Orphan Drug Pricing: An Original Exponential Model Relating Price to the Number of Patients
Abstract
:1. Introduction
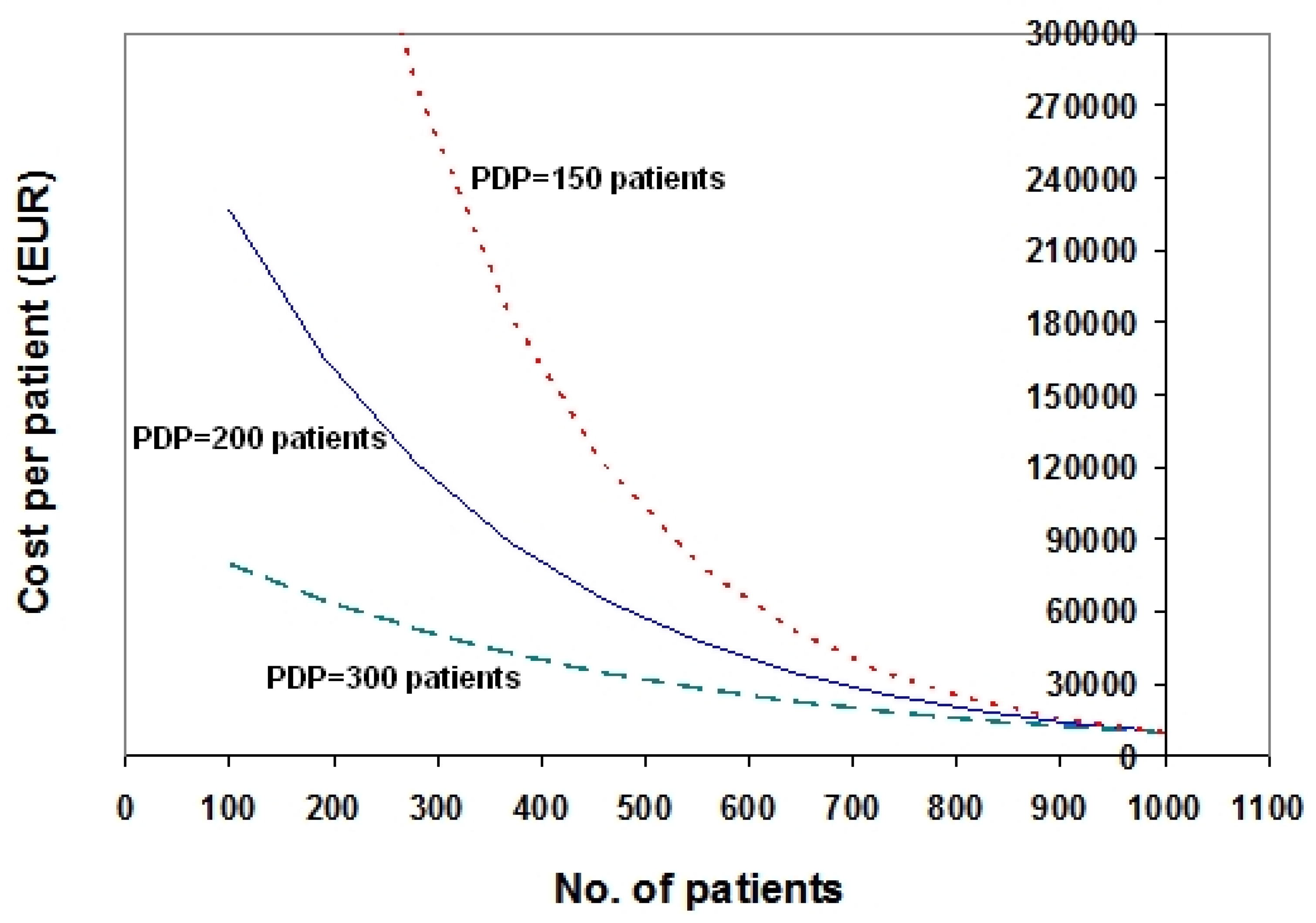
2. Description of the Exponential Model
- -
- Npt is the expected number of treated patients;
- -
- PRICE (in euro/patient) is the cost of the orphan drug treatment (expressed as a function of Npt) which is assumed to undergo an exponential increase as Npt decreases;
- -
- fPRICE (in euro) is the “baseline” price on the y axis attributed to the treatment (i.e., the full price with no discount) under the assumption that 1000 patients are treated; for the sake of simplicity, fPRICE has been set to 10,000 EUR in all examples described in this paper;
- -
- PDP (expressed as the number of patients) is defined as the “price-doubling population” and in the framework of this exponential model, represents the decrease in the number of patients that iteratively determines a doubling of drug price.
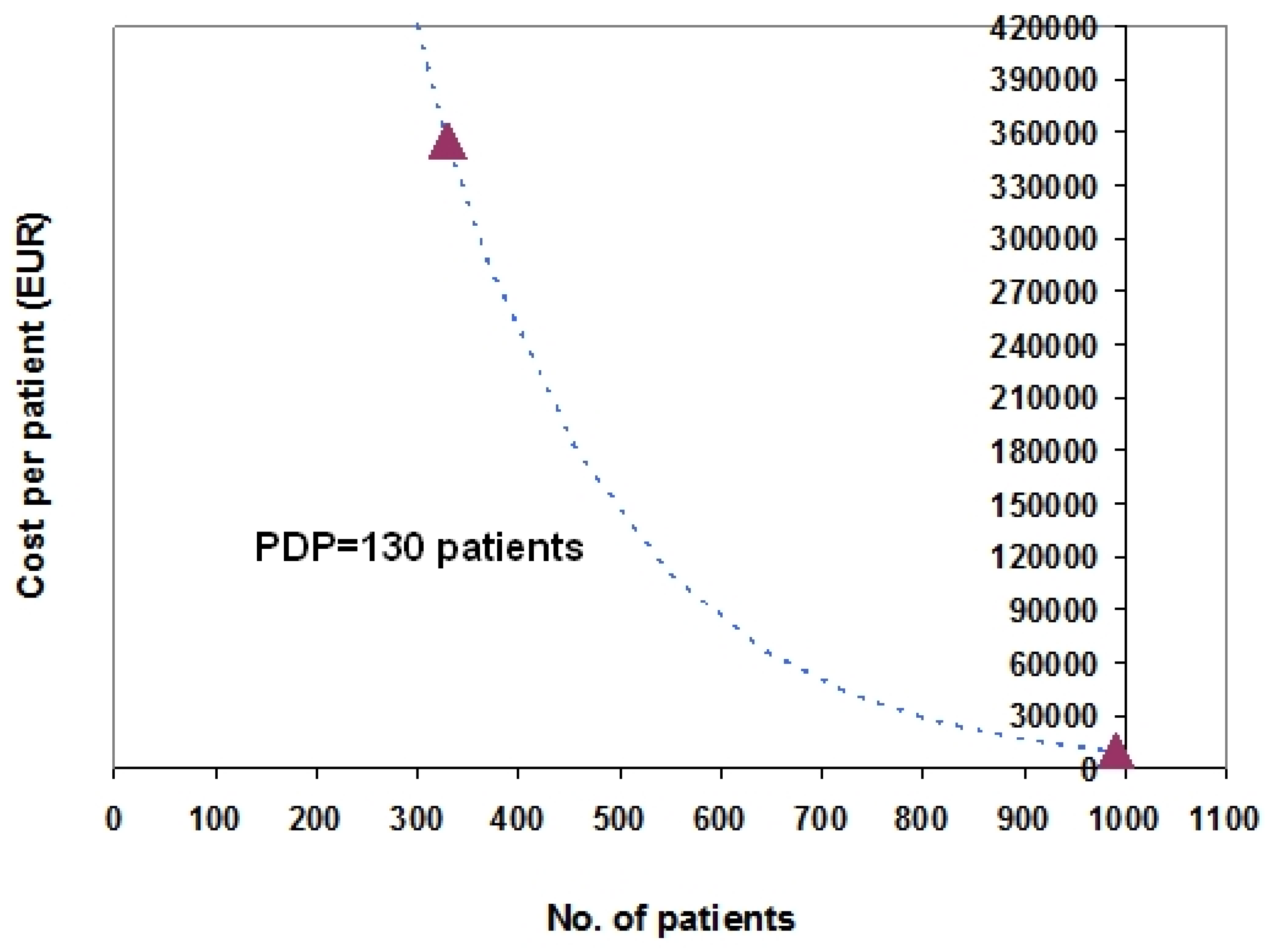
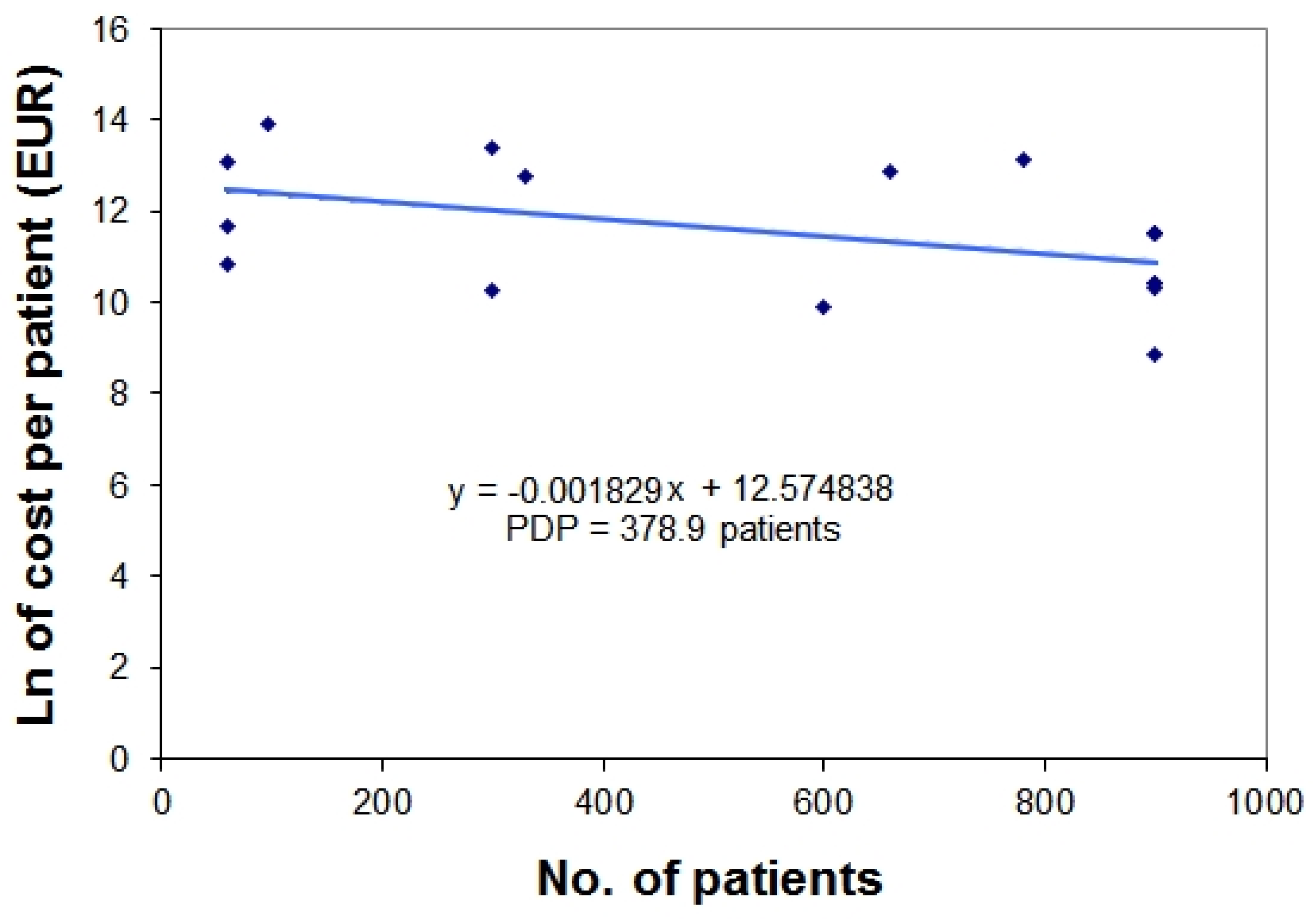
3. Examples Based on Real Data
4. Discussion
Conflicts of Interest
Appendix 1
-Bosentan: patients = 990; cost per patient = 10,491 EUR
Appendix 2
| Orphan Drug | Nationwide Number of Treated Patients (per year) * | Yearly Treatment Cost per Patient (EUR) | Natural Logarithm of Treatment Cost ** |
|---|---|---|---|
| mecasermin | 60 | 50,000 | 10.81978 |
| saproterin | 60 | 116,637 | 11.66682 |
| carglumic acid | 60 | 489,206 | 13.10054 |
| galsulfase | 96 | 1,072,800 | 13.88578 |
| deferasirox | 300 | 28,470 | 10.25661 |
| betaine anhydrous | 300 | 649,335 | 13.3837 |
| eculizumab | 330 | 340,400 | 12.73788 |
| icatibant | 600 | 20,192 | 9.913042 |
| alglucosidase alfa | 660 | 396,000 | 12.88917 |
| laronidase | 780 | 504,000 | 13.13033 |
| epoprostenol | 900 | 100,000 | 11.51293 |
| treprostinil | 900 | 100,000 | 11.51293 |
| sildenafil | 900 | 6,928 | 8.843326 |
| sitaxentan sodium | 900 | 32,879 | 10.40059 |
| bosentan | 900 | 33,084 | 10.40681 |
| iloprost | 900 | 100,000 | 11.51293 |
| ambrisentan | 900 | 30,879 | 10.33783 |
References
- Paulden, M.; Stafinski, T.; Menon, D.; McCabe, C. Value-based reimbursement decisions for orphan drugs: A scoping review and decision framework. Pharmacoeconomics 2015, 33, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Cicchetti, A.; Patregnani, L. Orphan drugs: Relating price determination to disease prevalence. BMJ 2010, 341, c4615. [Google Scholar] [CrossRef] [PubMed]
- Fadda, V.; Messori, A. Reducing Orphan Drugs Prices (Rapid Response). eBMJ, Published 23 November 2010. Available online: http://www.bmj.com/rapid-response/2011/11/03/reducing-orphan-drugs-prices (accessed on 27 September 2016).
- Messori, A. Criteria for drug pricing: Preliminary experiences with modeling the price-volume relationship. Sci. Pharm. 2015, 84, 73–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Messori, A. Evolocumab and alirocumab: Exploring original procurement models to manage the reimbursement of these innovative treatments. Int. J. Clin. Pharmacol. Ther. 2016, 54, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Fadda, V.; Trippoli, S. Lowering the High Cost of Cancer Drugs—II. Mayo Clin. Proc. 2016, 91, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, P.D. Pharmacokinetics (2nd Edition, Revised and Expanded); Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- McCabe, C.; Stafinski, T.; Menon, D. Is it time to revisit orphan drug policies? Yes, for equity’s sake. BMJ 2010, 341, c4777. [Google Scholar] [CrossRef] [PubMed]
- Desser, A.S.; Gyrd-Hansen, D.; Olsen, J.A.; Grepperud, S.; Kristiansen, I.S. Societal views on orphan drugs: Cross sectional survey of Norwegians aged 40 to 67. BMJ 2010, 341, c4715. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F.; Wilson, D.A.; Kanavos, P.; Ubel, P.; Rovira, J. Assessing the economic challenges posed by orphan drugs. Int. J. Technol. Assess Health Care 2007, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Tsuchiya, A.; Claxton, K.; Raftery, J. Assessing the economic challenges posed by orphan drugs: A comment on Drummond et al. Int. J. Technol. Assess Health Care 2007, 23, 397–401. [Google Scholar] [PubMed]
- McCabe, C.; Tsuchiya, A.; Claxton, K.; Raftery, J. Orphan drugs revisited. QJM 2006, 99, 341–345. [Google Scholar] [CrossRef] [PubMed]
- O'Sullivan, B.P.; Orenstein, D.M.; Milla, C.E. Pricing for orphan drugs: Will the market bear what society cannot? JAMA 2013, 310, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messori, A. Orphan Drug Pricing: An Original Exponential Model Relating Price to the Number of Patients. Sci. Pharm. 2016, 84, 618-624. https://doi.org/10.3390/scipharm84040618
Messori A. Orphan Drug Pricing: An Original Exponential Model Relating Price to the Number of Patients. Scientia Pharmaceutica. 2016; 84(4):618-624. https://doi.org/10.3390/scipharm84040618
Chicago/Turabian StyleMessori, Andrea. 2016. "Orphan Drug Pricing: An Original Exponential Model Relating Price to the Number of Patients" Scientia Pharmaceutica 84, no. 4: 618-624. https://doi.org/10.3390/scipharm84040618
APA StyleMessori, A. (2016). Orphan Drug Pricing: An Original Exponential Model Relating Price to the Number of Patients. Scientia Pharmaceutica, 84(4), 618-624. https://doi.org/10.3390/scipharm84040618




