Abstract
The aim of this study was to increase the antibacterial spectrum of modified hen egg white lysozyme (HEWL) with thermal and chemical treatments against Gram-negative bacteria. The antibacterial activity of heat-denatured HEWL and chemical denatured HEWL against Gram-negative and Gram-positive bacteria was evaluated in 15 h of incubation tests. HEWL was denatured by heating at pH 6.0 and pH 7.0 and chemical denaturing was carried out for 1.0, 1.5, 2.0, and 4.0 h with DL-Dithiothreitol (DTT). HEWL modified by thermal and chemical treatments was characterized using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis method. Heat-denatured HEWL lytic activity against Micrococcus lysodeikticus lessened with increasing temperature and time of incubation with the chemical agent (DTT). The loss of lytic activity in modified HEWL suggests that the mechanism of action of the antibacterial activity is not dependent on the lytic activity. Thermal and chemical treatments of HEWL enabled the production of oligoforms and increased antibacterial activity over a wider spectrum. Heat-denatured HEWL at pH 6.0 and chemically-denatured HEWL increased the HEWL antibacterial spectrum against Gram-negative bacteria (Escherichia coli ATCC 25922). HEWL at 120 °C and pH 6.0 (1.0 mg/mL) inhibited 78.20% of the growth of E. coli. HEWL/DTT treatment for 4.0 h (1.0 mg/mL) inhibited 68.75% of the growth E. coli. Heat-denatured HEWL at pH 6.0 and pH 7.0 and chemically-denatured HEWL (1.0, 1.5, 2.0, and 4.0 h with DTT) were active against Gram-positive bacteria (Staphylococcus carnosus CECT 4491T). Heat-denatured and chemical-denatured HEWL caused the death of the bacteria with the destruction of the cell wall. LIVE/DEAD assays of fluorescent dye stain of the membrane cell showed membrane perturbation of bacteria after incubation with modified HEWL. The cell wall destruction was viewed using electron microscopy. The results obtained in this study suggest that heat-denatured HEWL at pH 6.0 and chemical-denatured HEWL treatments increase the HEWL antibacterial activity against Gram-negative bacteria.
1. Introduction
Hen Egg White Lysozyme (HEWL) is a basic protein consisting of 129 amino acids where lysine (Lys) is the N-terminal sequence and leucine (Leu) is C-terminal sequence. HEWL has a molecular weight of 14.3 kDa (type c) [1]. The amino acids (6Cys–127Cys, 30Cys–115Cys, 64Cys–80Cys, and 76Cys–94Cys) are part of the four-disulfide bridge in HEWL. HEWL has a high isoelectric point (pI) with a pI value of 10.7 [2]. Hen’s egg is the richest HEWL source, accounting for 3.5% of total egg white proteins [3]. HEWL is a natural antibacterial agent categorized as Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration (FDA). This category indicates that it can be used in the food industry. As a well-known antibacterial protein, HEWL has been commercialized for applications as a natural additive, numbered E-1105, to control bacteria in meat products such as sausages, salami, pork, beef, or turkey, to prevent the growth of Clostridium tyrobutyricum in cheese making, or to control lactic bacteria in wine and beer production. HEWL can also be used in cosmetics applications and pharmaceuticals. For example, in the market, there are many drugs with lysozyme such as Lisozima Chiesi® and Lizipaina® recommended for treatment against herpes zoster, chickenpox, and measles for their antiviral activity [4,5,6,7,8,9,10,11,12].
HEWL belongs to a class of enzymes that lyses the cell wall of certain Gram-positive bacteria by breaking off β (1–4) linkages between N-acetyl-muramic acid and N-acetyl-glucosamine of the peptidoglycan, the components making up bacterial cell walls. HEWL is the most effective against Gram-positive bacteria but is largely ineffective against Gram-negative bacteria due to differences in the structure of their cell walls: Gram-negative bacteria have an outer membrane. This membrane is an additional barrier to HEWL, with the presence of lipopolysaccharide (LPS) on the bacterial cell wall [13,14,15,16,17]. To extend the HEWL antibacterial spectrum against Gram-negative bacteria, several strategies have been developed by different research groups. These methods include denatured treatment, high pressure, ultrafiltration treatment, electric pulses, the γ-irradiation method, HEWL modification by covalent attachment of polysaccharides and fatty acids, the attachment of C-terminal hydrophobic peptides to lysozyme by genetic modification, and the use of outer membranes permeabilizing agents such as ethylenediaminetetraacetic acid (EDTA) or polycations [18,19,20,21,22,23]. The native form of the proteins and their biological properties have often been studied. The denatured form of proteins can enhance the functional and biological properties of proteins. For example, thermally treated lactoferrin were found to increases their antibacterial activity. HEWL also increases its antibacterial capacity when treated thermally [14]. This antibacterial property may be due to an increase in hydrophobicity caused by exposure of the cellular regions that were previously protected. DTT is a reducing agent of disulfide bonds of proteins. The reduction of the disulfide bonds changes native HEWL to partially unfolded species with the physicochemical properties of the molten-globule form. Reduced proteins with DTT are not recommended to food applications, as DTT has toxicological properties. However, heated HEWL treatments are compatible with food applications [22].
Native HEWL antibacterial activity against many bacteria has been described. These bacteria include Staphylococcus aureus, Micrococcus luteus, Bacillus stearothermophilus, and Clostridium tyrobutyricum [24]. Modified HEWL has been studied for activity against Enterococcus faecalis, Bacillus subtilis, Listeria innocua, E. coli, E. coli K12, Salmonella typhimurium, Bacillus cereus, Yersinia enterocolitica, Shigella flexneri, E. coli O157:H7, Pseudomonas aeruginosa, and others [25].
Different hydrolysates have been obtained from HEWL with proteases such as pepsin, trypsin, chymotrypsin, and pancreatin. Mine et al. described a HEWL hydrolysate with antibacterial activity against Gram-negative bacteria (E. coli) [26]. Carrillo et al. examined native and heated HEWL hydrolysates with antibacterial activity against Gram-negative bacteria. The first hydrolysate was obtained by applying the batch method and the second hydrolysate was obtained by hydrolysis in situ with pepsin in a cationic column [27,28]. The fractions were analyzed using HPLC-MS-MS and 21 peptides were identified. Five peptides were synthetized; these peptides presented antibacterial activity against Gram-negative bacteria. In July 2018, the European Union (UE) approved the use of HEWL hydrolysates with a new food regulation UE 2018/991, allowing the use in food supplements for the adult population. Additionally, the Food and Drug Administration (FDA) recognized hen egg white lysozyme hydrolysate as generally recognized as safe (GRAS), allowing its use as an ingredient in supplements.
The aim of this study was to increase the antibacterial spectrum of modified HEWL with thermal and chemical treatments against Gram-negative bacteria.
2. Materials and Methods
2.1. Materials
HEWL (58,000 U/mg), Micrococcus lysodeikticus, and pepsin from porcine mucose (3440 U/mg) were obtained from Sigma Chemical Co. (Saint Louis, MO, USA). Tryptic soy agar (TSA) and trypticase soy broth (TSB) were obtained from Scharlau (Barcelona, Spain). DL-Dithiothreitol (DTT) and Iodoacetamide (IAM) were obtained from Sigma ((Saint Louis, MO, USA). The LIVE/DEAD BacLigth kit as also used (L-13152; Molecular Probes, Leiden, The Netherlands). All other reagents were of analytical grade.
2.2. Strains Bacteria
E. coli ATCC 25922 was from the American Type Culture Collection (ATCC) (Rockville, MD, USA) and S. carnosus CECT 4491T was from The Spanish Type Culture Collection (Colección Española de Cultivos Tipo, CECT; Valencia, Spain).
2.3. Preparation of Denatured HEWL with Heat Treatment
HEWL heat-induced inactivation (1.0 mg/mL) was performed by incubation at 80 °C, 90 °C, 95 °C, and 120 °C (HEWL/80 °C, HEWL/90 °C, HEWL/95 °C and HEWL/120 °C) for 20 min in a 10 mM potassium phosphate buffer (pH 6.0 and pH 7.0). Insoluble aggregates were removed by centrifugation (3000× g for 15 min). The supernatants obtained were used in the next experiments after the determination of the protein content using the BCA method, and the commercial diagnosis test of Pierce (Rockford, IL, USA). Then, samples were dialyzed in distilled water, lyophilized, and frozen at −80 °C [13]
2.4. Preparation of Denatured HEWL with a DTT Treatment
HEWL was reduced with DTT at given times to achieve different levels of reduction. HEWL solution in Tris-HCl buffer (10 mM, pH 8.0), at a final protein concentration of 1.0 mg/mL, was incubated with 2 mM DTT at 30 °C. Aliquots (10 mL) were withdrawn at intervals of 0, 1.0, 1.5, 2.0, and 4.0 h (HEWL/DTT 0 h, HEWL/DTT 1.0 h, HEWL/DTT 1.5 h, HEWL/DTT 2.0 h, and HEWL/DTT 4.0 h, respectively), and immediately allowed to react with three molar equivalents of iodoacetamide (IAM) with respect to DTT at 30 °C for 1.0 h in the dark. The supernatants obtained were used in the next experiments. Previously, the protein content was determined with the commercial BCA method by Pierce (Rockford, IL, USA). The samples, after being extensively dialyzed with distilled water at 5 °C, were freeze-dried [29].
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
Modified HEWL SDS-PAGE electrophoresis was performed using 20% polyacrylamide gels in a Mini-Protean electrophoresis system (Bio-Rad, Hercules, CA, USA). Polypeptide bands were stained with Kit silver nitrate (GE Healthcare, Uppsala, Sweden). Relative molecular masses of protein were determined comparing the masses with molecular weight markers (Bio-Rad, Hercules, CA, USA).
2.6. HEWL Lytic Activity of the Assay
The lysozyme lytic activity was determined by spectrophotometrically monitoring the decrease in turbidity of a suspension of Micrococcus lysodeikticus cells at 450 nm at 25 °C, using the Shugar, method [30]. One unit of HEWL was defined as a decrease in the absorbance at 450 nm of 0.001 min−1. The assay was performed with three reactions per sample.
2.7. Antibacterial Assay
To determine antimicrobial activity, 10 mL of trypticase soy broth (TSB) were inoculated in a colony of bacteria (E. coli or S. carnosus) and incubated overnight at 37 °C. One milliliter of bacterial suspension was then diluted (1:50) in TSB. Bacteria were grown at 37 °C until the logarithmic phase was reached as determined by the absorbance at 660 nm. Bacteria were centrifuged at 5000× g for 10 min, then washed with 10 mM sodium phosphate and 137 mM sodium chloride buffer, at pH 7.4, and suspended (3 × 105 CFU/mL) in the same media. One milliliter of the bacterial suspension was mixed with an equal volume of native HEWL or modified HEWL (2.0 mg/mL). The mixture of the sample and bacteria was incubated at 37 °C for 15 h, and the absorbance at 660 nm was measured using a Novospec 2 Pharmacia spectrophotometer Amersham Pharmacia Biotech (Orsay, France). A 100 μL aliquot was placed onto nutrient agar plates. Colony Forming Units (CFU) were obtained after incubation of plates at 37 °C for 24 h. The assays were performed in triplicate [26].
2.8. Fluorescence Microscopy
Cell lysis was observed using a phase-contrast microscopy at 100× magnification with a DM2500 microscopy equipped with a digital firewire camera (DFC 420 C) digital camera (Leica, Wetzlar, Germany). The LIVE/DEAD BacLigth kit (L-13152; Molecular Probes, Leiden, The Netherlands) was used to assay membrane integrity by selective nucleic acid staining. The kit contains two dyes: SYTO 9 (fluorescent green) penetrates and labels all bacteria. Propidium iodide (fluorescent red) penetrates only bacteria with damaged membranes, and in these cells, suppresses SYTO 9 staining. As a result, green fluorescent stains living cells stained, and red fluorescent stains dead cells. Five microliters of a stationary culture suspended in phosphate buffer (10 mM, pH 7.0) (with and without HEWL) was mixed with 5 μL of stained mixture to obtain a final concentration of 6 μM SYTO 9 and 30 μM propidium iodide. After 15 min of incubation in the dark; green and red cells were observed under a fluorescence microscopy with a long-pass filter (excitation, 420 to 490 nm and emission 515 nm). The fluorescent images were recorded to be subsequently analyzed [31].
2.9. Electron Microscopy
Suspensions of E. coli and S. carnosus at mid-log phase (108 cells/ mL), made in TSB at pH 7.4, were incubated with different HEWL doses at 37 °C for 15 h and fixed to the culture plate with 4% paraformaldehyde (Merck, Darmstadt, Germany) and 2% glutaraldehyde (SERVA, Heidelberg, Germany) in 0.05 M cacodylate buffer, pH 7.4, for 120 min at room temperature. Cells were then carefully scraped from the plate, centrifuged at 3000× g for 5 min and the washed pellet post-fixed with 1% OsO4 and 1% K3Fe (CN)6 (potassium ferrocyanide) in water for 60 min. Cells were dehydrated with ethanol and embedded in Epon TAAB 812 resin, TAAB Laboratories Equipment Limited (Berkshire, UK) according to standard procedures. Ultrathin sections were collected on collodion-carbon coated copper grids, stained with uranyl acetate and lead citrate, and examined at 80 kV with a Jeol 1010 (Tokyo, Japan) electron microscopy. Electron micrographs were recorded [32].
2.10. Statistical Analysis
Results are presented as means ± standard deviation from three replicates of each experiment. Differences between mean values were determined by the analysis of variance (ANOVA). The post hoc analysis was performed by the Tukey´s test. All tests were considered significant at p < 0.05 using the software GraphPad Prism 7 (La Jolla, CA, USA).
3. Results and Discussion
3.1. Denatured HEWL by Thermal and Chemical Treatments
HEWL was denatured using a thermal treatment at pH 6.0 and pH 7.0. HEWL was heated at 80 °C, 90 °C, 95 °C, and 120 °C for 20 min. HEWL was treated with DTT for 0, 1.0, 1.5, 2.0, and 4.0 h at pH 8.0 (Figure 1). HEWL has four disulfide bridges resulting in high thermal resistance and resistance to acids and bases [33,34]. Possible structural changes in HEWL were analyzed using SDS-PAGE electrophoresis. Figure 1A,B show that when the denaturation temperature increased, the formation of oligomeric structures increased. HEWL samples incubated at pH 7.0 have a frequent presence of dimer (28.6 kDa), trimer (42.9 kDa), and tetramer (57.2 kDa) forms. HEWL/90 °C and HEWL/120 °C, at pH 7.0, present more frequently in higher oligomeric forms. Native HEWL at pH 7.0 also presents in dimer forms. Monaco et al. described the formation of dimers (28.6 kDa) for storage of HEWL [35]. HEWL/DTT presents as dimers and other oligomer forms. HEWL/DTT was treated at pH 8.0; at this pH, HEWL can be slightly negatively charged. The pH contributes to the formation of new oligoforms. HEWL/DTT 1.5 h presented intensive bands in the dimer form. Tomizawa et al. [36] explained that the dimer formation can result from pH denaturation, the HEWL concentration, the time of denaturation, or the temperature and/or medium. In this study, pH 7.0 stimulated dimer formation in HEWL. Nakada et al. [37] reported impurity of dimer forms in the commercial HEWL proteins obtained from crystallization. Typical residual impurities contained in commercial lysozyme are formed with covalently bound lysozyme.
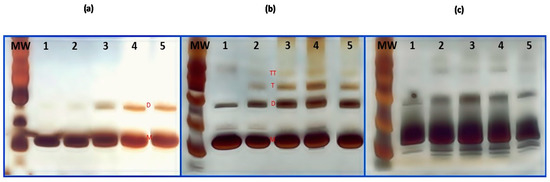
Figure 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of heat-denatured and chemically-denatured of hen egg white lysozyme (HEWL). (a) heat-denatured HEWL at pH 6.0, lane 1: native HEWL, lane 2: HEWL/80 °C, lane 3: HEWL/90 °C, lane 4: HEWL/95 °C, and lane 5: HEWL/120 °C. (b) Heat-denatured HEWL at pH 7.0, lane 1: native HEWL, lane 2: HEWL/80 °C, lane 3: HEWL/90 °C, lane 4: HEWL/95 °C, lane 5: HEWL/120 °C, and (c) HEWL treated with DTT, lane 1: HEWL/DTT 0 h, lane 2: HEWL/DTT 1 h, lane 3: HEWL/DTT 1.5 h, lane 4: HEWL/DTT 2.0 h, and lane 5: HEWL/DTT 4.0 h. M—monomer, D—dimer, T—trimer, and TT—tetramer.
Lesnierowski et al. [38] tested the formation of HEWL oligomeric forms with heat treatment at 90 °C and 95 °C for 30 min in the presence of resorcinol and H2O2, reporting the formation of dimer, trimer, and tetramer HEWL forms [39]. Ibrahim et al. [20] described HEWL as having one hydrophobic region inside the molecule and one hydrophilic region outside the molecule in contact with the medium. They also claimed that heat-denatured HEWL conformationally changes because of the denaturation. Circular dichroism (CD) spectroscopy and fluorescence methods indicate that during the HEWL heating process, two bridges are broken, exposing four cysteine amino acids, resulting in an increase in the molecule hydrophobicity. Tryptophan 62, 63, and 108 are inside the molecule. When 64Cys–80Cys and 76Cys–94Cys are broken due to the heating process, these amino acids are exposed. The increase in HEWL hydrophobicity is related to antibacterial activity, as these molecules may have greater affinity for the bacteria membrane and cause cell lysis. When disulfide bonds break, an increase in hydrophobicity in denatured HEWL is produced, resulting in an increase in HEWL affinity with the cell membranes, resulting in bacteria cellular perturbation and death of bacteria.
Touch et al. argued that reduced HEWL, with 2 mM dithiothreitol (DTT) at pH 8.0 and 30 °C for 0.5 to 4.0 h of incubation, released modified sulfhydryl groups [29]. Denatured HEWL presents an increase in surface hydrophobicity of the lysozyme upon reduction of the disulfide bonds with DTT. Siwicki et al. stated that the HEWL dimer is less toxic than the HEWL monomer, and the dimer is more active than monomer [39]. Kiczka (1994) [40] registered the dimeric HEWL form (named KLP-602) with patent No. 5,200,182, which presented better antibacterial (Staphylococcus aureus, E. coli, Pseudomonas aeruginosa Streptococcus) and antiviral (Sendai virus strain) activity and lower toxicity. Rymuszka et al. (2005) [41] described immunomodulatory effects of the dimer of lysozyme (KLP-602) in a carp (Cyprinus carpio L) in an in vivo study.
In this study, HEWL was denatured for incubation with the chemical agent DTT for 0, 1.0, 1.5. 2.0, and 4.0 h (Figure 1C). DTT is able to reduce the four HEWL disulfide bonds (6Cys–127Cys, 30Cys–115Cys, 64Cys–80Cys, and 76Cys–94Cys). After incubation of HEWL with DTT, HEWL was treated with iodoacetamide (IAM) for alkylation of SH groups. Partial reduction of native HEWL produces a denatured HEWL form. This reduced HEWL form can increase the antibacterial spectrum against Gram-negative bacteria. HEWL incubated for 1.0, 1.5. 2.0, and 4.0 h presented a higher frequency of oligomeric forms (dimer, trimer, and tetramer forms). HEWL chemical denaturation produces oligomeric forms. Touch et al. (2003) described reduced HEWL with the food-compatible reducing agent cysteine (Cys) and glutathione (GSH) [21]. The disulfide bonds were partially reduced by thiol-disulfide exchange reactions under heat-induced denaturing conditions from 55 °C to 90 °C. The results showed that treatment of lysozyme with Cys and GSH resulted in the introduction of new half-cystine residues (2–3 residues/moL of protein). Both reduced lysozymes displayed a significantly higher antimicrobial activity than native or heated lysozyme against Salmonella enteritidis in a sodium phosphate buffer (10 mM, pH 7.2) at 30 °C for one hour. Touch et al. (2004) also reported HEWL reduction by incubation of DTT for 0.5 to 4 h with a reduction of S–S bonds and an exposition to the hydrophobicity region (Trp 63, Trp 111 and Trp 123) from HEWL [29].
3.2. Denatured HEWL Lytic Enzymatic Activity
Denatured HEWL residual lytic activity was evaluated against Micrococcus lysodeikticus using the spectrophotometric method. The activity was compared to 100% native HEWL (Figure 2). Heated-HEWL at pH 6.0 presented decreased lytic activity proportional to the denaturation temperature. HEWL/80 °C presented a lytic enzymatic value of 95% with only a 5% loss in lytic activity. HEWL/90 °C presented a 76.5% lytic enzymatic activity. HEWL/95 °C presented a 41.8% of residual lytic enzymatic activity. HEWL/120 °C only presented a 5.8% residual lytic activity compared to native HEWL. At higher denaturation temperatures, the loss in lytic enzymatic activity was higher. All heated HEWL samples at pH 7.0 had lower percentages of residual lytic enzymatic activity compared to heated-HEWL at pH 6.0. For example, HEWL/80 °C, at pH 7.0 had a value of 16.5%, whereas the HEWL/80 °C at pH 6.0 had 95% residual lytic activity. HEWL/90 °C at pH 7.0 only presented a 1.8% residual lytic activity with a higher loss.
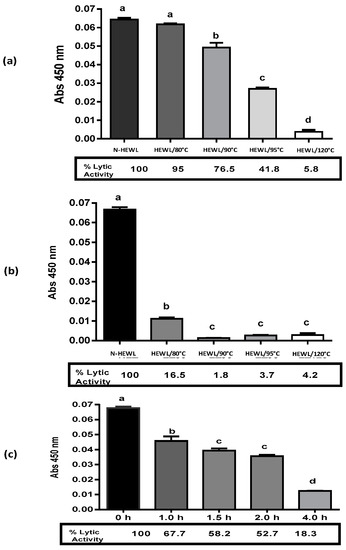
Figure 2.
Lytic activity of modified hen egg white lysozyme (HEWL) by heat and chemical treatment. (a) Heat treatment HEWL at pH 6.0, native HEWL (N-HEWL), (b) heat treatment HEWL at pH 7.0, N-HEWL, and (c) HEWL treated with DTT for 0 h, 1.0 h, 2.0 h, and, 4.0 h of incubation. Data are expressed as the mean ± standard deviation (n = 3). Different letters present significant differences (p < 0.05) in ANOVA and Tukey’s test.
Lesnierowski et al. (2012) [38] explained that HEWL heated at 90 °C and 95 °C for 30 min at pH 4.0 demonstrated a loss in lytic enzymatic activity. HEWL treated at 90 °C for 30 min at pH 4.0 showed 63.6% lytic activity. HEWL treated at 95 °C for 30 min at pH 4.0 presented a value of 76.7% of lytic activity. Ibrahim et al. (1998) described modified HEWL at 80 °C for 20 min at pH 6.0 with 50% lytic activity [42]. The values of lytic activity of heated HEWL reported in the literature are similar to the ones obtained in this study. Touch et al. (2003) [21] reported heated-HEWL at 85 °C with 70% lytic enzymatic, and denatured HEWL with Cys and GSH showed values of 45% and 38%, respectively. Our results for the denatured HEWL and the loss in lytic enzymatic activity are consistent with the values reported by these authors.
HEWL was incubated at pH 8.0 with DTT for 0, 1.0, 1.5, 2.0, and 4.0 h and then treated with iodoacetamide. HEWL/DTT 0 h, HEWL/DTT 1.0 h, HEWL/DTT 1.5 h p, HEWL/DTT 2.0 h, and HEWL/DTT 4.0 h demonstrated lytic enzymatic activities of 100%, 67.7%, 58.2%, 52.7%, and 18.3%, respectively. The decrease in HEWL lytic enzymatic activity was proportional to the incubation time with the DTT agent. The higher the incubation time, the higher the loss in HEWL lytic enzymatic activity. Touch et al. (2004) [29] described the loss of HEWL lytic enzymatic activity by incubation with DTT for 0.5 h at 4 h, at pH 8.0. HEWL/DTT 0.5 h, HEWL/DTT 1.0 h, HEWL/DTT 1.5 h, HEWL/DTT 2.0 h, HEWL/DTT 3.0 h, and HEWL/DTT 4.0 h showed lytic enzymatic activities of 80%, 60%, 45%, 30%, 15%, and 0.2%, respectively. Our results for the denatured HEWL/DTT loss in lytic enzymatic activity agreed with the values reported by Touch et al. [29]. An increase in HEWL incubation time with the DTT agent increased the loss of HEWL lytic enzymatic activity.
3.3. Antibacterial Activity of Modified HEWL
Antibacterial activity from native HEWL, heated-HEWL, and HEWL/DTT at 2.0 mg/mL was evaluated against E. coli ATCC 25922 and S. carnosus CECT 4491T for a 15 h incubation period. Antibacterial activity is expressed as Log N0/Nf where N0 refers to the control number of colonies without antibacterial material and Nf refers to the number of colonies containing antibacterial sample (HEWL) after an incubation period of 15h. Modified HEWL produced a visible precipitate in the Falcon tube. This precipitate was an aggregation of bacteria. Only HEWL at pH 7.0 did not produce bacteria precipitation against Gram-negative bacteria (E. coli).
Heated-HEWL at pH 6.0 and pH 7.0 at 80 °C, 90 °C, 95 °C, and 120 °C for 20 min was assayed. Heated-HEWL at pH 6.0 at 80 °C, 90 °C, 95 °C, and 120 °C for 20 min increased the antibacterial spectrum, killing Gram-negative bacteria (E. coli) (Table 1). Native HEWL was used as the control and showed a reduction of 1.6 units Log of E. coli. Native HEWL dissolved in pH 6.0 showed antibacterial activity against Gram-negative bacteria. At pH 6.0, HEWL had the highest positive charge. HEWL has a high isoelectric point (pI) 10.7 and pH. The positive charge increases and can have an affinity for membrane bacteria, as membrane bacteria has a negative charge. HEWL/80 °C, at pH 6.0, was able reduce 4.3 units Log of E. coli, heated-HEWL/90 °C was able to reduce 3.8 units Log of E. coli, and HEWL/95 °C reduced 3.9 units Log against E. coli. HEWL/120 °C at pH 6.0 was able to reduce 3.7 units Log of Escherichia coli. HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 7.0 showed no antibacterial activity against E. coli. This pH of denaturation was unable to produce an increase in antibacterial activity in HEWL. A higher activity was obtained with HEWL/80 °C (4.3 units Log). The antibacterial activity decreased with the increase in the temperature of heated HEWL, i.e., HEWL/120 °C at pH 6.0 (3.7 units Log). The statistical analysis only supported statistical differences between HEWL/80 °C and HEWL/90 °C, and HEWL/95 °C and HEWL/120 °C at pH 6.0. In S. carnosus bacteria, the activity was the opposite: antibacterial activity against Gram-positive bacteria increased with increasing temperature of denatured HEWL.

Table 1.
Antibacterial activity of modified hen egg white lysozyme (HEWL) against at Escherichia. coli and Staphylococcus carnosus.
HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 6.0 and pH 7.0 showed higher antibacterial activity against Gram-positive bacteria (S. carnosus) (Table 1). Native HEWL showed the ability to reduce 7.8 units Log. Native HEWL showed bactericidal activity against Gram-positive bacteria. HEWL/80 °C reduced 4.9 units Log, and HEWL/90 °C and HEWL/95 °C both reduced 5.2 units Log of S. carnosus. HEWL/120 °C reduced 5.6 units Log of S. carnosus. This sample presented higher activity against Gram-positive bacteria. Heated HEWL at pH 7.0 has lytic activity values from 1.8% to 16.5%. HEWL/90 °C at pH 7.0 had only 1.8% lytic activity, suggesting that its antibacterial activity is not dependent on the lytic activity. The mechanism of action is then different.
Ibrahim et al. described heated HEWL at pH 6.0 and pH 7.0 as having antibacterial activity against Gram-negative bacteria (E. coli k-12) and Gram-positive bacteria (S. aureus). HLz80/6 was the sample with the highest activity compared to HEWL at pH 7.0 [19]. Masschalck et al. described heated denatured HEWL at pH 7.0, applying the methodology of Ibrahim et al. [19]. Denatured HEWL at pH 7.0 showed an absence of antibacterial activity against any Gram-negative bacteria E. coli K-12 strain MG1655, Pseudomonas fluorescens LMMBM07, Salmonella enterica serovar Typhimurium LMMBM01, Salmonella enteritidis ATCC13076, Shigella flexneri LMG10472, and Shigella sonnei LMG10473. In this study, Gram-positive bacteria were not assayed [14]. There is controversy about the antibacterial activity of thermally denatured lysozyme. Our results confirm that heated HEWL at pH 7.0 is not effective against Gram-negative bacteria (E. coli). Heated HEWL at pH 7.0 had antibacterial activity against Gram-positive bacteria. This indicates that the antibacterial activity of denatured HEWL is independent of lytic activity according to the genetic evidence found. Other studies have confirmed this fact [43,44].
Denatured HEWL with DTT (0, 1.0, 1.5, 2.0, and 4.0 h) was assayed and antibacterial activity against Gram-negative and Gram-negative bacteria was measured. Denatured HEWL/DTT (1.0, 1.5, 2.0, and 4.0 h) was active against both bacteria. HEWL/DTT 0 h had no antibacterial activity against Gram-negative bacteria. HEWL/DTT 1.5 h had higher activity with a reduction of 5.5 units Log of E. coli. HEWL/DTT 0 had bactericidal activity against Gram-positive bacteria reducing 8 units Log. HEWL/DTT 1.0 h reduced 5.2 units Log of S. carnosus. Touch et al. [29] examined the antibacterial activity of HEWL/DTT (0.5 h to 4.0 h) against Salmonella enteritidis, E. coli, Proteus mirabilis, and Serratia marcescens. This activity was related to the increase in HEWL hydrophobicity due to the exposition of tryptophan amino acids in their sequence. The antibacterial activity was related to the formation of oligomeric forms due to the reduction in S–S bonds.
3.4. Fluorescent Analysis
3.4.1. Denatured HEWL at pH 6.0
E. coli and S. carnosus incubated with or without modified HEWL by heat and chemical treatments were dyed with a LIVE/DEAD fluorescence kit to analyze membrane integrity. E. coli and S. carnosus were incubated with native HEWL at different concentrations (0.5 mg/mL to 10 mg/mL). Cell aggregation was absent in E. coli when incubated with native HEWL at the concentrations assayed. Only at the 10 mg/mL concentration were red cell bacteria observed, with typical movements (Figure 3a–c). When S. carnosus was incubated with native HEWL, cell aggregation was observed, presenting a bactericidal effect at 0.5 and 5.0 mg/mL (Figure 3d–f). Bacteria under fluorescence were observed with a red-orange color.
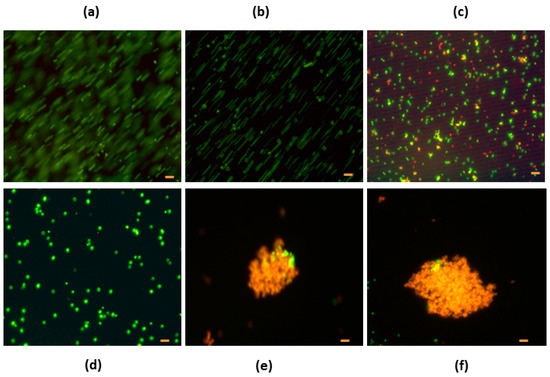
Figure 3.
Escherichia coli and Staphylococcus carnosus incubated with native hen egg white lysozyme (HEWL) (a) E. coli control without HEWL, (b) E. coli with native HEWL (0.5 mg/mL), (c) E. coli with native HEWL (10 mg/mL), (d) S. carnosus (control without HEWL), (e) S. carnosus (0.5 mg/mL), and (f) S. carnosus with HEWL (5 mg/mL). Bar = 2 µm.
We observed green fluorescence in E. coli, the control bacteria without sample, and typical movements of bacteria (Figure 4a,b). HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 6.0 showed cell aggregation and red-orange fluorescence (Figure 4b–f). For example, HEWL/80 °C at pH 6.0 and at a concentration of 0.5 mg/mL showed aggregation of cell bacteria (E. coli) with red fluorescence (Figure 4c). Red-orange fluorescence indicates damaged membranes with dead cells. Bacteria were different with respect to bacteria control and characteristic bacteria movement was absent, presenting a product aggregation as a result of the breaking membranes.
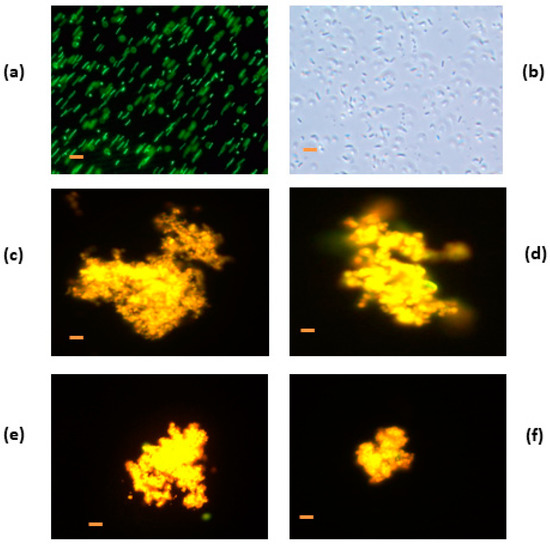
Figure 4.
Fluorescence micrographs of Escherichia coli incubated with heat-treated hen egg white lysozyme (HEWL) at pH 6.0. (a) E. coli control without HEWL, (b) E. coli control without HEWL without fluorescence, (c) E. coli incubated with HEWL/80 °C at pH 6.0, (d) E. coli incubated with HEWL/90 °C at pH 6.0, (e) E. coli incubated with HEWL/95 °C at pH 6.0, and (f) E. coli incubated with HEWL/120°C, pH 6.0. Bacteria control and bacteria with sample were incubated for 15 h at a concentration of 0.5 mg/mL. Bar = 2 µm.
S. carnosus, the control bacteria without HEWL, showed green fluorescence, which indicated an absence of membrane damage in the cell. All cells observed moved normally and were spherical in form, characteristic of coccus bacteria (Figure 5). HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 6.0 were cellularly aggregated and fluoresced red (Figure 5). For example, heated HEWL/80 °C at pH 6.0 and at a concentration of 0.5 mg/mL showed cell bacteria aggregation (S. carnosus) with red-orange fluorescence (Figure 5). Red-orange fluorescence indicates membrane damage with cellular death. A red-orange color using the LIVE/DEAD kit was observed by Carrillo et al. (2014) [13] and Zdybicka-Barabas et al. (2012) [45], indicating cell damage. Bacteria were different with respect to bacteria control and bacteria characteristic movement was absent, showed an aggregation of product as a result of the breaking membranes.
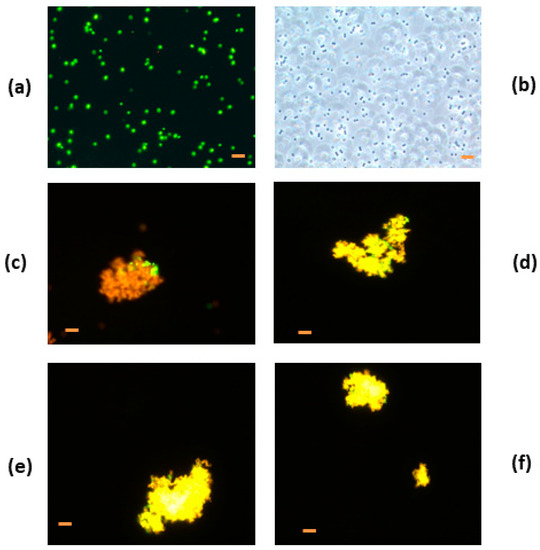
Figure 5.
Fluorescence micrographs of Staphylococcus carnosus incubated with heat-treated hen egg white lysozyme (HEWL) at pH 6.0. (a) S. carnosus control without HEWL, (b) S. carnosus control without HEWL without fluorescence, (c) S. carnosus incubated with HEWL/80 °C, (d) S. carnosus incubated with HEWL/90 °C, (e) S. carnosus incubated with HEWL/95 °C, and (f) S. carnosus incubated with heat-treated HEWL/120 °C. Bacteria control and bacteria with sample were incubated for 15 h at a concentration of 0.5 mg/mL. Bar = 2 µm.
3.4.2. Denatured HEWL at pH 7.0
When E. coli was incubated with HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 7.0 (Figure 6c–f) under fluorescence, green fluorescence with the same movement characteristic of the bacteria control (Figure 6a) was observed in all samples. For example, heated HEWL at pH 7.0 at 80 °C at a concentration of 0.5 mg/mL under fluorescence was green with characteristic movements (Figure 6c). Aggregation of cells was nonexistent. This confirms that heated HEWL at pH 7.0 has no antibacterial activity against Gram-negative bacteria, as the membranes were not damaged. This situation is correlated with the results obtained for antibacterial activity against E. coli incubated with denatured HEWL at pH 7.0, as these samples were not active. Modified HEWL at pH 7.0 produced no damage in bacteria membranes.
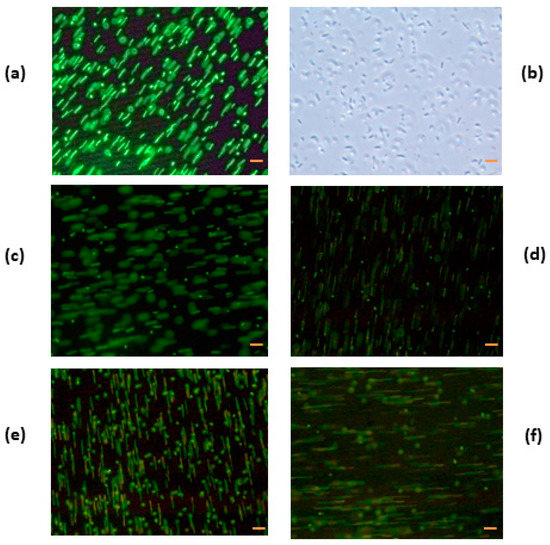
Figure 6.
Fluorescence micrographs of Escherichia coli incubated with heat-treated hen egg white lysozyme (HEWL) at pH 7.0. (a) E. coli control without HEWL, (b) E. coli control without HEWL without fluorescence, (c) E. coli incubated with HEWL/80 °C, (d) E. coli incubated with HEWL/90 °C, (e) E. coli incubated with HEWL/95 °C, and (f) E. coli incubated with HEWL/120 °C. Bacteria control and bacteria with sample were incubated for 15 h at a concentration of 0.5 mg/mL. Bar = 2 µm.
Gram-positive bacteria (S. carnosus) were incubated with heated HEWL at pH 7.0 (80 °C, 90 °C, 95 °C, and 120 °C) under a fluorescence assay. Bacteria control (without modified HEWL) showed green fluorescence indicating no damage to the bacteria membrane. HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C at pH 7.0 showed a strong cellular aggregation and red-orange fluorescence indicating damage of cell membrane.
3.4.3. Denatured HEWL/DTT
Cellular aggregation and red fluorescence also occurred with HEWL/DTT incubation for 1.0, 1.5, 2.0, and 4.0 h indicating membrane damage (Figure 7). Characteristic movements of E. coli bacteria were absent. We observed normal morphological aspect and normal movements in E. coli control without denatured HEWL. When bacteria were compared with the control, they were different.
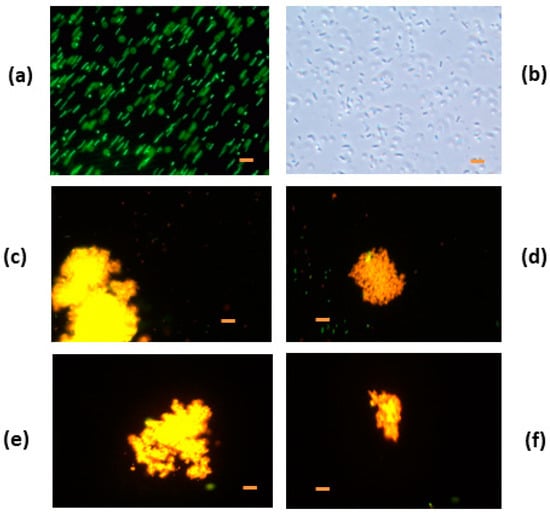
Figure 7.
Fluorescence micrographs of Escherichia coli incubated with denatured hen egg white lysozyme (HEWL)-DTT for 1.0 h, 1.5, 2.0, and 4.0 h of incubation (a) E. coli control without HEWL, (b) E. coli control without HEWL without fluorescence, (c) E. coli incubated with HEWL/DTT 1.0 h, (d) E. coli incubated with HEWL/DTT 1.5 h, (e) E. coli incubated with HEWL/DTT 2.0 h, and (f) E. coli incubated with HEWL/DTT 4.0 h. Bacteria control and bacteria with sample were incubated for 15 h at 0.5 mg/mL. Bar = 2 µm.
HEWL/DTT 2.0 h also showed cellular aggregation and red fluorescence indicating membrane damage. Characteristic S. carnosus movement was absent. When S. carnosus was incubated with HEWL/80 °C, HEWL/90 °C, HEWL/95 °C, and HEWL/120 °C, at pH 6. under fluorescence, red-orange fluorescence was observed, indicating damaged membranes and cell death.
3.5. Electron Microscopy Analysis
Electron microscopy was used to observe possible changes in cell morphology after incubation of E. coli and S. carnosus with modified HEWL. The bacteria control (E. coli without HEWL) had normal morphology of bacillus Gram-negative bacteria (Figure 8A). The outer membrane appeared normal under an electron microscope. E. coli incubated for 15 h with HEWL/90 °C pH 6.0 (0.5 mg/mL) showed different morphology with respect to the control bacteria. The outer membrane was broken, and cellular pieces were observed in the medium (Figure 8B). The cells were lysed with HEWL/90 °C at pH 6.0. The same situation was observed when E. coli was incubated with HEWL/120 °C at pH 6.0 (0.5 mg/mL) (Figure 8C). Pieces of bacteria were viewed in the medium product of lysis with heated HEWL. This same situation was observed with heated HEWL at pH 6.0 at different temperatures and different concentrations. The morphology of E. coli incubated for 15 h with HEWL/DTT (2.0 h) at 0.5 mg/mL was different compared with the control bacteria. A black cytoplasm was observed in the cells and white spaces (loss of cytoplasm) were seen under the electron microscope (Figure 8D). The same was noticed when E. coli was incubated with HEWL/DTT at different reduction times and concentrations of the assay. Gram-positive bacteria (S. carnosus with or without modified HEWL) were analyzed with an electron microscope. Control bacteria without HEWL are shown in Figure 9A.
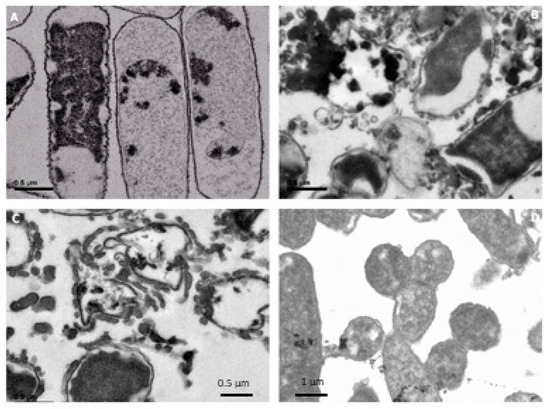
Figure 8.
Electron microscopy micrographs (40×) analysis of Escherichia coli nonincubated and incubated with modified hen egg white lysozyme (HEWL) for 15 h at concentration of 0.5 mg/mL. (A) Bacteria control without HEWL, (B) HEWL/90 °C at pH 6.0, (C) HEWL/120 °C at pH 6.0, and (D) HEWL/DTT 2.0 h.
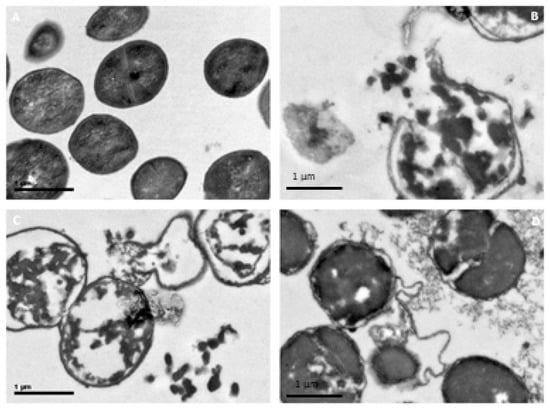
Figure 9.
Electron microscopy micrographs (40×) analysis of S. carnosus nonincubated and incubated with modified hen egg white lysozyme (HEWL) (0.5 mg/mL): (A) Bacteria control without HEWL, (B) HEWL/90 °C at pH 6.0, (C) HEWL/120 °C at pH 6.0, and (D) HEWL/DTT 2.0 h.
Normal morphology from coccus Gram-positive bacteria was observed, with spherical form, cell wall, and normal cytoplasm. Normal cellular division was observed in the microphotography. Figure 9b shows S. carnosus incubated for 15 h with HEWL/90 °C at pH 6.0 (0.5 mg/mL). We can see expulsion of the cytoplasmic content in the medium with the breaking of the cell wall. In the case of S. carnosus incubated for 15 h with HEWL/90 °C at pH 6.0 (0.5 mg/mL), cells were different with respect to the control bacteria morphology. Figure 9C shows S. carnosus incubated for 15 h with HEWL/120 °C at pH 6.0 (0.5 mg/mL) expelling cytoplasmic content. Many cells expelled all cytoplasm content to the medium, and cellular pieces were observed in the microphotography. This same situation was seen with heated-HEWL at pH 6.0 and pH 7.0 at different temperatures and different concentrations. Figure 9D shows S. carnosus incubated HEWL/DTT 2.0 h at 0.5 mg/mL with breaking cell walls and black cytoplasm with white spaces in the cytoplasm. Cellular components were viewed in the medium. The same occurred when S. carnosus was incubated with HEWL/DTT at different reduction times and different concentrations of assay.
4. Conclusions
For the first time, we described the formation of dimers, trimers, and other oligomeric forms in the reduction of HEWL with DTT. This study also shows the antibacterial activity of HEWL denatured by heat and a chemical agent against the Staphylococcus carnosus strain CECT 4491T. The morphological changes experienced by Gram-positive and -negative bacteria were determined by exposure to denatured HEWL. HEWL at pH 6.0 and HEWL/DTT increased the HEWL antibacterial spectrum against Gram-negative bacteria (Escherichia coli). These treatments maintain the HEWL antibacterial activity against Gram-positive bacteria (S. carnosus). Heated HEWL at pH 6.0 and denatured HEWL with DTT ruptured the cell wall with expulsion of the cytoplasm to the culture medium. Increases in temperature in HEWL denaturation were proportional to the loss of the lytic enzymatic activity of HEWL. The increase in incubation time with DTT in denatured HEWL caused a reduction in the lytic enzymatic activity of HEWL. The fluorescence microscopy revealed cellular aggregation for both bacteria with loss of cellular viability. The longer the incubation time with the reducing agent, the higher the loss of lytic enzymatic in HEWL. Heated denatured HEWL could be used as an alternative antibacterial agent in the food industry. Denatured HEWL can be used as a functional ingredient for different proposes.
Author Contributions
Conceptualization, W.C. and R.W.; methodology, R.V.; W.R.; W.C.; formal analysis, W.C.; investigation, W.C.; writing-original draft preparation, P.M.; W.R.; A.P.; W.C.; writing-review and editing, P.M.; W.C.; supervision, W.C. Writing—Review & Editing, English P.M.
Funding
This research received no external funding.
Acknowledgments
Wilman Carrillo thanks Universidad Técnica de Babahoyo. This work is part of Project number CPU-1373-2014 of Universidad Técnica de Ambato, (Ecuador) Emilio Labrador Jimenez has reviewed and edited the English language for the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lesnierowski, G.; Kijowski, J. Lysozyme: Bioactive Compounds; Huopalahti, R., López-Fandiño, R., Amton, M., Schade, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 33–42. ISBN 978-3-540-37883-9. [Google Scholar]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Gordon, M.E.; Carrillo, W. Hen Egg White Lysozyme: Antimicrobial Activity & Allergenicity. Lysozymes: Sources, Functions and Role in Disease; Nova Science Publishers: New York, NY, USA, 2013; pp. 215–226. ISBN 978-1-62257-842-9. [Google Scholar]
- Carrillo, W.; Tubón, J.; Vilcacundo, R. Isolation of hen egg white lysozyme by cation exchange chromatography, analysis of its digestibility and evaluation of the inhibition lipid peroxidation in the zebrafish model. Asian J. Pharm. Clin. Res. 2016, 9, 345–349. [Google Scholar]
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Use of hen egg white lysozyme in the food industry. In Egg Innovations and Strategies for Improvements; Academic Press: London, UK, 2017; pp. 233–242. ISBN 978-0-12-800879-9. [Google Scholar]
- Carrillo, W.; Barrio, D.; Welbaum, J.; Carpio, C.; Vilcacundo, R.; Morales, D.; Ortiz, J. Antimicrobial and antioxidant peptides obtained from food proteins. In Bioactive Peptides: Types, Roles and Research; Powell, K., Ed.; Nova Science Publisher: New York, NY, USA, 2017; pp. 37–58. ISBN 978-1-53610-994-8. [Google Scholar]
- Jiménez-Saiz, R.; Martos, G.; Carrillo, W.; López-Fandiño, R.; Molina, E. Susceptibility of lysozyme to in-vitro digestion and immunoreactivity of its digests. Food Chem. 2011, 127, 1719–1726. [Google Scholar] [CrossRef]
- Carrillo, W.; Tubón, J. Digestibility and enzymatic activity in vitro of hen egg white lysozyme. Asian J. Pharm. Clin. Res. 2016, 9, 376–378. [Google Scholar]
- Lesnierowski, G.; Cegielska-Radziejewska, R.; Kijowski, J. Thermally and chemical-thermally modified lysozyme and its bacteriostatic activity. World’s Poult. Sci. J. 2004, 60, 303–310. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion? A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Carrillo, W.; Spindola, H.; Ramos, M.; Recio, I.; Carvalho, J.E. Anti-inflammatory and anti-nociceptive activities of native and modified hen egg white lysozyme. J. Med. Food. 2016, 19, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; García-Ruiz, A.; Recio, I.; Moreno-Arribas, M.V. Antibacterial activity of hen egg white lysozyme modified by heat and enzymatic treatments against oenological lactic acid bacteria and acetic acid bacteria. J. Food Prot. 2014, 77, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Masschalck, B.; Van Houdt, R.; Van Haver, E.G.; Michiels, C.W. Inactivation of gram-negative bacteria by lysozyme, denatured lysozyme, and lysozyme-derived peptides under high hydrostatic pressure. Appl. Environ. Microbiol. 2001, 67, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cegielska-Radziejewska, R.; Lesnierowski, G.; Kijowski, J. Antibacterial activity of hen egg white lysozyme modified by thermochemical technique. Eur. Food Res. Technol. 2009, 228, 841–845. [Google Scholar] [CrossRef]
- Mann, K. Proteomics of Egg White. In Proteomics in Food Science; Kuddus, M., Ed.; Academic Press: London, UK, 2017; pp. 261–276. ISBN 9780128132807. [Google Scholar]
- Gorbenko, G.P.; Loffe, V.M.; Kinnunen, P.K.J. Binding of lysozyme to phospholipid bilayers: Evidence for protein aggregation upon membrane association. Biophys. J. 2007, 93, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Yamada, M.; Matsushita, K.; Kobayashi, K.; Kato, A. Enhanced bactericidal action of lysozyme to E. coli by inserting a hydrophobic pentapeptide into its C terminus. J. Biol. Chem. 1994, 269, 5059–5063. [Google Scholar] [PubMed]
- Ibrahim, H.R.; Higashiguchi, S.; Keats, M.; Juneja, F.; Kim, M.; Yamamoto, T.; Sugimoto, Y.; Aoki, T. Partially unfolded lysozyme at neutral pH agglutinates and kills Gram-negative and Gram-positive bacteria through membrane damage mechanism. J. Agric. Food Chem. 1996, 44, 3799–3806. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Higashiguchi, S.; Koketsu, M.; Juneja, L.R.; Kim, M.; Yamamoto, T. A structural phase of heat-denatured lysozyme with novel antimicrobial action. J. Agric. Food Chem. 1996, 44, 1416–1423. [Google Scholar] [CrossRef]
- Touch, V.; Hayakawa, S.; Fukada, K.; Aratani, Y.; Sun, Y. Preparation antimicrobial reduced lysozyme compatible in Food Applications. J. Agric. Food Chem. 2003, 53, 5154–5161. [Google Scholar] [CrossRef] [PubMed]
- Lesnierowski, G.; Kijowski, J.; Cegielska-Radziejewska, R. Ultrafiltration modified chicken egg white lysozyme and its antibacterial action. Int. J. Food Sci. Technol. 2009, 44, 305–311. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Aboueleinin, A.M.; Kim, M.; Ibrahim, H.R. Antimicrobial peptides derived from hen egg lysozyme with inhibitory effect against Bacillus species. Food Cont. 2007, 18, 173–178. [Google Scholar] [CrossRef]
- Aminlari, L.; Hashemi, M.M.; Aminlari, M. Modified Lysozymes as Novel Broad Spectrum Natural Antimicrobial Agents in Foods. J. Food Sci. 2014, 79, R1077–R1090. [Google Scholar] [CrossRef] [PubMed]
- Nakimbugwe, D.; Masschalck, B.; Atanassova, M.; Zewdie-Bosüner, A.; Michiels, C.W. Comparison of bactericidal activity of six lysozymes at atmospheric pressure and under high hydrostatic pressure. Int. J. Food Microbiol. 2006, 108, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Ma, F.P.; Lauriau, S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J. Agric. Food Chem. 2004, 58, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Lucio, A.; Gaibor, J.; Morales, D.; Vásquez, G. Isolation of antibacterial hydrolysates from hen egg white lysozyme and identification of antibacterial peptides. J. Med. Food. 2018, 21, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Ramos, M. Identification of antimicrobial peptides of native and heated hydrolysates from hen egg white lysozyme. J. Med. Food. 2018, 21, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Touch, V.; Hayakawa, S.; Saitoh, K. Relationships between conformational changes and antimicrobial activity of lysozyme upon reduction of its disulfides bonds. Food Chem. 2004, 84, 421–428. [Google Scholar] [CrossRef]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
- Masschalck, B.; Deckers, D.; Michiels, C.W. Lytic and nonlytic mechanism of inactivation of Gram-positive bacteria by lysozyme under atmospheric and high hydrostatic pressure. J. Food Prot. 2002, 65, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, V.; Rodríguez, N.; Amils, R. Immunocytochemical analysis of the subcellular distribution of ferritin in imperata cylindrica (L.) Raeuschel, an iron hyperaccumulator plant. Acta Histochem. 2012, 114, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Jollès, P.; Jollès, J. What’s new in lysozyme research? Mol. Cell Biochem. 1984, 63, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Cegielska-Radziejewska, R.; Leśnierowski, G.; Kijowski, J. Antibacterial activity of lysozyme modified by the membrane technique. Electr. J. Pol. Agric. Univ. 2003, 6, 2. [Google Scholar]
- Monaco, L.A.; Rosenberger, F. Growth and etching kinetics of tetragonal lysozyme. J. Cryst. Growth 1993, 129, 465–484. [Google Scholar] [CrossRef]
- Tomizawa, H.; Yamada, H.; Tanigawa, K.; Imoto, T. Stabilization of lysozyme against irreversible inactivation by suppression of chemical reactions. J. Biochem. 1995, 117, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Sazaki, G.; Miyashita, S.; Durbin, S.D.; Komatsu, H. Direct AFM observations of impurity effects on a lysozyme crystal. J. Cryst. Growth. 1999, 196, 503–510. [Google Scholar] [CrossRef]
- Leśnierowski, G.; Cegielska-Radziejewska, R. Potential possibilities of production, modification and practical application of lysozyme. Acta Sci. Pol. Technol. Aliment. 2012, 11, 223–230. [Google Scholar] [PubMed]
- Siwicki, A.K.; Klein, P.; Morand, M.; Kiczka, W.; Studnicka, M. Immunostimulatory effects of dimerized lysozyme (KLP-602) on the nonspecific defense mechanisms and protection against furunculosis in salmonids. Vet. Immunol. Immunopathol. 1998, 62, 369–378. [Google Scholar] [CrossRef]
- Kiczka. From lysozyme monomer to lysozyme dimmer. In Proceedings of the 18th Buiatrics Congress, Bologna, Italy, 29 August–2 September, 1994; pp. 897–900. [Google Scholar]
- Rymuszka, A.; Studnicka, M.; Siwicki, A.K.; Sierosławska, A.; Bownik, A. The immunomodulatory effects of the dimer of lysozyme (KLP-602) in carp (Cyprinus carpio L) in vivo study. Ecotoxicol. Environ. Saf. 2015, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]
- Ibrahim, H.R. On the novel catalytically-independent antimicrobial function of hen egg-white lysozyme: A conformation-dependent activity. Food-Nahrung 1998, 42, 187–193. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Stączek, S.; Mak, P.; Piersiak, T.; Skrzypiec, K.; Cytryńska, M. The effect of Galleria mellonella apolipophorin III on yeasts and filamentous fungi. J. Insect Physiol. 2012, 58, 164–177. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).