Abstract
Coronavirus Disease 2019 (COVID-19) has spread globally with the number of cases exceeding seventy million. Although trials on potential treatments of COVID-19 Acute Respiratory Distress Syndrome (ARDS) are promising, the introduction of an effective therapeutic intervention seems elusive. In this review, we explored the potential therapeutic role of volatile anesthetics during mechanical ventilation in the late stages of the disease. COVID-19 is thought to hit the human body via five major mechanisms: direct viral damage, immune overactivation, capillary thrombosis, loss of alveolar capillary membrane integrity, and decreased tissue oxygenation. The overproduction of pro-inflammatory cytokines will eventually lead to the accumulation of inflammatory cells in the lungs, which will lead to ARDS requiring mechanical ventilation. Respiratory failure resulting from ARDS is thought to be the most common cause of death in COVID-19. The literature suggests that these effects could be directly countered by using volatile anesthetics for sedation. These agents possess multiple properties that affect viral replication, immunity, and coagulation. They also have proven benefits at the molecular, cellular, and tissue levels. Based on the comprehensive understanding of the literature, short-term sedation with volatile anesthetics may be beneficial in severe stages of COVID-19 ARDS and trials to study their effects should be encouraged.
1. Introduction
The first case of COVID-19 (Coronavirus Disease 2019) was reported in the city of Wuhan, China. Soon after, cases were reported in more than 170 countries across the world. As of December 2020, the number of cases worldwide has exceeded 70 million, with more than 1.7 million deaths. Although many ongoing trials of potential treatments are promising, the introduction of an effective therapeutic intervention to the medical market seems elusive. Hence, shedding light on the possible roles of some of the regularly used drugs could lead us to achieve a fair degree of success in facing the pandemic.
As the name of the virus ‘SARS-CoV-2’ (Severe Acute Respiratory Syndrome Coronavirus 2) implies, COVID-19 can progress to a cytokine storm that strikes the lungs, leading to ARDS (Acute Respiratory Distress Syndrome) [1,2]. In patients presenting with COVID-19 pneumonia, 42% can develop ARDS [3]. The typical ARDS mortality rate in the ICU (Intensive Care Unit) reaches 35% [4], while mortality from COVID-19 ARDS in patients admitted to ICU is nearly 60% and reaches 90% if the patient requires mechanical ventilation [5,6]. Hence, respiratory failure resulting from ARDS is the most common cause of death in COVID-19 [7]. Through a comprehensive understanding of the pathophysiology of the disease, insights from the literature could lead us to possible therapeutic solutions. We aim to discover the potential role of inhalational anesthetics in reversing or slowing down the progress of COVID-19 ARDS when given as short-term sedative agents for mechanically ventilated patients.
2. COVID-19 ARDS Pathophysiology
The pathophysiology of SARS-CoV-2 infection resembles that of SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) infection, with inflammatory responses being responsible for damaging the airways [8]. Some patients may be at risk of secondary bacterial and fungal infections [8,9]. When the virus is introduced to the body, the RBD (Receptor-Binding Domain) on the S1 subunit of S protein of the virus will bind to the ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane Serine Protease 2) proteins on the epithelial cell surface, triggering endocytosis [10,11,12,13,14,15,16,17]. The virus then replicates to release many copies of itself. The cell then undergoes pyroptosis (which is a form of programmed cell death that potently triggers immune responses [18,19]) and releases damage-associated molecular patterns like ATP (Adenosine Triphosphate), nucleic acids and ASC oligomers [11]. This inciting event is recognized by the neighboring resident epithelial cells, endothelial cells, and macrophages, causing the release of pro-inflammatory cytokines such as IL-6 (Interleukin-6), IP-10 (Interferon gamma-induced protein 10), G-CSF (Granulocyte colony-stimulating factor) and macrophage inflammatory proteins [7,20,21]. Viral infection can also activate the NLRP3 inflammasome (Nod-like receptor family, pyrin domain-containing 3) through cellular hyperpolarization, which occurs due to disturbances in the K+ and Ca2+ flux mediated by 3a and E viral proteins [22,23,24,25,26,27,28,29,30,31], the release of reactive oxygen species, or direct signals from mitochondrial DNA (Deoxyribonucleic acid) after cell damage [32,33]. When activated, the NLRP3 inflammasome binds to mitochondrial proteins to catalyze a proteolytic process of pro-IL-1β and pro-IL-18 into their active forms [34,35]. These cytokines will attract neutrophils, natural killer cells, monocytes, macrophages and T cells to the site of injury. These cells will eventually accumulate in the lungs, leading to the overproduction of pro-inflammatory cytokines and a cytokine storms [20,36,37,38].
COVID-19 ARDS is a spectrum of COVID-19 cytokine storms, which reflects the vast release of cytokines from T cells and macrophages in response to the viral infection [7,39]. It is confirmed when a patient with viral pneumonia meets Berlin ARDS diagnostic criteria [4,5]. As severity increases and mechanical ventilation becomes mandatory, high levels of CRP (C-reactive protein), CK (Creatinine Kinase), neutrophil count, interleukins (IL-2, IL-6, IL-7, IL-1β), TNF-α (Tumor Necrosis Factor α), interferon γ and D-dimers are usually detected in serum [6,21,40]. This implies that severe stages of the disease are strongly related to immune overactivation. In addition, lymphocytic count decreases as the disease progresses [6,41,42]. Notably, levels of immune-suppressive IL-10 (Interleukin 10) markedly increase in parallel with disease severity [43]. Cellular damage along with the released cytokines will activate the adaptive immunity, represented by T and B lymphocytes [21]. Mass cytometry on lung tissues demonstrated diffuse infiltration of CD4+ T lymphocytes and macrophages, with patchy infiltrations of natural killer cells, neutrophils, and mature T cells [44]. Histological findings include diffuse alveolar damage along with severe pulmonary microthrombosis [38,45].
The main pathology in COVID-19 ARDS is damage to the alveolar epithelial cells and endothelial cells through inflammatory cell infiltration that accumulates proteases and reactive oxygen species [37,38,46]. In severe status, high viral load is directly related to poor outcome [47]. Thrombotic Disseminated Intravascular Coagulation (DIC) was noted in most deaths related to COVID-19 ARDS [48]. Autopsy tissues showed very high levels of TNF-α, IL-1β, and IL-6 [49]. Interestingly, highly inflammatory monocyte-derived FCN1+ macrophages with CD14+ and CD16+ monocytes were found in the bronchoalveolar lavage fluid of patients with severe COVID-19 [20,50].
Despite the fact that COVID-19 ARDS is mainly a manifestation of overactive immunity, the immune response plays an important role in fighting the virus. CD4+ T cells are very important for priming the response of CD8+ T cells and B cells and recruiting more immune cells through cytokine production [51]. The CD8+ T cells are directly involved in attacking the virus-infected cells [52]. The depletion of CD4+ and CD8+ T cells can slow viral clearance and hasten lung inflammation [53,54]. The B cells attack the N (Nucleocapsid) protein and the S protein of the virus [55,56], and polyclonal antibodies from convalescent plasma have proven efficacy in decreasing viral load and mortality [57].
In this sense, we conclude that severe stages of COVID-19 ARDS are mostly related to immune dysregulation, direct viral damage, and possibly pulmonary thrombosis. Hence, a comprehensive approach at this late stage of the disease should target these three pathological mechanisms [20].
3. The Potential Therapeutic Role of Volatile Anesthetics
To balance the facts, when COVID-19 worsens, the host response is more damaging than the virus itself [20]. This view is supported by the ongoing trials on potential treatments of the disease, where the main mechanism of action of most drugs tested is immunosuppression [58,59,60,61,62,63,64,65,66]. Hence, agents that show major immunosuppressive effects with some balancing activating effects should be ideal for countering the disease.
Macrophages are the first responders to infection as they reside in tissues [36]. Sevoflurane and Desflurane reduce macrophage levels in bronchial alveolar fluid, with overall cellular infiltration also reduced [67]. Isoflurane was shown to decrease macrophage release of TNF-α, IL-6, IL-1β, Monocyte Chemotactic Protein-1 (MCP-1) and Macrophage Inflammatory Protein-2 (MIP-2) [68,69]. Regarding cytokines, there are two main classes that regulate the immune response: pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and Interferon-γ, and anti-inflammatory cytokines like IL-1α, IL-10, TNF-β [36]. Multiple studies were conducted to evaluate the precise effect of each volatile agent on the different classes of cytokines. Though some studies showed some volatile agents might potentiate inflammatory cytokines, the prominent general model extracted from numerous other studies points to a potent inhibitory effect of volatile agents on pro-inflammatory cytokines, with a stimulatory effect on anti-inflammatory cytokines [36,68,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90]. In brief, volatile agents seem to have a rather balancing effect on immunity where many studies proved their ability to attenuate pro-inflammatory and potentiate anti-inflammatory cytokines, but there are some studies that suggest these agents might not impact or paradoxically potentiate the immune response. While keeping in mind that immunosuppression is mostly in favor of other effects, the balancing effects reported in the literature can be viewed as a beneficial aspect of these agents [68].
Neutrophils are among the first and most lethal immune cells recruited to the site of inflammation [91]. Inhalational anesthetics decrease neutrophilic adhesion, phagocytic function, and reactive oxygen species production [79,92,93]. Sevoflurane was also shown to decrease neutrophilic count [92]. The inhibition of neutrophilic adhesion spares the deleterious effects of Polymorphonuclear (PMN) cell accumulation and protects the remaining healthy tissues [36]. This can be supported by the reported beneficial effects of general anesthesia to post-ischemic cardiac function [94,95]. Trials on mice exposed to volatile agents showed decreased PMN cells level in BAL (Bronchoalveolar Lavage), neutrophil-attracting chemokines, and severity of symptoms when mice were injected with a sublethal dose of the Influenza A virus [96,97]. Some studies suggested that volatile agents do not affect proliferation of PMN cells, which might be a balancing effect [98]. Other cells affected include natural killer cells, which have a vital role against viral infections. Isoflurane was shown to decrease natural killer cells’ response to interferon, while Sevoflurane reduces their cytokine release [71,99,100]. A decrease in the number of natural killer cells was also noted following the use of volatile agents [100,101]. Regarding microglia, they are resident tissue cells that potentiate inflammation through cytokine release, but data regarding the effects of volatile agents on these cells are conflicting [83,102,103].
Platelets play an important role in cellular adhesion and coagulation. Sevoflurane enhances P-selectin expression on platelets to potentiate the immune cells’ binding ability [104]. Though it did not affect intra-operative blood loss, nor coagulation studies, Sevoflurane can significantly inhibit platelet aggregation [105,106], a property that can be very beneficial in COVID-19 induced microthrombosis [107].
With regard to adaptive immunity, volatile agents decrease proliferation and increase apoptosis of T lymphocytes [93,108,109,110]. Desflurane preserves Th1/Th2 (T helper cells) and IL-2/IL-4 values [111], while Isoflurane and Sevoflurane decrease Th1/Th2 values [101,112]. B lymphocytes modify their surface receptors or immunoglobulins to be able to recognize pathogens [91]. Their products constitute the most selective and specific part of the immune system. Sevoflurane, Isoflurane and Desflurane can cause B lymphocyte damage through the enhancement of Ca2+ release from its endoplasmic reticulum [113,114]. Reports regarding the effects of inhalational agents on the complement system are lacking [36], though general anesthesia is generally associated with a decrease in complement levels [93]. In brief, volatile agents were mostly immunosuppressive with some selective activation, which is exactly what we need in the late stage of COVID-19 ARDS.
As an overview, volatile agents were able to affect all forms of innate and adaptive immunity. They can also inhibit platelet aggregation. Interestingly, volatile agents can inhibit and potentiate macrophages at the same time, depending on the anatomical site of inflammation [36]. Although the literature points to uncountable benefits, long-term exposure to volatile agents can lead to immune dysfunction, increase the viral and the bacterial load, and worsen the outcome in settings of sepsis [115,116,117]. Hence, in patients with a septic profile, this aspect should be kept in mind, as the patient might already have impaired immunity. Another drawback of volatile agents is their ability to enhance hyperpolarization of the cellular membrane, which is a key step in the pathway of cytokine production through NLRP3 inflammasome activation [118,119,120,121].
Among the important effects of volatile agents in the speculation about their ability to treat COVID-19 ARDS is their antiviral effect. The replication of many animal viruses was moderately inhibited by halothane exposure at 2.2% concentration [122]. The studies that evaluated measles virus replication in cellular culture showed a reduction in the appearance of viral replicants at relevant clinical concentrations of the commonly used volatile agents [123,124]. In addition, ether solvents possess the property of disinfection, and diethyl ether was one of the commonly used agents in general anesthesia induction. Since the MAC value of diethyl ether is 2%, its blood concentration might reach a level that is enough to affect the virus directly [125].
4. Favorable Specifics of Volatile Anesthetics in ARDS Sedation
The ideal sedative agent should have fast onset and offset, with a short context-sensitive half-life. Currently, none of the IV (Intravascular) anesthetic agents used is ideal [126]. Volatile anesthetics have been used as sedatives in intensive care settings in Europe and Canada [127,128]. The commonly used agents are Isoflurane, Sevoflurane and Desflurane [129]. Inhalational agents’ context-sensitive half-lives are comparable and do not increase in relation to period of administration [130]. All forms of anesthesia are able to modulate the immune system and affect innate and adaptive immunity, but volatile agents possess favorable properties [98,108,131,132]. Multiple studies have shown that sedation with volatile anesthetics over midazolam and propofol was associated with a decrease in time of mechanical ventilation [133,134,135,136,137]. Early extubation is very beneficial in terms of reduction in ventilator-associated complications like volutrauma, atelectasis, and pneumonia [129]. Other studies showed a reduction in post-extubation agitation and delusion, and preservation of factual ICU memory when patients were sedated with volatile agents [138,139,140]. Studies concerning the effects of volatile agents on ICU delirium are lacking [129]. In a study on patients with lung diseases on ventilators, the group sedated with Isoflurane had significantly lower mortality than those sedated with propofol or midazolam [140]. Volatile agents can also improve consequences of ARDS. In a prospective study, researchers compared PaO2/FiO2 in ARDS patients who received midazolam or Sevoflurane for a 48-h sedation, they found that the Sevoflurane group showed higher PaO2/FiO2 [141]. Other studies showed that general anesthesia with inhalational agents over IV anesthetics was associated with lower pulmonary complications and reduced mortality after cardiac and non-cardiac surgeries [142,143]. In surgeries requiring one-lung ventilation, Sevoflurane was associated with fewer pulmonary complications, better oxygenation, and lower pro-inflammatory mediator levels than propofol [117,144]. In sepsis models, Isoflurane showed the ability to decrease neutrophil recruitment over propofol [145,146]. Additionally, Isoflurane attenuated lung injury in post-hemorrhagic shock, but phenobarbital did not [147]. In a ventilator induced lung injury model, Isoflurane and Sevoflurane could attenuate neutrophilic recruitment more than ketamine and Desflurane [148]. However, though never evaluated in diseased lungs, volatile anesthetics worsen V/Q mismatching in healthy individuals [129,149,150].
Volatile anesthetics can also directly affect lung cells in ARDS. Isoflurane could decrease the cytokine-based recruitment of neutrophils following LPS (Lipopolysaccharide) instillation induced injury [97,151]. In other studies, both Isoflurane and Sevoflurane were able to attenuate the pro-inflammatory response of alveolar epithelial cells through their effect on type A γ-aminobutyric acid receptors (GABAa receptors) [152,153]. They also cause bronchodilation through activation of GABAa receptors, hence improving lung and tissue oxygenation [154,155]. Due to unknown reasons, volatile agents can shift the oxygen–hemoglobin dissociation curve to the right, increasing the release of O2 from hemoglobin to tissues and improving tissue oxygenation [156]. Regarding effects at the tissue level, Sevoflurane mitigates pulmonary oedema through the activation of the amiloride-sensitive Na+ channel (ENaC) and Na+/K+-ATPase on the epithelial cell surface [157], and was also associated with less oxidative burst and lower levels of inflammatory mediators in BAL during mechanical ventilation [158]. In an LPS hit model of lung injury, Isoflurane and Desflurane were able to maintain the integrity of alveolar–capillary membrane [122]. In brief, adding to their immunomodulatory, antiviral and antithrombotic effects, volatile anesthetics were more beneficial than IV agents at the cellular and tissue levels in the settings of ARDS. Their short-term use can also spare some of the complications of the long-term use of IV anesthetics like PRIS (propofol infusion syndrome) [156].
5. Practical Obstacles and Physiological Barriers of Inhalational Sedation
The conduction of inhalational sedation in the ICU settings still has many considerations and practical obstacles. Volatile anesthetics are used in the operation rooms where ventilators are specifically equipped for this purpose, but ICU ventilators use high flow non-rebreathing circuits, which cannot incorporate traditional plenum vaporizers [129]. A couple of solutions to counter this problem were raised, including the use of miniature vaporizers, but issues like large dead space and lack of proper scavenging systems still limit their use at wide ranges [159]. Other limitations are typically circumstantial, for example, sedation using volatile agents should only be conducted by certified anesthesiologists or anesthesiology trained intensivists [160]. The potential complications of volatile anesthetics and the consequences of environmental air pollution with these agents should be properly monitored [161]. The education of ICU staff in the recognition and management of malignant hyperthermia reaction should accompany the implementation of inhalational sedation, and adequate stocks of dantrolene and activated charcoal filters should be readily available.
Though multiple studies suggested the relative safety of volatile anesthetics in patients with multiple comorbidities [133,141,162], these agents are not risk-free. Volatile agents cause dose-dependent respiratory depression and hypotension, along with varying degrees of hypnosis in response to adopted dosing regimens [160]. One study suggested that long-term use of Sevoflurane may rarely be associated with diabetes insipidus [163]. Other known side effects include malignant hyperthermia, allergy, and hepatitis. Arrhythmogenic agents like halothane must be avoided in the ICU setting, as halothane–epinephrine induced arrhythmias in ICU patients could be fatal [164]. Once sedation commences, regular assessment of pain, delirium and predetermined sedation score is mandatory [160]. For light sedation purposes, dosing regimens as low as 2–5 mL/h Isoflurane or 3–8 mL/h Sevoflurane can be adequate as starting levels. Increments of 0.5–1 mL/h can be used when needed [160]. Routine monitoring as recommended by ASA (American Society of Anesthesiology) guidelines is required, with the addition of the monitoring of end-tidal Minimal Alveolar Concentration (MAC). An end-tidal MAC of 0.2–0.5 typically achieves most sedation goals, with some variations seen in elderly and people with multiple comorbidities and encephalopathy [165]. The provision of volatile agents comes with the price of extra care for maintenance; this includes some daily device changes to preserve humidity and antimicrobial functions, taking extra caution during disconnection of circuits to prevent aerosolization of SARS-CoV-2 with vapor droplets, and the regular checking of scavenging systems to keep occupational levels of volatile agents acceptable.
6. Conclusions
Though mostly mild, COVID-19 might manifest as severe pneumonia progressing to ARDS and eventually death. Severely ill patients may require mechanical ventilation with sedation. Due to practical reasons, volatile agents are not widely used or adequately studied for sedation in ICU [141]. Till now, studies on patients with ARDS from causes other than COVID-19 have proven the beneficial effects of inhalational over IV sedation in terms of tissue oxygenation and mortality [141]. Since the severity of lung injury in COVID-19 is directly related to the levels of cytokines and viral load [166], we suggest that inhalational agents could potentially decrease the severity of the disease. Inhalational agents may mitigate the progression of the disease through many mechanisms: near-balanced immunosuppression, antiviral properties, antithrombotic effects, preservation of membranous and cellular integrity, improvement of tissue oxygenation and bronchodilation. Inhalational sedation is practical, of low cost, and easily controlled. It also meets ASA safety guidelines for COVID-19 patients’ sedation [167,168]. Trials on inhalational sedation for COVID-19 ARDS are being conducted [169]. Multiple literature reviews suggested the relative safety and efficacy of using inhalational sedation in late stages of COVID-19 [160,170]. Another review suggested the usage of inhalational sedation as a reliable substitute in the case of an IV anesthetics shortage [171]. The COVID-19 pandemic constitutes an emerging opportunity to study the scientific and practical aspects of inhalational sedation implementation in ICU settings. According to the insights mentioned above, the use of volatile anesthetics for short-term sedation may be beneficial in settings of severe COVID-19 ARD. Reliable recommendations can be constructed after clinical trials confirm benefits. Figure 1 discloses a summary of the beneficial and adverse events associated with the use of volatile anesthetics for sedating mechanically ventilated COVID-19 ARDS patients.
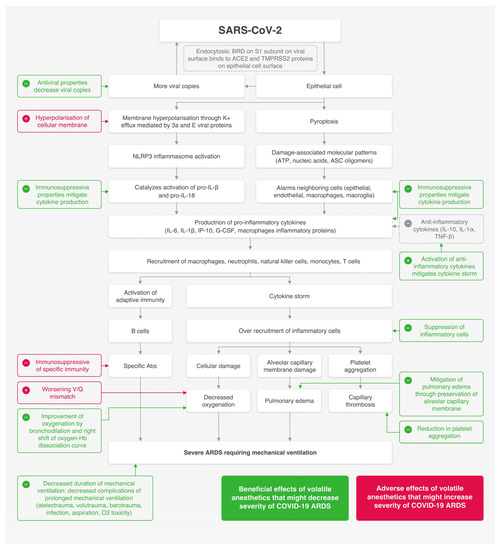
Figure 1.
A summary of beneficial and adverse events associated with the use of volatile anesthetics for sedating mechanically ventilated COVID-19 ARDS patients.
Author Contributions
Conceptualization, methodology and writing (draft): A.S. (Aiman Suleiman) and A.B.Q.; writing and review: M.A. (Mazen Alnouti), M.Y., B.S., M.E.J. and G.A.; resources and review: I.B.; project administration and summary figure: A.S. (Abeer Santarisi); project administration and supervision: M.A. (Muaweih Ababneh). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S. COVID-19 ARDS: Clinical features and differences to “usual” pre-COVID ARDS. Med. J. Aust. 2020, 213, 54–56. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.K.; Wu, A.K.L.; Ip, W.K.; Lee, N.L.S.; Chan, I.H.S.; Lit, L.C.W.; Hui, D.S.C.; Chan, M.H.M.; Chung, S.S.C.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. SSRN Electron. J. 2020, 395, 10223. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Shi, L.; Hickey, M.; Pewe, L.; Netland, J.; Farzan, M.; Wohlford-Lenane, C.; Perlman, S.; McCray, P.B. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J. Virol. 2005, 79, 14614–14621. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the putative receptor of Wuhan COVID-19. BioRxiv 2020. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Xiao, X.; Chakraborti, S.; Dimitrov, A.S.; Gramatikoff, K.; Dimitrov, D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003, 312, 1159–1164. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Arcos, J.M.G.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817.e22. [Google Scholar] [CrossRef]
- Wong, S.K.; Li, W.; Moore, M.J.; Choe, H.; Farzan, M. A 193-Amino Acid Fragment of the SARS Coronavirus S Protein Efficiently Binds Angiotensin-converting Enzyme 2. J. Biol. Chem. 2003, 279, 3197–3201. [Google Scholar] [CrossRef]
- Yang, M. Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Torres, J.; Maheswari, U.; Parthasarathy, K.; Ng, L.; Liu, D.X.; Gong, X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007, 16, 2065–2071. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, B.-J.; Xu, K.; Schwarz, W.; Du, L.; Wong, C.K.L.; Chen, J.; Duan, S.; Deubel, V.; Sun, B. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc. Natl. Acad. Sci. USA 2006, 103, 12540–12545. [Google Scholar] [CrossRef]
- Wilson, L.; McKinlay, C.; Gage, P.; Ewart, G. SARS coronavirus E protein forms cation-selective ion channels. Virology 2004, 330, 322–331. [Google Scholar] [CrossRef]
- Wang, K.; Xie, S.; Sun, B. Viral proteins function as ion channels. Biochim. Biophys. Acta Biomembr. 2011, 1808, 510–515. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef]
- Petrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Fernandesalnemri, T.; Wu, J.; Yu, J.-W.; Datta, P.K.; Miller, B.; Jankowski, W.; Rosenberg, S.A.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Ablasser, A.; Bartok, E.; Kim, S.; Schmid-Burgk, J.; Cavlar, T.; Hornung, V. Inflammasomes: Current understanding and open questions. Cell. Mol. Life Sci. 2010, 68, 765–783. [Google Scholar] [CrossRef]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Subramanian, N.; Natarajan, K.; Clatworthy, M.R.; Wang, Z.; Germain, R.N. The Adaptor MAVS Promotes NLRP3 Mitochondrial Localization and Inflammasome Activation. Cell 2013, 153, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.L.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Dreher, M.; Kersten, A.; Bickenbach, J.; Balfanz, P.; Hartmann, B.; Cornelissen, C.; Daher, A.; Stöhr, R.; Kleines, M.; Lemmen, S.W.; et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch. Aerzteblatt Online 2020, 117, 271–278. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lu, Z.; Zhang, L.; Fan, T.; Xiong, R.; Shen, X.; Feng, H.; Meng, H.; Lin, W.; Jiang, W.; et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020, 127, 104361. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, H.; Yuan, C.; Yao, C.; Luo, W.; Shen, X.; Wang, J.; Shao, J.; Xiang, Y. Clinical and Immune Features of Hospitalized Pediatric Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw. Open 2020, 3, e2010895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Qiao, L.; Wang, W.; Chen, D. Inflammatory Response Cells During Acute Respiratory Distress Syndrome in Patients with Coronavirus Disease 2019 (COVID-19). Ann. Intern. Med. 2020, 173, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, J.; Hajizadeh, N.; Moore, E.E.; McIntyre, R.C.; Moore, P.K.; Veress, L.A.; Yaffe, M.B.; Moore, H.B.; Barrett, C.D. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J. Thromb. Haemost. 2020, 18, 1752–1755. [Google Scholar] [CrossRef]
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J.; et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006, 210, 288–297. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Wong, R.S.M.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.K.; Wong, C.K.; Chan, P.K.S.; Ng, M.H.L.; Yu, L.M.; Hui, D.S.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qiu, Z.; Zhang, L.; Han, Y.; He, W.; Liu, Z.-Y.; Ma, X.; Fan, H.; Lu, W.; Xie, J.; et al. Significant Changes of Peripheral T Lymphocyte Subsets in Patients with Severe Acute Respiratory Syndrome. J. Infect. Dis. 2004, 189, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.; Perlman, S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-J.; Goh, P.-Y.; Fielding, B.C.; Shen, S.; Chou, C.-F.; Fu, J.-L.; Leong, H.N.; Leo, Y.S.; Ooi, E.E.; Ling, A.E.; et al. Profiles of Antibody Responses against Severe Acute Respiratory Syndrome Coronavirus Recombinant Proteins and Their Potential Use as Diagnostic Markers. Clin. Diagn. Lab. Immunol. 2004, 11, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-S.; Hsieh, Y.-C.; Su, I.-J.; Lin, T.-H.; Chiu, S.-C.; Hsu, Y.-F.; Lin, J.-H.; Wang, M.-C.; Chen, J.-Y.; Hsiao, P.-W.; et al. Early detection of antibodies against various structural proteins of the SARS-associated coronavirus in SARS patients. J. Biomed. Sci. 2004, 11, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Du, B.; Wang, Y.S.; Kang, H.Y.J.; Wang, F.; Sun, B.; Qiu, H.B.; Tong, Z.H. The keypoints in treatment of the critical coronavirus disease 2019 patient (2). Chin. J. Tuberc. Respir. Dis. 2020, 43, 277–281. [Google Scholar]
- Efficacy and Safety of Corticosteroids in COVID-19 US National Library of Medicine: ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04273321 (accessed on 20 July 2020).
- CellMed AG. GLP-1 CellBeads® for the Treatment of Stroke Patients with Space-Occupying Intracerebral Hemorrhage. NCT01298830. Available online: http://www.clinicaltrials.gov (accessed on 13 May 2016).
- Roivant Sciences, Inc. Roivant Announces Development of Anti-GM-CSF Monoclonal Antibody to Prevent and Treat Acute Respiratory Distress Syndrome (ARDS) in Patients with COVID-19. 2020. Available online: https://www.biospace.com/article/releases/roivant-announces-development-of-anti-gm-csf-monoclonal-antibody-to-prevent-and-treat-acute-respiratory-distress-syndrome-ards-in-patients-with-covid-19/ (accessed on 3 September 2020).
- Bioscience Izana. Initiation of Two-Centre Compassionate Use Study Involving Namilumab in the Treatment of Individual Patients with Rapidly Worsening COVID-19 Infection in Italy. 2020. Available online: https://izanabio.com/initiation-of-two-centre-compassionate-use-study-involving-namilumab-in-the-treatment-of-individual-patients-with-rapidly-worsening-covid-19-infection-in-italy (accessed on 5 September 2020).
- Ye, Q.; Wang, B.; Mao, J. Cytokine storm in COVID-19 and treatment. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Chen, C.; Qi, F.; Shi, K.; Li, Y.; Li, J.; Chen, Y.; Pan, J.; Zhou, T.; Lin, X.; Zhang, J.; et al. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumonia. 2020. Preprints. [Google Scholar]
- Tobinick, E. TNF-α inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr. Med. Res. Opin. 2004, 20, 39–40. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Majumdar, S.; Singh, R.; Misra, A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Kalimeris, K.; Christodoulaki, K.; Karakitsos, P.; Batistatou, A.; Lekka, M.E.; Bai, M.; Kitsiouli, E.; Nakos, G.; Kostopanagiotou, G. Influence of propofol and volatile anaesthetics on the inflammatory response in the ventilated lung. Acta Anaesthesiol. Scand. 2011, 55, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Flondor, M.; Hofstetter, C.; Boost, K.; Betz, C.; Homann, M.; Zwissler, B. Isoflurane Inhalation after Induction of Endotoxemia in Rats Attenuates the Systemic Cytokine Response. Eur. Surg. Res. 2007, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Giraud, O.; Molliex, S.; Rolland, C.; Leçon-Malas, V.; Desmonts, J.M.; Aubier, M.; Dehoux, M. Halogenated anesthetics reduce interleukin-1beta-induced cytokine secretion by rat alveolar type II cells in primary culture. Anesthesiology 2003, 98, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhu, S.; Wang, L.; He, Y.; Xie, H.; Zheng, S. Sevoflurane Protects against Acute Kidney Injury in a Small-Size Liver Transplantation Model. Am. J. Nephrol. 2010, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhata, H.; Shimizu, R.; Yokoyama, M.M. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int. J. Immunopharmacol. 1995, 17, 529–534. [Google Scholar] [CrossRef]
- Kim, M.; Ham, A.; Kim, J.Y.; Brown, K.M.; D’Agati, V.D.; Lee, H.T. The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013, 84, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Altay, O.; Suzuki, H.; Hasegawa, Y.; Ostrowski, R.P.; Tang, J.; Zhang, J. Isoflurane on brain inflammation. Neurobiol. Dis. 2014, 62, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Dong, Y.; Zhang, G.; Zhang, Y.; Xu, Z.; Culley, D.J.; Crosby, G.; Marcantonio, E.R.; Tanzi, R.E.; et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol. Aging 2012, 33, 1364–1378. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.-F.; Cao, D.-H.; Yang, Y.; Wang, H.-Q.; Zhu, L.-J.; Ruan, B.-H.; Du, J.; Wang, M.-C. Isoflurane Protects Against Injury Caused by Deprivation of Oxygen and Glucose in Microglia Through Regulation of the Toll-Like Receptor 4 Pathway. J. Mol. Neurosci. 2014, 54, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kai, S.; Matsuyama, T.; Adachi, T.; Fukuda, K.; Hirota, K. General Anesthetics Inhibit LPS-Induced IL-1β Expression in Glial Cells. PLoS ONE 2013, 8, e82930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.; Jiang, W.; Zuo, Z. The Choice of General Anesthetics May Not Affect Neuroinflammation and Impairment of Learning and Memory After Surgery in Elderly Rats. J. Neuroimmune Pharmacol. 2015, 10, 179–189. [Google Scholar] [CrossRef]
- Kotani, N.; Takahashi, S.; Sessler, D.I.; Hashiba, E.; Kubota, T.; Hashimoto, H.; Matsuki, A. Volatile Anesthetics Augment Expression of Proinflammatory Cytokines in Rat Alveolar Macrophages during Mechanical Ventilation. Anesthesiologists 1999, 91, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Yoon, J.H.; Hong, S.J.; Lee, S.H.; Sim, S.B. The Effects of Sevoflurane on Systemic and Pulmonary Inflammatory Responses After Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2009, 23, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Canakci, C.F.; Cicek, Y.; Canakci, V. Reactive Oxygen Species and Human Inflammatory Periodontal Diseases. Biochem. Mosc. 2005, 70, 619–628. [Google Scholar] [CrossRef]
- Rancan, L.; Huerta, L.; Cusati, G.; Erquicia, I.; Isea, J.; Paredes, S.D.; García, C.; Garutti, I.; Simón, C.; Vara, E. Sevoflurane Prevents Liver Inflammatory Response Induced by Lung Ischemia-Reperfusion. Transplantation 2014, 98, 1151–1157. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S.-Y.; Li, Y.-W.; Zhang, L.; Shou-Yuan, T.; Li, J.; Chen, Y.-Y.; Wang, Y.-X.; Liang, Y.; Zhang, X.-S.; et al. Sevoflurane preconditioning improving cerebral focal ischemia–reperfusion damage in a rat model via PI3K/Akt signaling pathway. Gene 2015, 569, 60–65. [Google Scholar] [CrossRef]
- Shen, X.; Dong, Y.; Xu, Z.; Wang, H.; Miao, C.; Soriano, S.G.; Sun, D.; Baxter, M.G.; Zhang, Y.; Xie, Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 2013, 118, 502–515. [Google Scholar] [CrossRef]
- Burburan, S.M.; Silva, J.D.; Abreu, S.C.; Samary, C.S.; Guimarães, I.H.L.; Xisto, D.G.; Morales, M.M.; Rocco, P.R. Effects of inhalational anaesthetics in experimental allergic asthma. Anaesthesia 2014, 69, 573–582. [Google Scholar] [CrossRef]
- Shen, Q.Y.; Fang, L.; Wu, H.M.; Wu, L.; He, F.; Liu, R.Y. Effect of Toll-like receptor 2 on the inhibition role of sevoflurane on airway inflammation in asthmatic mice. Zhonghua Yi Xue Za Zhi 2016, 96, 138–141. [Google Scholar] [PubMed]
- Polak, P.E.; Dull, R.O.; Kalinin, S.; Sharp, A.J.; Ripper, R.; Weinberg, G.; Schwartz, D.E.; Rubinstein, I.; Feinstein, D.L. Sevoflurane reduces clinical disease in a mouse model of multiple sclerosis. J. Neuroinflamm. 2012, 9, 272. [Google Scholar] [CrossRef]
- Cata, J.P.; Bauer, M.; Sokari, T.; Ramírez, M.F.; Mason, D.; Plautz, G.; Kurz, A. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J. Clin. Anesth. 2013, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C.A.; Murray, D.; Doran, P.; Moriarty, D.C.; Sessler, D.I.; Mascha, E.; Kavanagh, B.P.; Buggy, D.J. Anesthetic Technique and the Cytokine and Matrix Metalloproteinase Response to Primary Breast Cancer Surgery. Reg. Anesth. Pain Med. 2010, 35, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Kim, M.; Song, J.H.; Chen, S.W.; Gubitosa, G.; Emala, C.W. Sevoflurane-mediated TGF-beta1 signaling in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2008, 294, F371–F378. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, J.; Shin, S.; Baek, S.; Choi, K.-U.; Jeon, Y.; Kim, W.-S.; Bae, J.; Choi, H.-J.; Kim, H.-K.; et al. Effects of sevoflurane on collagen production and growth factor expression in rats with an excision wound. Acta Anaesthesiol. Scand. 2009, 54, 885–893. [Google Scholar] [CrossRef]
- Parham, P. The Immune System; Informa UK Limited: London, UK, 2014. [Google Scholar]
- Fröhlich, D.; Rothe, G.; Schwall, B.; Schmid, P.; Schmitz, G.; Taeger, K.; Hobbhahn, J. Effects of volatile anaesthetics on human neutrophil oxidative response to the bacterial peptide FMLP. Br. J. Anaesth. 1997, 78, 718–723. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hall, S.C.; Rudnick, S.; Seleny, F.L.; Stevenson, H.C. The Effect of Anesthetic Agents on the Human Immune Response. Anesthesiologists 1990, 72, 542–552. [Google Scholar] [CrossRef]
- Kowalski, C.; Zahler, S.; Becker, B.F.; Flaucher, A.; Conzen, P.F.; Gerlach, E.; Peter, K. Halothane, Isoflurane, and Sevoflurane Reduce Postischemic Adhesion of Neutrophils in the Coronary System. Anesthesiologists 1997, 86, 188–195. [Google Scholar] [CrossRef]
- Heindl, B.; Reichle, F.M.; Zahler, S.; Conzen, P.F.; Becker, B.F. Sevoflurane and Isoflurane Protect the Reperfused Guinea Pig Heart by Reducing Postischemic Adhesion of Polymorphonuclear Neutrophils. Anesthesiologists 1999, 91, 521–530. [Google Scholar] [CrossRef]
- Penna, A.; Johnson, K.; Camilleri, J.; Knight, P. Alterations in Influenza A Virus Specific Immune Injury in Mice Anesthetized with Halothane or Ketamine. Intervirology 1990, 31, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Reutershan, J.; Chang, D.; Hayes, J.K.; Ley, K. Protective Effects of Isoflurane Pretreatment in Endotoxin-induced Lung Injury. Anesthesiology 2006, 104, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Schneemilch, C.E.; Hachenberg, T.; Ansorge, S.; Ittenson, A.; Bank, U. Effects of different anaesthetic agents on immune cell function in vitro. Eur. J. Anaesthesiol. 2005, 22, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of Natural Killer Cell Activity and Promotion of Tumor Metastasis by Ketamine, Thiopental, and Halothane, but Not by Propofol: Mediating Mechanisms and Prophylactic Measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Knight, P.R.; Murasko, D.M. Inhibition of Interferon Stimulation of Natural Killer Cell Activity in Mice Anesthetized with Halothane or Isoflurane. Anesthesiologists 1993, 78, 700–706. [Google Scholar] [CrossRef]
- Wada, H.; Seki, S.; Takahashi, T.; Kawarabayashi, N.; Higuchi, H.; Habu, Y.; Sugahara, S.; Kazama, T. Combined Spinal and General Anesthesia Attenuates Liver Metastasis by Preserving Th1/Th2 Cytokine Balance. Anesthesiologists 2007, 106, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhen, Y.; Dong, Y.; Xu, Z.; Yue, Y.; Golde, T.E.; Tanzi, R.E.; Moir, R.D.; Xie, Z. Anesthetic Propofol Attenuates the Isoflurane-Induced Caspase-3 Activation and Aβ Oligomerization. PLoS ONE 2011, 6, e27019. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, Y.; Wang, A.; Dong, Y.; Xie, Z. Vitamin C Attenuates Isoflurane-Induced Caspase-3 Activation and Cognitive Impairment. Mol. Neurobiol. 2015, 52, 1580–1589. [Google Scholar] [CrossRef]
- Horn, N.A.; De Rossi, L.; Robitzsch, T.; Hecker, K.E.; Hutschenreuter, G.; Rossaint, R. The effects of sevoflurane and desflurane in vitro on platelet-leukocyte adhesion in whole blood. Anaesthesia 2003, 58, 312–319. [Google Scholar] [CrossRef]
- Elrashidy, A.A.; Abdelrahman, R.S.; Ghali, A.M.; Elsheikh, A.M.; Elsheikh, M.M. Effects of sevoflurane and isoflurane on coagulation system: A comparative study. Tanta Med. Sci. J. 2007, 2, 11-10. [Google Scholar]
- Law, N.; Ng, K.; Irwin, M.; Man, J. Comparison of coagulation and blood loss during anaesthesia with inhaled isoflurane or intravenous propofol. Br. J. Anaesth. 2001, 86, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Use of low-molecular-weight heparin in COVID-19 patients. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Kato, M. Anesthetics, immune cells, and immune responses. J. Anesth. 2008, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Picq, C.A.; Clarençon, D.; Sinniger, V.E.; Bonaz, B.L.; Mayol, J.-F.S. Impact of Anesthetics on Immune Functions in a Rat Model of Vagus Nerve Stimulation. PLoS ONE 2013, 8, e67086. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Kurosawa, S.; Horinouchi, T.; Kato, M.; Hashimoto, Y. Inhalation Anesthetics Induce Apoptosis in Normal Peripheral Lymphocytes In Vitro. Anesthesiologists 2001, 95, 1467–1472. [Google Scholar] [CrossRef]
- Woo, J.H.; Baik, H.J.; Kim, C.H.; Chung, R.K.; Kim, D.Y.; Lee, G.Y.; Chun, E.H. Effect of Propofol and Desflurane on Immune Cell Populations in Breast Cancer Patients: A Randomized Trial. J. Korean Med. Sci. 2015, 30, 1503–1508. [Google Scholar] [CrossRef]
- Inada, T.; Yamanouchi, Y.; Jomura, S.; Sakamoto, S.; Takahashi, M.; Kambara, T.; Shingu, K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004, 59, 954–959. [Google Scholar] [CrossRef]
- Yang, H.; Liang, G.; Hawkins, B.J.; Madesh, M.; Pierwola, A.; Wei, H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008, 109, 243–250. [Google Scholar] [CrossRef]
- Puig, N.; Ferrero, P.; Bay, M.; Hidalgo, G.; Valenti, J.; Amerio, N.; Elena, G. Effects of sevoflurane general anesthesia: Immunological studies in mice. Int. Immunopharmacol. 2002, 2, 95–104. [Google Scholar] [CrossRef]
- Imanaka, H.; Shimaoka, M.; Matsuura, N.; Nishimura, M.; Ohta, N.; Kiyono, H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth. Analg. 2001, 92, 428–436. [Google Scholar] [CrossRef]
- Stollings, L.M.; Jia, L.-J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiologists 2016, 125, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Voigtsberger, S.; Lachmann, R.A.; Leutert, A.C.; Schläpfer, M.; Booy, C.; Reyes, L.; Urner, M.; Schild, J.; Schimmer, R.C.; Beck-Schimmer, B. Sevoflurane Ameliorates Gas Exchange and Attenuates Lung Damage in Experimental Lipopolysaccharide-induced Lung Injury. Anesthesiologists 2009, 111, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Sauviat, M.-P.; Frizelle, H.P.; Descorps-Declère, A.; Mazoit, J.-X. Effects of halothane on the membrane potential in skeletal muscle of the frog. Br. J. Pharmacol. 2000, 130, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, J.; Chen, M.X. General anesthesia mediated by effects on ion channels. World J. Crit. Care Med. 2012, 1, 80–93. [Google Scholar] [CrossRef]
- Winegar, B.D.; Owen, D.F.; Yost, C.S.; Forsayeth, J.R.; Mayeri, E. Volatile general anesthetics produce hyperpolarization of Aplysia neurons by activation of a discrete population of baseline potassium channels. Anesthesiology 1996, 85, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Bedows, E.; Davidson, B.A.; Knight, P.R. Effect of halothane on the replication of animal viruses. Antimicrob. Agents Chemother. 1984, 25, 719–724. [Google Scholar] [CrossRef]
- Matthay, M.A.; McAuley, D.F.; Ware, L.B. Clinical trials in acute respiratory distress syndrome: Challenges and opportunities. Lancet Respir. Med. 2017, 5, 524–534. [Google Scholar] [CrossRef]
- Knight, P.R.; Nahrwold, M.L.; Bedows, E. Inhibiting Effects of Enflurane and Isoflurane Anesthesia on Measles Virus Replication: Comparison with Halothane. Antimicrob. Agents Chemother. 1981, 20, 298–306. [Google Scholar] [CrossRef]
- Knight, P.R.; Nahrwold, M.L.; Bedows, E. Anesthetic action and virus replication: Inhibition of measles virus replication in cells exposed to halothane. Antimicrob. Agents Chemother. 1980, 17, 890–896. [Google Scholar] [CrossRef]
- Vetter, P.; Eckerle, I.; Kaiser, L. Covid-19: A puzzle with many missing pieces. BMJ 2020, 368, m627. [Google Scholar] [CrossRef]
- Misra, S.; Koshy, T. A review of the practice of sedation with inhalational anaesthetics in the intensive care unit with the AnaConDa ® device. Indian J. Anaesth. 2012, 56, 518–523. [Google Scholar] [CrossRef] [PubMed]
- L’Her, E.; Dy, L.; Pili, R.; Prat, G.; Tonnelier, J.-M.; Lefevre, M.; Renault, A.A.; Boles, J.-M. Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: A prospective observational study. Respir. Care 2008, 53, 1295–1303. [Google Scholar]
- Baron, R.; Binder, A.; Biniek, R.; Braune, S.; Buerkle, H.; Dall, P.; Demirakca, S.; Eckardt, R.; Eggers, V.; Eichler, I.; et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)—Short version. Ger. Med. Sci. 2015, 13, 19. [Google Scholar]
- Sophia, K.; Shimaoka, M.; Yuki, K. The Use of Volatile Anesthetics as Sedatives for Acute Respiratory Distress Syndrome. Transl. Perioper. Pain Med. 2019, 6, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.M. Context-sensitive half-times and other decrement times of inhaled anesthetics. Anesth. Analg. 1997, 85, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Homburger, J.A.; Meiler, S.E. Anesthesia drugs, immunity, and long-term outcome. Curr. Opin. Anaesthesiol. 2006, 19, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pirbudak, C.L.; Ugur, M.G.; Karadasli, H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr. Ther. Res. Clin. Exp. 2012, 73, 41–51. [Google Scholar] [CrossRef]
- Jerath, A.; Panckhurst, J.; Parotto, M.; Lightfoot, N.; Wasowicz, M.; Ferguson, N.D.; Steel, A.; Beattie, W.S. Safety and Efficacy of Volatile Anesthetic Agents Compared with Standard Intravenous Midazolam/Propofol Sedation in Ventilated Critical Care Patients: A Meta-analysis and Systematic Review of Prospective Trials. Anesth. Analg. 2017, 124, 1190–1199. [Google Scholar] [CrossRef]
- Bellgardt, M.; Bomberg, H.; Herzog-Niescery, J.; Dasch, B.; Vogelsang, H.; Weber, T.P.; Steinfort, C.; Uhl, W.; Wagenpfeil, S.; Volk, T.; et al. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: Retrospective analysis. Eur. J. Anaesthesiol. 2016, 33, 6–13. [Google Scholar] [CrossRef]
- Röhm, K.D.; Wolf, M.W.; Schöllhorn, T.; Schellhaass, A.; Boldt, J.; Piper, S.N. Short-term sevoflurane sedation using the Anaesthetic Conserving Device after cardiothoracic surgery. Intensiv. Care Med. 2008, 34, 1683–1689. [Google Scholar] [CrossRef]
- Jerath, A.; Beattie, S.W.; Chandy, T.; Karski, J.; Djaiani, G.; Rao, V.; Yau, T.; Wasowicz, M.; Perioperative Anesthesia Clinical Trials Group. Volatile-based short-term sedation in cardiac surgical patients: A prospective randomized controlled trial. Crit. Care Med. 2015, 43, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Pasin, L.; Cabrini, L.; Scandroglio, A.M.; Redaelli, M.B.; Votta, C.D.; Bellandi, M.; Borghi, G.; Zangrillo, A.; Scandroglio, M. Volatile Agents in Medical and Surgical Intensive Care Units: A Meta-Analysis of Randomized Clinical Trials. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hellström, J.; Öwall, A.; Sackey, P.V. Wake-up times following sedation with sevoflurane versus propofol after cardiac surgery. Scand. Cardiovasc. J. 2012, 46, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, M.; Capdevila, X.; Bringuier, S.; Trine, P.-O.; Falquet, Y.; Charbit, J.; Roustan, J.-P.; Chanques, G.; Jaber, S. Long-term sedation in intensive care unit: A randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensiv. Care Med. 2011, 37, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Sackey, P.V.; Martling, C.-R.; Carlswärd, C.; Sundin, Ö.; Radell, P.J. Short- and long-term follow-up of intensive care unit patients after sedation with isoflurane and midazolam—A pilot study*. Crit. Care Med. 2008, 36, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Jabaudon, M.; Boucher, P.; Imhoff, E.; Chabanne, R.; Faure, J.-S.; Roszyk, L.; Thibault, S.; Blondonnet, R.; Clairefond, G.; Guérin, R.; et al. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am. J. Respir. Crit. Care Med. 2017, 195, 792–800. [Google Scholar] [CrossRef]
- Uhlig, C.; Bluth, T.; Schwarz, K.; Deckert, S.; Heinrich, L.; De Hert, S.; Landoni, G.; Serpa Neto, A.; Schultz, M.J.; Pelosi, P.; et al. Effects of Volatile Anesthetics on Mortality and Postoperative Pulmonary and Other Complications in Patients Undergoing Surgery: A Systematic Review and Meta-analysis. Anesthesiology 2016, 124, 1230–1245. [Google Scholar] [CrossRef]
- Grabitz, S.D.; Farhan, H.N.; Ruscic, K.J.; Timm, F.P.; Shin, C.H.; Thevathasan, T.; Staehr-Rye, A.K.; Kurth, T.; Eikermann, M. Dose-Dependent Protective Effect of Inhalational Anesthetics Against Postoperative Respiratory Complications: A Prospective Analysis of Data on File from Three Hospitals in New England. Crit. Care Med. 2017, 45, 30–39. [Google Scholar] [CrossRef]
- De Conno, E.; Steurer, M.P.; Wittlinger, M.; Zalunardo, M.P.; Weder, W.; Schneiter, D.; Schimmer, R.C.; Klaghofer, R.; Neff, T.A.; Schmid, E.R.; et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology 2009, 110, 1316–1326. [Google Scholar] [CrossRef]
- Koutsogiannaki, S.; Schaefers, M.M.; Okuno, T.; Ohba, M.; Yokomizo, T.; Priebe, G.P.; Dinardo, J.A.; Sulpicio, S.G.; Yuki, K. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol. Sci. 2016, 156, 402–411. [Google Scholar] [CrossRef]
- Yuki, K.; Bu, W.; Xi, J.; Sen, M.; Shimaoka, M.; Eckenhoff, R.G. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 2012, 26, 4408–4417. [Google Scholar] [CrossRef] [PubMed]
- Harr, J.N.; Moore, E.E.; Stringham, J.; Wohlauer, M.V.; Fragoso, M.; Jones, W.L.; Gamboni, F.; Silliman, C.C.; Banerjee, A. Isoflurane prevents acute lung injury through ADP-mediated platelet inhibition. Surgery 2012, 152, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Strosing, K.M.; Faller, S.; Gyllenram, V.; Engelstaedter, H.; Buerkle, H.; Spassov, S.; Hoetzel, A. Inhaled Anesthetics Exert Different Protective Properties in a Mouse Model of Ventilator-Induced Lung Injury. Anesth. Analg. 2016, 123, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nyrén, S.; Radell, P.; Mure, M.; Petersson, J.; Jacobsson, H.; Lindahl, S.G.E.; Sánchez-Crespo, A. Inhalation Anesthesia Increases V/Q Regional Heterogeneity during Spontaneous Breathing in Healthy Subjects. Anesthesiologists 2010, 113, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Kozian, A.; Kretzschmar, M.; Huth, C.; Welte, T.; Bühling, F.; Hedenstierna, G.; Hachenberg, T. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br. J. Anaesth. 2007, 99, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Wang, H.; Li, W.; Wang, L.-F.; Hou, L.-C.; Mu, J.-L.; Liu, X.; Chen, H.-J.; Xie, K.-L.; Li, N.-L.; et al. Anesthetic Isoflurane Posttreatment Attenuates Experimental Lung Injury by Inhibiting Inflammation and Apoptosis. Mediat. Inflamm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-Y.; Chen, X.; Li, J.; Wang, S.; Faclier, G.; Macdonald, J.F.; Hogg, J.C.; Osrser, B.A.; Lu, W.-Y. Isoflurane Regulates Atypical Type-A γ-Aminobutyric Acid Receptors in Alveolar Type II Epithelial Cells. Anesthesiologists 2013, 118, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, Y.; Su, H.; Yang, J. The anesthetic agent sevoflurane attenuates pulmonary acute lung injury by modulating apoptotic pathways. Braz. J. Med Biol. Res. 2017, 50, e5747. [Google Scholar] [CrossRef]
- Forkuo, G.S.; Nieman, A.N.; Kodali, R.; Zahn, N.M.; Li, G.; Rashid-Roni, M.S.; Stephen, M.R.; Harris, T.W.; Jahan, R.; Guthrie, M.L.; et al. A Novel Orally Available Asthma Drug Candidate That Reduces Smooth Muscle Constriction and Inflammation by Targeting GABA(A) Receptors in the Lung. Mol. Pharm. 2018, 15, 1766–1777. [Google Scholar] [CrossRef]
- Fortis, S.; Spieth, P.M.; Lu, W.-Y.; Parotto, M.; Haitsma, J.J.; Slutsky, A.S.; Zhong, N.; Mazer, C.D.; Zhang, H. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intensiv. Care Med. 2012, 38, 1548–1555. [Google Scholar] [CrossRef]
- Gropper, M.A.; Miller, R.D.; Eriksson, L.I.; Fleisher, L.A.; Wiener-Kronish, J.P.; Cohen, N.H.; Leslie, K. Miller’s Anesthesia; Elsevier Health Sciences: Philadelphia, PA, USA, 2019; Volume 2. [Google Scholar]
- Schläpfer, M.; Leutert, A.C.; Voigtsberger, S.; Lachmann, R.A.; Booy, C.; Beck-Schimmer, B. Sevoflurane reduces severity of acute lung injury possibly by impairing formation of alveolar oedema. Clin. Exp. Immunol. 2012, 168, 125–134. [Google Scholar] [CrossRef]
- Wagner, J.; Strosing, K.M.; Spassov, S.; Lin, Z.; Engelstaedter, H.; Tacke, S.; Hoetzel, A.; Faller, S. Sevoflurane posttreatment prevents oxidative and inflammatory injury in ventilator-induced lung injury. PLoS ONE 2018, 13, e0192896. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Ferguson, N.D.; Steel, A.; Wijeysundera, D.N.; Macdonald, J.; Wasowicz, M. The use of volatile anesthetic agents for long-term critical care sedation (VALTS): Study protocol for a pilot randomized controlled trial. Trials 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Ferguson, N.D.; Cuthbertson, B. Inhalational volatile-based sedation for COVID-19 pneumonia and ARDS. Intensiv. Care Med. 2020, 46, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.D.; Le, C.; Lamers, V.; Eckelman, M.J. Life Cycle Greenhouse Gas Emissions of Anesthetic Drugs. Anesth. Analg. 2012, 114, 1086–1090. [Google Scholar] [CrossRef]
- Jerath, A.; Steel, A.; Ferguson, N.; Wasowicz, M. High fluoride levels with isoflurane critical care sedation does not cause nephrotoxicity. Am. J. Respir. Crit. Care Med. 2016, 193, A3639. [Google Scholar]
- Maussion, E.; Combaz, S.; Cuisinier, A.; Chapuis, C.; Payen, J.-F. Renal dysfunction during sevoflurane sedation in the ICU. Eur. J. Anaesthesiol. 2019, 36, 377–379. [Google Scholar] [CrossRef]
- Zink, J.; Sasyniuk, B.I.; Dresel, P.E. Halothane-Epinephrine-induced Cardiac Arrhythmias and the Role of Heart Rate. Anesthesiologists 1975, 43, 548–555. [Google Scholar] [CrossRef]
- Wong, K.; Wasowicz, M.; Grewal, D.; Fowler, T.; Ng, M.; Ferguson, N.D.; Steel, A.; Jerath, A. Efficacy of a simple scavenging system for long-term critical care sedation using volatile agent-based anesthesia. Can. J. Anesth. J. Can. Anesth. 2015, 63, 630–632. [Google Scholar] [CrossRef]
- Osrser, B.A.; Wang, D.-S.; Lu, W.-Y. Sedating ventilated COVID-19 patients with inhalational anesthetic drugs. EBioMedicine 2020, 55, 102770. [Google Scholar] [CrossRef]
- Jerath, A.; Parotto, M.; Wasowicz, M.; Ferguson, N.D. Volatile Anesthetics. Is a New Player Emerging in Critical Care Sedation? Am. J. Respir. Crit. Care Med. 2016, 193, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Anesthesiologists ASo. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators: Anesthesia Patient Safety Foundation. 2020. Available online: https://wwwasahqorg/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators (accessed on 16 October 2020).
- Zurich, U. Sevoflurane in COVID-19 ARDS (SevCov) US National Library of Medicine: ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04355962 (accessed on 16 October 2020).
- Nieuwenhuijs-Moeke, G.J.; Jainandunsing, J.S.; Struys, M.M. Sevoflurane, a sigh of relief in COVID-19? Br. J. Anaesth. 2020, 125, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ferrière, N.; Bodenes, L.; Bailly, P.; L’Her, E. Shortage of anesthetics: Think of inhaled sedation! J. Crit. Care 2020, S0883-9441, 30686–30689. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).