The extraordinary mitochondrion and unusual citric acid cycle in Trypanosoma brucei. Biochem. Soc. Trans. 2005, 33, 967–971. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2016, 17, e49–e55. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Cheenpracha, S.; Karalai, C. HIV-1 Protease and HIV-1 Integrase inhibitory substances from Eclipta prostrata. Phytother. Res. 2007, 21, 1092–1095. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
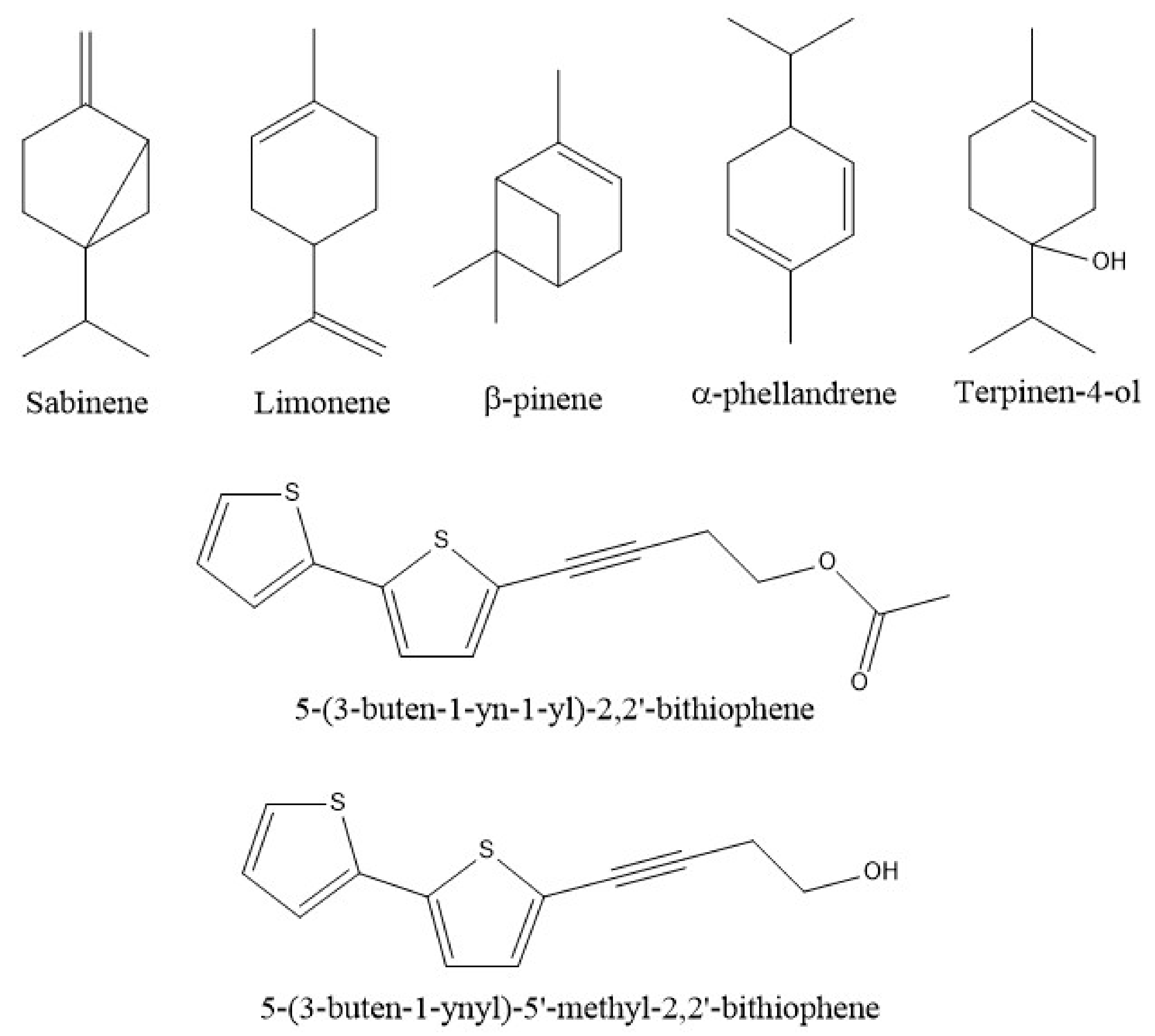
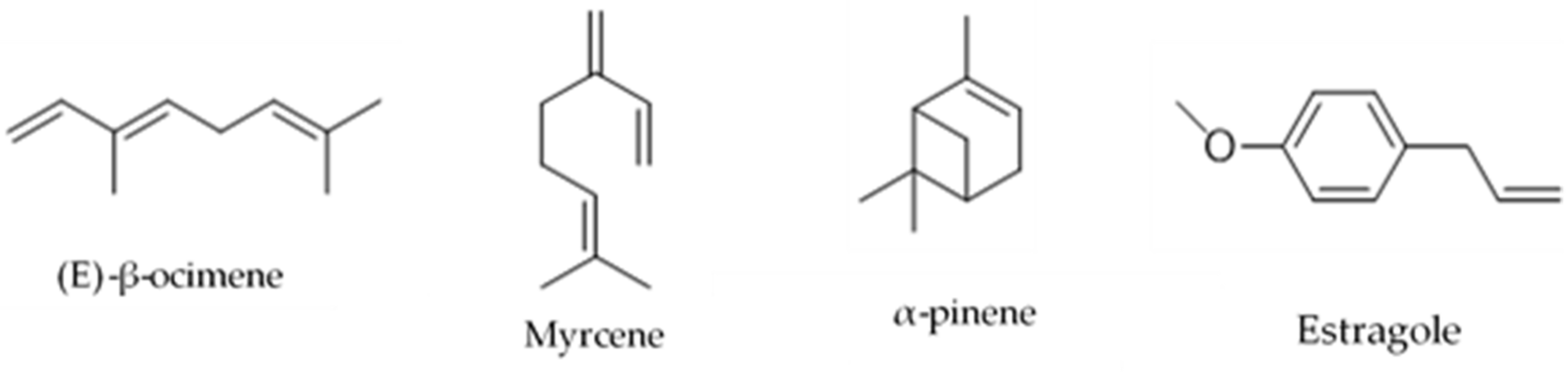
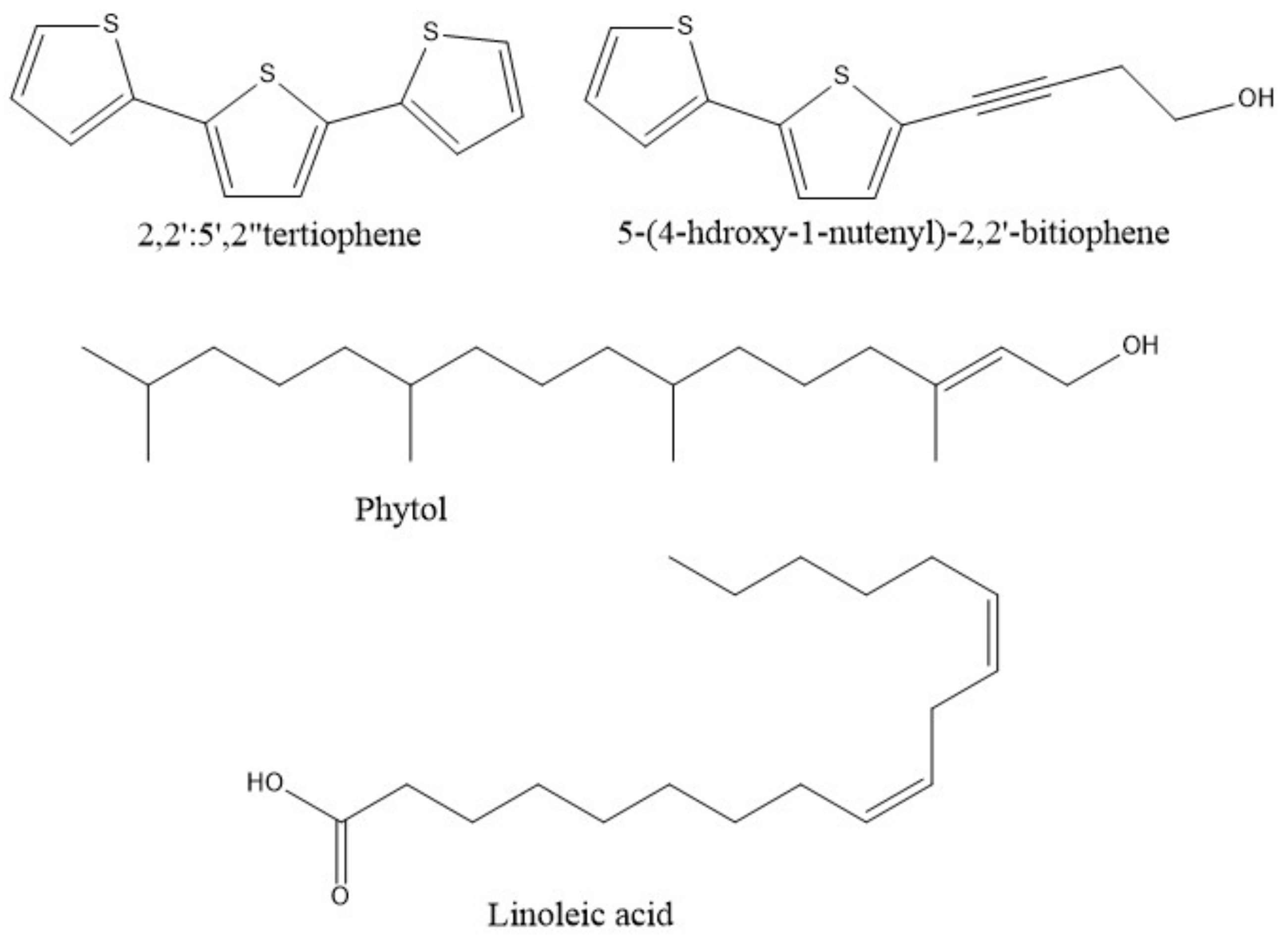
| Species | Common Name | Part of the Plant | Extraction System | Chemical Analysis Performed | Isolation/Identification | Chemical Compounds and Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| P. gracile | “hierba del venado” | Aerial parts | Static head-space technique. | Gas chromatography (GC) with Flame Ionization Detector (FID) | Identification | α-pinene (17.7) Sabinene (3.5) Myrcene (62.7) β-cubenene (9.1) | [14] |
| P. linaria | “pipicha, pepicha o chepiche” | Leaves | Hidro-distillation using a Clevenger system equipped with a microwave set at 100% potency. Aromatic water recirculation system using a Clevenger-type apparatus adapted with a conventional microwave. | Gas Chromatography–Mass Spectrometry (GC–MS) | Identification | Phytol (25.50) Linoleic acid (29.50) β-myrcene (41.94) D-limonene (20.29) Estragole (20.03) 1-undecene (8.02) 3-(4-methyl-3-pentenyl)-furan (3.72) Terpinen-4-ol (8.51) | [5,15] |
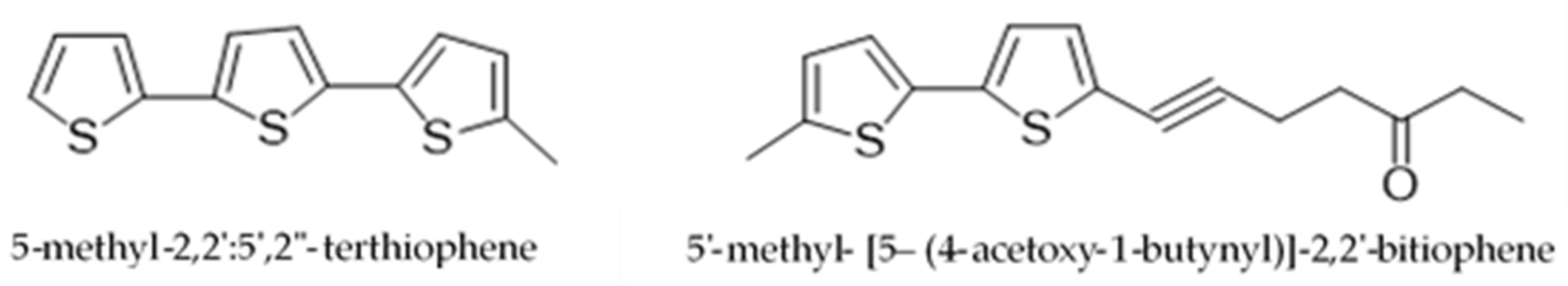
| P.obscurum | “kilkina, pus-pus, quirquiña, ruda blanca, hierba de la gama, hierba del ciervo” | Aerial parts | Maceration with methanol Hhydro-distillation in an all-glass Clevenger-type apparatus Maceration with hexane | GC GC-MS Thin layer chromatography (TLC) | Identification and isolation | β-pinene (16.30) trans-sabinene hydrate (12.8) Undec-1-ene (12.4) 5-(3-buten-1-ynyl)-2,2′-bithiophene (0.018) 5-(4-acetoxy-1-butenyl)-2,2′-bithiophene (0.008) 5-(4-hydroxy-1-butenyl)-2,2′-bithiophene (0.010) 2,2′:5′,2″-terthiophene (0.039) | [16,17,18] |
| P.ruderale | “papaloquelite, pápalo” | Aerial parts | Maceration with Et2O Modified Clevenger-type distillation Maceration with dichlorometane Hidro-distillation | 1H Nuclear Magnetic Resonance (NMR) GC-MS 1H and 13C NMR and MS | Identification and isolation | Myrcene (3.37) (Z)-β-ocimene (1.38) (E)-β-ocimene (93.95) β-pinene (0.27) α-terpineol (0.3) Terpinen-4-ol (10.3) 5′-acetoxymethylen-2-[4-acetoxy-but-3-inyl]-ditiophene (0.005) 5′-methyl-2-[4-acetoxy-but-3-inyl]-bitiophene (0.010) 3-but-1-en-3-inyldithyenil (0.003) 5,methyl-2,2′:5,2″-terthiophene (0.007) 5′-methyl-[5-4(4-acetoxy-1-butynyl)]-2,2′ bi-thiophene (0.018). | [19,20,21,22] |
| P. tagetoides | “pipicha” | Leaves and stems | Maceration with ethanol and water, oil emulsion | Headspace GC-MS | Identification | 1-noneno (4.05) α-terpinene (0.52) Perilleno (6.49) Nonanal (1.88) 2-decenal (6.46) p-thymol (0.82) 8-phenyloctan-1-ol (1.10) Dodecyl hexanoate (1.01) β-myrcene (1.6) D-limonene (9.67) Nonanal (50.87) Decanal (19.52) (-)-trans-pineno (43.48) (Z)-8-dodecen1-ol (17.27) | [23] |
| P. scoparia | “jarilla, romerillo” | Root and aerial parts | Maceration with methanol/ether/petrolether, 1:1:1 | 1H-NMR | Isolation | 3-butyn-2-ol,4-[2,2′-bithiophene]-5-yl-1-chloro-2-acetate (0.002) 5-(3-buten-1-yn-1-yl)-2,2′-bithiophene (0.003) 5-(4-hydroxybut-1-ynyl)-2,2′-bithiophene (0.002) 2,2′:5′,2′′-terthiophene (0.002) 2,4,3-butyne-1,2-diol,4-[2,2′-bithiophene]-5-yl-1,2-diacetate (0.0006) 3-butyne-1,2-diol,4-[2,2′ bithiophene]-5-yl-isovalerate (0.0007) Sakuranetin (naringenin 7-methyl ether) (0.0007) | [24] |
| P. riedelli | Not specified | Aerial parts | Et2O-petrol (1:2) | 1H-NMR | Isolation | 5-(3-buten-1-yn-1-yl)-2,2′ bithiophene (0.005) 2,2′:5′,2′′-terthiophene (0.01) 2-methoxy-9-tygloyloxy-8,10-epoxy thymol isobutyrate (0.003) 2-methoxy-9- isobutyryloxy-8,10-epoxy thymol tiglate (0.003) 2-methoxy-9-tigloyloxy-8,10 epoxythymol tiglate (0.0002) 2-methoxy-9-isobutyryloxy-8,10-epoxythymol-isobutyrate (0.002) α-isocomene (0.003) β-isocomene (0.002) Modhephene (0.001) Squalene (0.014) Caryophyllene (0.001) Germacrene D (0.002) Bicyclogermacrene (0.0009) | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Atanacio, M.J.; Bautista-Ávila, M.; Velázquez-González, C.; Castañeda-Ovando, A.; González-Cortazar, M.; Sosa-Gutiérrez, C.G.; Ojeda-Ramírez, D. Porophyllum Genus Compounds and Pharmacological Activities: A Review. Sci. Pharm. 2021, 89, 7. https://doi.org/10.3390/scipharm89010007
Vázquez-Atanacio MJ, Bautista-Ávila M, Velázquez-González C, Castañeda-Ovando A, González-Cortazar M, Sosa-Gutiérrez CG, Ojeda-Ramírez D. Porophyllum Genus Compounds and Pharmacological Activities: A Review. Scientia Pharmaceutica. 2021; 89(1):7. https://doi.org/10.3390/scipharm89010007
Chicago/Turabian StyleVázquez-Atanacio, María José, Mirandeli Bautista-Ávila, Claudia Velázquez-González, Araceli Castañeda-Ovando, Manasés González-Cortazar, Carolina Guadalupe Sosa-Gutiérrez, and Deyanira Ojeda-Ramírez. 2021. "Porophyllum Genus Compounds and Pharmacological Activities: A Review" Scientia Pharmaceutica 89, no. 1: 7. https://doi.org/10.3390/scipharm89010007
APA StyleVázquez-Atanacio, M. J., Bautista-Ávila, M., Velázquez-González, C., Castañeda-Ovando, A., González-Cortazar, M., Sosa-Gutiérrez, C. G., & Ojeda-Ramírez, D. (2021). Porophyllum Genus Compounds and Pharmacological Activities: A Review. Scientia Pharmaceutica, 89(1), 7. https://doi.org/10.3390/scipharm89010007