Isothiochromenothiazoles—A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
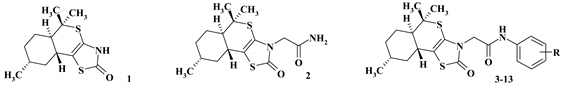
2.2. General Procedure for the Synthesis of 3-N-Substituted (5aR,8R,9aR)-5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-ones (2–13)
2.2.1. 2-{5,5,8-Trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}- acetamide (2)
2.2.2. N-(Phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (3)
2.2.3. N-(4-Methylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (4)
2.2.4. N-(3-Methylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (5)
2.2.5. N-(4-Isopropylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (6)
2.2.6. N-(4-Sulfonamido-phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (7)
2.2.7. N-(4-Acetylamino-phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (8)
2.2.8. 4-[2-(5,5,8-Trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl)- acetylamino]-benzoic acid ethyl ester (9)
2.2.9. N-(4-Chlorophenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (10)
2.2.10. N-(3,4-Dichlorophenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (11)
2.2.11. N-(2-Trifluoromethylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno [4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (12)
2.2.12. N-(3-Trifluoromethylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno [4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (13)
2.3. Pharmacology
2.3.1. Antitrypanosomal Activity Assay
2.3.2. Acute Toxicity In Vivo
3. Results and Discussion
3.1. Chemistry
3.2. Pharmacology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trypanosomiasis, Human African (Sleeping Sickness)/Fact Sheets. Available online: http://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 28 June 2018).
- Kryshchyshyn, A.; Kaminskyy, D.; Grellier, P.; Lesyk, R. Trends in research of antitrypanosomal agents among synthetic heterocycles. Eur. J. Med. Chem. 2014, 85, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Khan, M.O.; Austin, S.E.; Croft, S.L.; Yardley, V.; Rock, P.; Douglas, K.T. Antitrypanosomal, antileishmanial, and antimalarial activities of quaternary arylalkylammonium 2-amino-4-chlorophenyl phenyl sulfides, a new class of trypanothione reductase inhibitor, and of N-acyl derivatives of 2-amino-4-chlorophenyl phenyl sulfide. J. Med. Chem. 2005, 48, 8087–8097. [Google Scholar] [CrossRef] [PubMed]
- Bakunov, S.A.; Bakunova, S.M.; Wenzler, T.; Ghebru, M.; Werbovetz, K.A.; Brun, R.; Tidwell, R.R. Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1H-1,2,3-triazoles. J. Med. Chem. 2009, 53, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, I.H. Inhibitors of dihydrofolate reductase in Leishmania and trypanosomes. Biochim. Biophys. Acta 2002, 1587, 249–257. [Google Scholar] [CrossRef]
- Bodley, A.L.; Shapiro, T.A. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. USA 1995, 92, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.B.; Zimenkovsky, B.S.; Kaminskyy, D.V.; Kryshchyshyn, A.P.; Havrylyuk, D.Y.; Atamanyuk, D.V.; Subtel’na, I.Y.; Khylyuk, D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell 2011, 27, 107–117. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, T.; Peterlin Mašič, L. Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation. Expert Opin. Drug Discov. 2012, 7, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.; Farahat, A.A.; Abdel-Wahab, B.F. 2-Amino-4-thiazolidinones: Synthesis and reactions. J. Sulfur Chem. 2010, 31, 315–349. [Google Scholar] [CrossRef]
- Gomes, P.A.; Oliveira, A.R.; Cardoso, M.V.; Santiago, E.F.; Barbosa, M.O.; de Siqueira, L.R.; Moreira, D.R.; Bastos, T.M.; Brayner, F.A.; Soares, M.B.; et al. Phthalimido-thiazoles as building blocks and their effects on the growth and morphology of Trypanosoma cruzi. Eur. J. Med. Chem. 2016, 111, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes Moreira, D.R.; de Oliveira, A.D.; Teixeira de Moraes Gomes, P.A.; de Simone, C.A.; Villela, F.S.; Ferreira, R.S.; da Silva, A.C.; dos Santos, T.A.; Brelaz de Castro, M.C.; Pereira, V.R.; et al. Conformational restriction of aryl thiosemicarbazones produces potent and selective anti-Trypanosoma cruzi compounds which induce apoptotic parasite death. Eur. J. Med. Chem. 2014, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, J.W.; Cardoso, M.V.; Filho, G.B.; Oliveira E Silva, D.A.; Moreira, D.R.; Bastos, T.M.; Simone, C.A.; Soares, M.B.; Villela, F.S.; Ferreira, R.S.; et al. Synthesis and structure-activity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma cruzi cruzain. Eur. J. Med. Chem. 2015, 101, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; Faundez, M. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Siqueira, L.R.; da Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M.; Ferreira, R.S.; da Cruz, L.F.; Moreira, D.R.; Pereira, V.R.; et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: Structural design, synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; de Lima, R.S.; Moreira, D.R.; Cardoso, M.V.; Gouveia de Brito, A.C.; Farias Dos Santos, L.M.; Hernandes, M.Z.; Kiperstok, A.C.; de Lima, R.S.; Soares, M.B. Synthesis, docking, and in vitro activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acylthiazolidones against Trypanosoma cruzi. Bioorg. Med. Chem. 2006, 14, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Moreira, D.R.; Cardoso, M.V.; Hernandes, M.Z.; Alves Pereira, V.R.; Silva, R.O.; Kiperstok, A.C.; Lima, M.S.; Soares, M.B. Synthesis, Cruzain docking, and in vitro studies of aryl-4-oxothiazolylhydrazones against Trypanosoma cruzi. ChemMedChem 2007, 2, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.Z.; Rabello, M.M.; Leite, A.C.; Cardoso, M.V.; Moreira, D.R.; Brondani, D.J.; Simone, C.A.; Reis, L.C.; Souza, M.A.; Pereira, V.R.; et al. Studies toward the structural optimization of novel thiazolylhydrazone-based potent antitrypanosomal agents. Bioorg. Med. Chem. 2010, 18, 7826–7835. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.M.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Ferreira, R.S.; de Simone, C.A.; Meira, C.S.; Guimaraes, E.T.; da Silva, A.C.; dos Santos, T.A.; et al. Structural design, synthesis and structure-activity relationships of thiazolidinones with enhanced anti-Trypanosoma cruzi activity. ChemMedChem 2014, 9, 177–188. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; Espíndola, J.W.; Ferreira, L.F.; de Simone, C.A.; Ferreira, R.S.; Coelho, P.L.; Meira, C.S.; Magalhaes Moreira, D.R.; Soares, M.B.; et al. Structural design, synthesis and pharmacological evaluation of 4-thiazolidinones against Trypanosoma cruzi. Bioorg. Med. Chem. 2015, 23, 7478–7486. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Day, C.W.; Smee, D.F.; Grellier, P.; Lesyk, R. Synthesis and biological activity evaluation of 5-pyrazoline substituted 4-thiazolidinones. Eur. J. Med. Chem. 2013, 66, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Karpenko, O.; Grellier, P.; Lesyk, R. Synthesis of pyrazoline–thiazolidinone hybrids with trypanocidal activity. Eur. J. Med. Chem. 2014, 85, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Nektegayev, I.; Vasylenko, O.; Grellier, P.; Lesyk, R. Isothiocoumarin-3-carboxylic acid derivatives: Synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017—Utility and Limitations. ACS Chem. Biol. 2017, 13, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of isothiochromeno[3,4-d]thiazole derivatives. Ann. Univ. Mar. Curie Sklodowska DDD Pharm. 2008, 21, 247–251. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Atamanyuk, D.; Lesyk, R. Fused thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 2012, 80, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn, A.P.; Atamanyuk, D.V.; Kaminskyy, D.V.; Grellier, P.; Lesyk, R.B. Investigation of anticancer and anti-parasitic activity of thiopyrano[2,3-d]thiazoles bearing norbornane moiety. Biopolym. Cell 2017, 33, 183–205. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Roman, O.; Lozynskyi, A.; Lesyk, R. Thiopyrano[2,3-d]thiazoles as New Efficient Scaffolds in Medicinal Chemistry. Sci. Pharm. 2018, 86, 26. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D.; Wang, X. Trypanocidal activity of the proteasome inhibitor and anti-cancer drug bortezomib. Parasit. Vectors 2009, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Deterding, A.; Dungey, F.; Thompson, K.; Steverding, D. Anti-trypanosomal activities of DNA topoisomerase inhibitors. Acta Trop. 2005, 93, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Matiychuk, V.; Lesyk, R.; Obushak, M.; Gzella, A.; Atamanyuk, D.; Ostapiuk, Y.; Kryshchyshyn, A. A new domino-Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2008, 49, 4648–4651. [Google Scholar] [CrossRef]
- Smith, W.G. 1 Pharmacological Screening Tests. Prog. Med. Chem. 1961, 1, 1–33. [Google Scholar]
- Lethu, S.; Bosc, D.; Mouray, E.; Grellier, P.; Dubois, J. New protein farnesyltransferase inhibitors in the 3-arylthiophene 2-carboxylic acid series: Diversification of the aryl moiety by solid-phase synthesis. J. Enzym. Inhib. Med. Chem. 2013, 1, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Diego Maya, J.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Kryshchychyn, A.; Lesyk, R. Synthesis and antitumor activity study of new isothiochromeno[4a,4-d]thiazole derivatives. Pharm. Rev. 2010, 2, 6–10. (In Ukrainian) [Google Scholar] [CrossRef]

| Compound | R | Trypanosoma brucei brucei, IC50 (µM) | Anticancer Activity DTP 60 Lines Assay1,2 [27] | Acute Toxicity LD50 (mg/kg) |
|---|---|---|---|---|
 | ||||
| 1 | - | 29.32 | 240 | |
| 2 | - | 44.41 | 10 µM assay: mean GP—106%; range of growth—78.80–144.06%; EKVX (non-small-cell lung cancer)—78.80% 1 | |
| 3 | H | 10.18 | ||
| 4 | 4-Me | 10.80 | 10 µM assay: mean GP—85.70%; range of growth—5.23–119.66%; UACC (melanoma)—5.23%, OVCAR-8 (ovarian cancer)—34.80%, MCF-7 (breast cancer)—52.50%, NCI-H522 (non-small-cell lung cancer)—58.10 | |
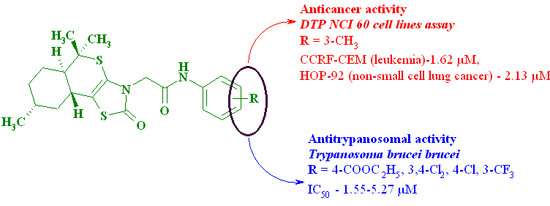
| 5 | 3-Me | 10.08 | 0.1–100 µM assay: GI50: CCRF-CEM (leukemia)—1.62 µM, MOLT-4 (leukemia)—5.94 µM, RPMI-8226 (leukemia)—5.69 µM, SR (leukemia)—12.1 µM, HOP-92 (non-small-cell lung cancer)—2.13 µM, NCI-H460 (non-small-cell lung cancer)—79.6 µM, SK-MEL-5 (melanoma)—53.9 µM, PC-3 (prostate cancer)—7.43 µM 2 | |
| 6 | 4-i-Pr | 14.17 | ||
| 7 | 4-SO2NH2 | 14.12 | ||
| 8 | 4-AcNH | 18.28 | ||
| 9 | 4-COOEt | 1.55 | 320 | |
| 10 | 4-Cl | 5.27 | 480 | |
| 11 | 3,4-Cl2 | 4.67 | 10 µM assay: mean GP—85.89%; range of growth—46.28–111.66%; HOP-92 (non-small-cell lung cancer)—46.90%, HS 578T (breast cancer)—46.28%, RPMI-8226 (leukemia)—46.87%, MOLT-4 (leukemia)—58.86% | |
| 12 | 2-CF3 | 8.50 | 10 µM assay: mean GP—84.74%; range of growth—0.76–116.02%; MOLT-4 (leukemia)—36.02%, OVCAR-8 (ovarian cancer)—28.87, UACC-257 (melanoma)—0.76%, MCF-7 (breast cancer)—56.32% | |
| 13 | 3-CF3 | 2.97 | 270 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryshchyshyn, A.; Kaminskyy, D.; Nektegayev, I.; Grellier, P.; Lesyk, R. Isothiochromenothiazoles—A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei. Sci. Pharm. 2018, 86, 47. https://doi.org/10.3390/scipharm86040047
Kryshchyshyn A, Kaminskyy D, Nektegayev I, Grellier P, Lesyk R. Isothiochromenothiazoles—A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei. Scientia Pharmaceutica. 2018; 86(4):47. https://doi.org/10.3390/scipharm86040047
Chicago/Turabian StyleKryshchyshyn, Anna, Danylo Kaminskyy, Igor Nektegayev, Philippe Grellier, and Roman Lesyk. 2018. "Isothiochromenothiazoles—A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei" Scientia Pharmaceutica 86, no. 4: 47. https://doi.org/10.3390/scipharm86040047
APA StyleKryshchyshyn, A., Kaminskyy, D., Nektegayev, I., Grellier, P., & Lesyk, R. (2018). Isothiochromenothiazoles—A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei. Scientia Pharmaceutica, 86(4), 47. https://doi.org/10.3390/scipharm86040047