Chitosan-Montmorillonite Polymer Composites: Formulation and Evaluation of Sustained Release Tablets of Aceclofenac
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer Composites
2.3. Powder Characterization
2.3.1. Micrometric Studies
2.3.2. Swelling Index
2.3.3. pH
2.3.4. Loss on Drying
2.3.5. Effective Pore Radius
2.4. Fourier Transform Infra Red- Attenuated Total Reflectance
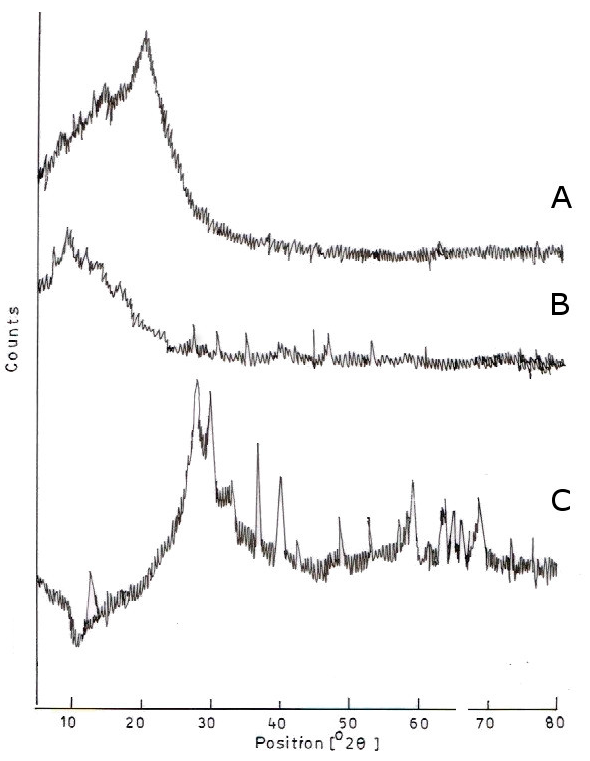
2.5. X-Ray Diffraction
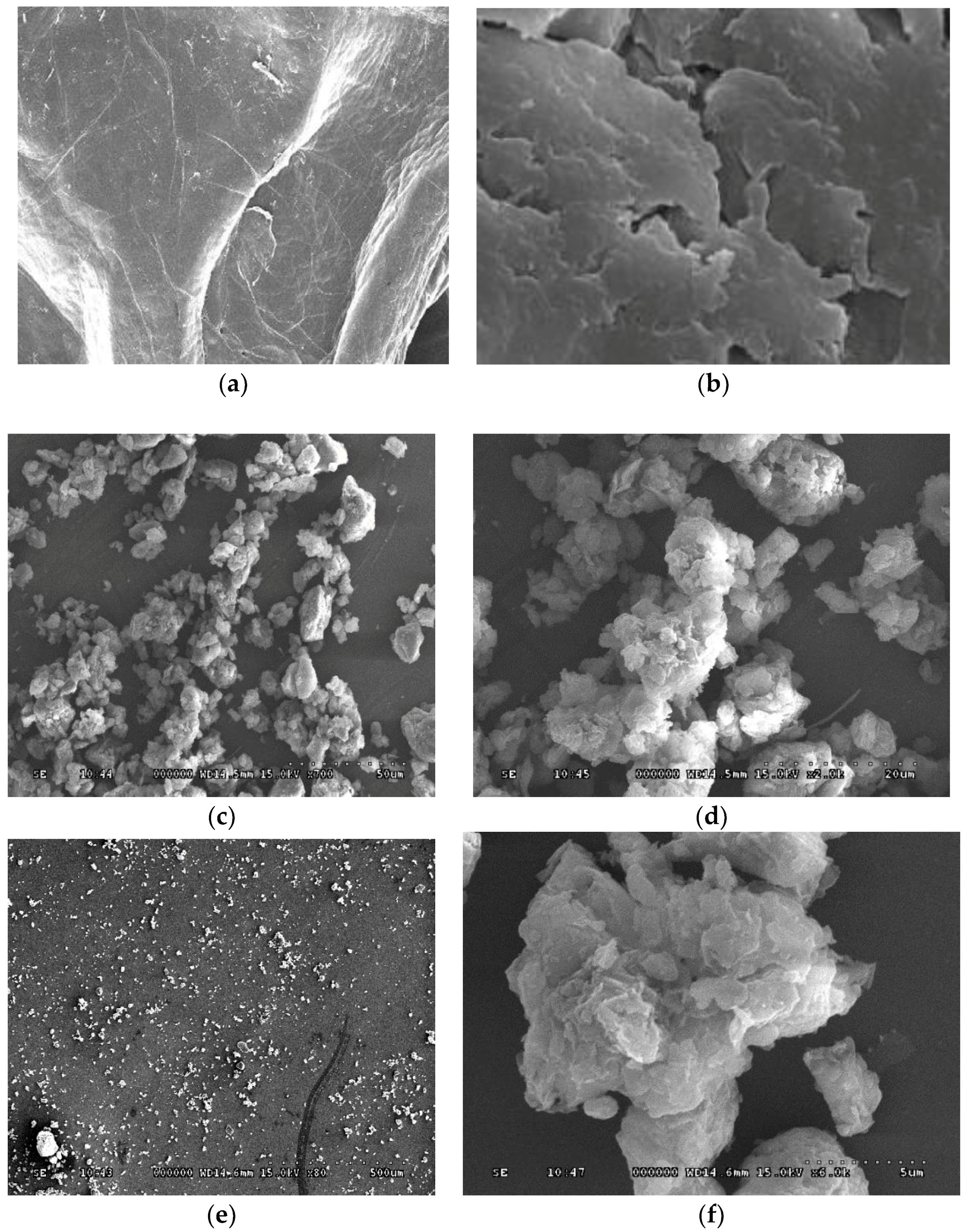
2.6. Scanning Electron Microscopy
2.7. Compression Studies
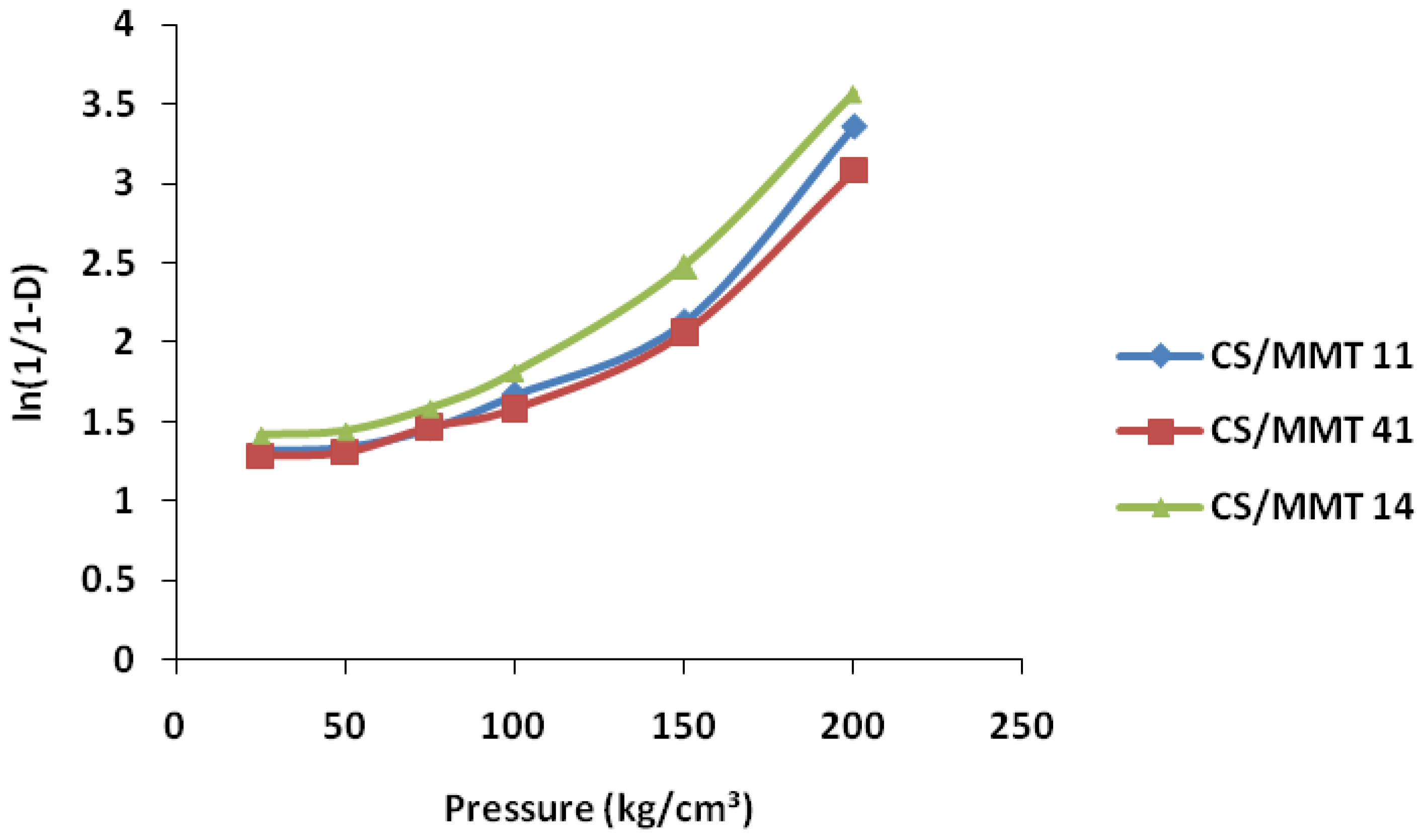
2.7.1. Heckel Function
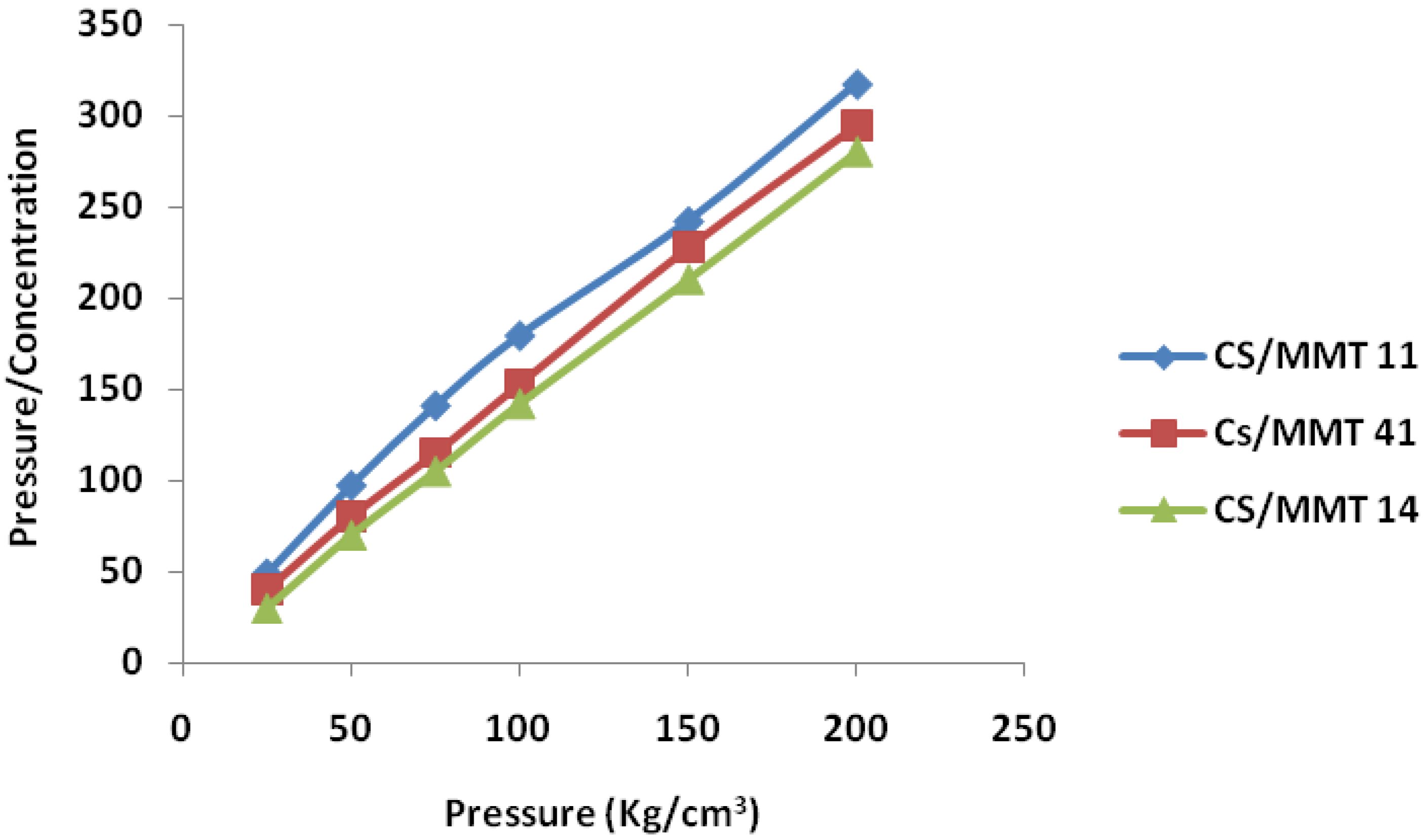
2.7.2. Kawakita Function
2.8. Formulation of Sustained Release Tablets
2.9. Evaluation of Sustained Release Tablets
2.9.1. Diameter and Thickness
2.9.2. Hardness
2.9.3. Friability
2.9.4. Tensile Strength
2.9.5. Drug Content
2.10. In Vitro Dissolution Studies
2.11. Stability Studies
3. Results and Discussions
3.1. Micrometric Studies
3.2. FTIR-ATR Analysis
3.3. XRD Analysis
3.4. SEM Analysis
3.5. Compression Study
3.5.1. Heckel Function Analysis
3.5.2. Kawakita Function Analysis
3.6. Tablets Properties
3.7. In Vitro Dissolution Studies
3.8. Stability Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, T.M.; Wu, C.Y. Biodegradable poly(lactic acid)/chitosan-modified montmorillonite nanocomposites: Preparation and characterization. Poly. Degrad. Stab. 2006, 91, 2198–2204. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Poly. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Abdeen, R.; Salahuddin, S. Modified Chitosan-clay nanocomposite as a drug delivery system intercalation and in vitro release of ibuprofen. J. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR study of montmorillonite–chitosan nanocomposite materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Khedr, M.A.; Waly, A.I.; Hafez, A.A.H. Synthesis of modified chitosan-montmorillonite nanocomposite. Aus. J. Basic App. Sci. 2012, 6, 216–226. [Google Scholar]
- Yang, H.; Wang, W.; Zhang, J.; Wang, A. Preparation, characterization, and drug-release behaviors of a pH-sensitive composite hydrogel bead based on guar gum, attapulgite, and sodium alginate. Int. J. Poly. Mat. Poly. Biomat. 2013, 62, 369–376. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Richhaiya, R.; Singh, V.K.; Singh, A.K.; Kumar, S.; Chaudhary, R.K. Formulation and evaluation of once daily sustained release matrix tablet of aceclofenac using natural gums. J. Drug Del. Therapeutics. 2012, 2, 16–25. [Google Scholar]
- Odeku, O.A. Assessment of Albiziazygiagum as a binding agent in tablet formulations. Acta Pharm. 2005, 55, 263–276. [Google Scholar] [PubMed]
- Bulk density and tapped density of powders. In European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourgh, France, 2011; p. 305.
- Goel, H.; Kaur, G.; Rana, V.; Tiwari, A.K. Formulation development of stronger and quick disintegrating tablets; a crucial effect of chitin. Yakugaku Zasshi. 2010, 130, 729–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonnergaard, J.M. Impact of particle density and initial volume on mathematical compression models. Eur. J. Pharm. Sci. 2000, 11, 307–315. [Google Scholar] [CrossRef]
- Gunister, E.; Pestreli, D.; Unln, C.H.; Atıcı, O.; Gung, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carb. Poly. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Y.; Szeto, Y.S.; Liao, L. A novel method to prepare chitosan/montmorillonite nanocomposites in the presence of hydroxy-aluminum oligomeric cations. Composites Sci. Tech. 2008, 68, 2917–2921. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mat. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Faruk, O.; Matuana, L.M. Nanoclay reinforced HDPE as a matrix for wood-plastic composites. Composites Sci. Tech. 2008, 68, 2073–2077. [Google Scholar] [CrossRef]
- Celis, R.; Adelino, M.A.; Hermosin, M.C.; Cornejo, J. Montmorillonite–Chitosan bionanocomposites as adsorbents of the herbicide clopyralid in aqueous solution and soil/water suspensions. J. Hazard. Mat. 2012, 209–210, 67–76. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (mg) | CS/MMT 11 | CS/MMT 41 | CS/MMT 14 |
|---|---|---|---|
| Acelofenac | 100 | 100 | 100 |
| Lactose | 70 | 70 | 70 |
| CS/MMT polymer composite | 25 | 25 | 25 |
| PVP K 30 | 25 | 25 | 25 |
| Talc | 2.5 | 2.5 | 2.5 |
| Magnesium stearate | 2.5 | 2.5 | 2.5 |
| Total Weight (mg) | 225 | 225 | 225 |
| S. No. | Parameter | CS/MMT 11 | CS/MMT 41 | CS/MMT 14 |
|---|---|---|---|---|
| 1 | Bulk density (g/cm3) | 0.54 ± 0.09 | 0.42 ± 0.11 | 0.47 ± 0.12 |
| 2 | Tapped density (g/cm3) | 0.62 ± 0.05 | 0.47 ± 0.08 | 0.52 ± 0.10 |
| 3 | Carr’s index (%) | 12.90 ± 0.42 | 10.63 ± 0.58 | 9.61 ± 0.72 |
| 4 | Hausner ratio | 1.15 ± 0.11 | 1.12 ± 0.08 | 1.11 ± 0.15 |
| 5 | Angle of repose (°) | 29.45 ± 0.89 | 31.14 ± 0.07 | 27.21 ± 0.98 |
| 6 | Swelling index (%) | 83.3 | 95 | 78.57 |
| 7 | pH | 5.1 | 4.9 | 5.2 |
| 8 | LOD (%) | 12.5 ± 0.56 | 16.5 ± 1.02 | 4.5 ± 0.34 |
| 9 | Effective pore radius (µm) | 12.8 ± 0.88 | 14.84 ± 0.95 | 11.37 ± 1.02 |
| Samples | Heckel Analysis | Kawakita Analysis | ||||
|---|---|---|---|---|---|---|
| D0 | DA | DB | Py | DI | Pk | |
| CS/MMT 11 | 0.28 | 0.40 | 0.21 | 83.3 | 0.33 | 14.5 |
| CS/MMT 41 | 0.29 | 0.52 | 0.23 | 90.9 | 0.30 | 5.6 |
| CS/MMT 14 | 0.31 | 0.54 | 0.23 | 100 | 0.31 | 6.4 |
| Formulation Code | Diameter (mm) | Thickness (mm) | Friability (%) | Hardness (kg/cm2) | Tensile Strength (mN/cm2) | Drug Content (%) |
|---|---|---|---|---|---|---|
| CS/MMT 11 | 6.73 ± 0.04 | 3.81 ± 0.04 | 0.71 ± 0.10 | 4.0 ± 0.11 | 0.60 ± 0.05 | 98.89 ± 0.82 |
| CS/MMT 41 | 6.73 ± 0.04 | 3.75 ± 0.02 | 0.85 ± 0.12 | 4.5 ± 0.18 | 0.47 ± 0.09 | 99.12 ± 0.66 |
| CS/MMT 14 | 6.74 ± 0.02 | 3.75 ± 0.04 | 0.91 ± 0.08 | 4.5 ± 0.27 | 0.73 ± 0.08 | 98.87 ± 0.91 |
| Batch No. | Zero Order | First Order | Higuchi | Korsmeyer-Peppas | Hixon Crowell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R² | k0 | R² | k1 | R² | kH | R² | kKP | n | R² | kHC | |
| CS/MMT 11 | 0.9694 | 0.1548 | 0.9348 | −0.0004 | 0.9719 | 3.9467 | 0.9859 | 0.2938 | 0.5946 | 0.9652 | −0.0038 |
| CS/MMT 41 | 0.9662 | 0.1484 | 0.9871 | −0.0012 | 0.9914 | 3.9577 | 0.9899 | 0.244 | 0.790 | 0.9953 | −0.0033 |
| CS/MMT 14 | 0.9629 | 0.1403 | 0.9865 | −0.001 | 0.9878 | 3.8081 | 0.9787 | 0.6316 | 0.827 | 0.9946 | 0.003 |
| Batch | Time Interval (months) | Test Parameters | ||
|---|---|---|---|---|
| Hardness (kg/cm2) | Friability (%) | Drug Content (%) | ||
| CS/MMT 11 | 0 | 3.56 ± 0.08 | 0.73 ± 0.05 | 99.1 ± 0.15 |
| 1 | 3.51 ± 0.15 | 0.79 ± 0.09 | 98.7 ± 0.20 | |
| 2 | 3.47 ± 0.10 | 0.79 ± 0.07 | 98.1 ± 0.56 | |
| 3 | 3.41 ± 0.20 | 0.82 ± 0.03 | 97.8 ± 0.15 | |
| CS/MMT 41 | 0 | 3.80 ± 0.15 | 0.54 ± 0.05 | 98.92 ± 0.70 |
| 1 | 3.71 ± 0.80 | 0.59 ± 0.06 | 98.67 ± 0.35 | |
| 2 | 3.65 ± 0.27 | 0.63 ± 0.02 | 98.1 ± 0.59 | |
| 3 | 3.50 ± 0.41 | 0.63 ± 0.07 | 97.34 ± 0.65 | |
| CS/MMT 14 | 0 | 4.25 ± 0.20 | 0.42 ± 0.02 | 98.99 ± 0.20 |
| 1 | 4.11 ± 0.17 | 0.44 ± 0.04 | 98.61 ± 0.19 | |
| 2 | 4.04 ± 0.31 | 0.47 ± 0.09 | 98.28 ± 0.35 | |
| 3 | 3.98 ± 0.24 | 0.49 ± 0.01 | 97.91 ± 0.55 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, G.; Singh, A.; Singh, I. Chitosan-Montmorillonite Polymer Composites: Formulation and Evaluation of Sustained Release Tablets of Aceclofenac. Sci. Pharm. 2016, 84, 603-617. https://doi.org/10.3390/scipharm84040603
Thakur G, Singh A, Singh I. Chitosan-Montmorillonite Polymer Composites: Formulation and Evaluation of Sustained Release Tablets of Aceclofenac. Scientia Pharmaceutica. 2016; 84(4):603-617. https://doi.org/10.3390/scipharm84040603
Chicago/Turabian StyleThakur, Garima, Amrinder Singh, and Inderbir Singh. 2016. "Chitosan-Montmorillonite Polymer Composites: Formulation and Evaluation of Sustained Release Tablets of Aceclofenac" Scientia Pharmaceutica 84, no. 4: 603-617. https://doi.org/10.3390/scipharm84040603
APA StyleThakur, G., Singh, A., & Singh, I. (2016). Chitosan-Montmorillonite Polymer Composites: Formulation and Evaluation of Sustained Release Tablets of Aceclofenac. Scientia Pharmaceutica, 84(4), 603-617. https://doi.org/10.3390/scipharm84040603




