Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models
Abstract
:1. Introduction
2. Results
2.1. The Effect of BEY on SOX5 Regulation in MOG-Induced Splenocytes
2.2. Upregulation of the Epigenetic Factors (miRNA218-5p) by BEY Treatment
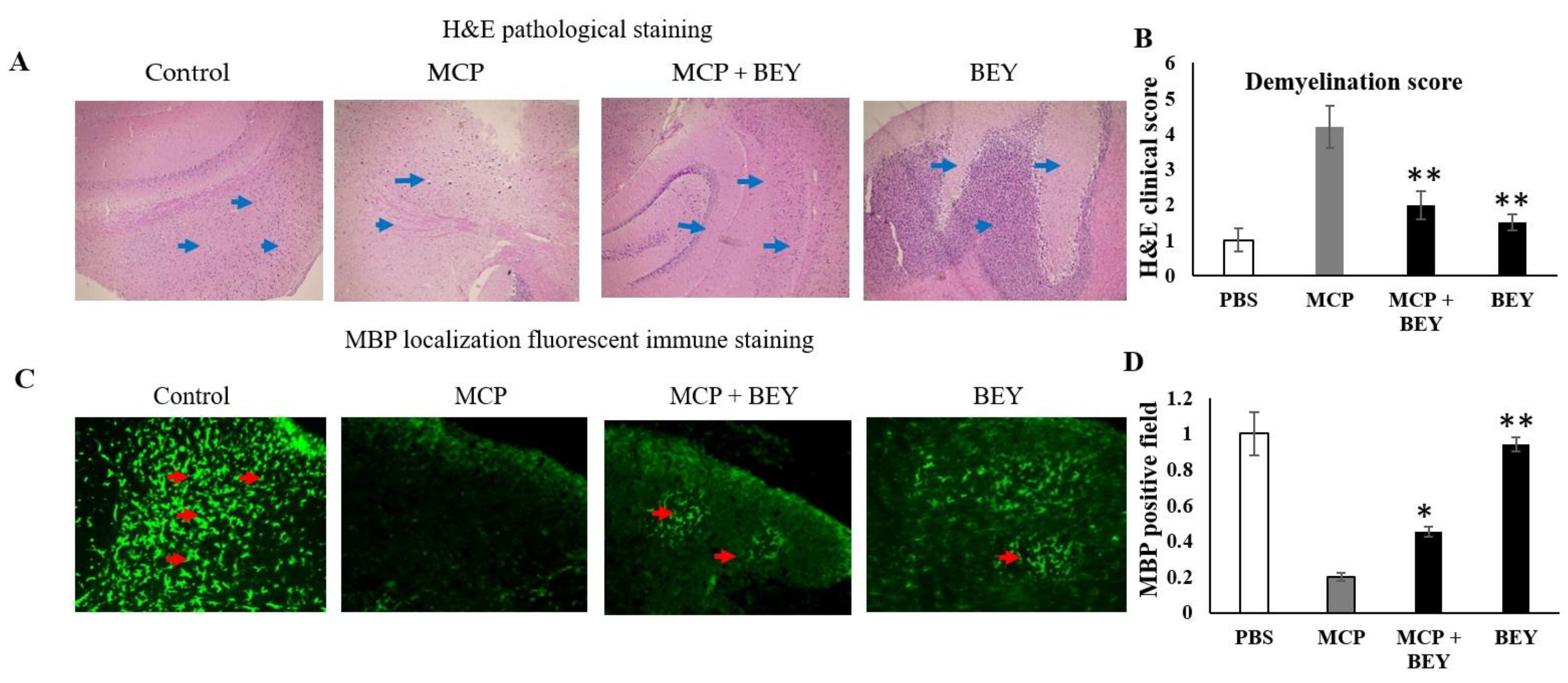
2.3. Pathology of EAE C57BL6j Mice Treated with BEY
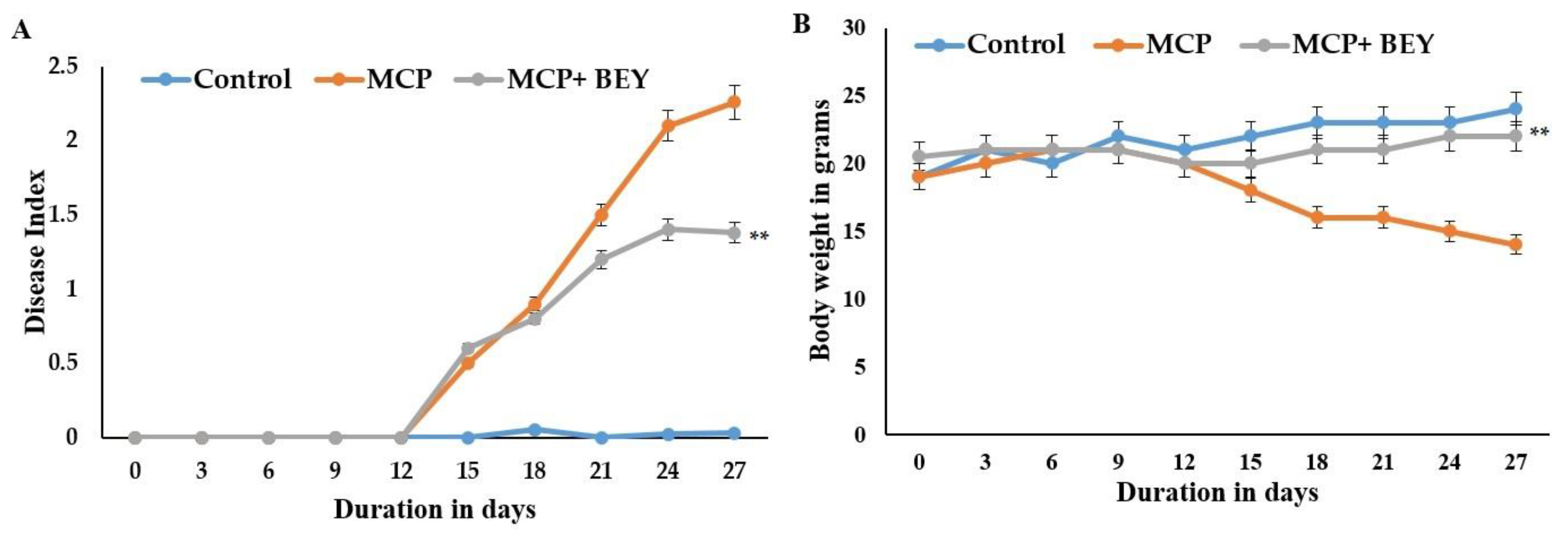
2.4. The Positive Effect of BEY on EAE Pathology in C57Bl6j Mice
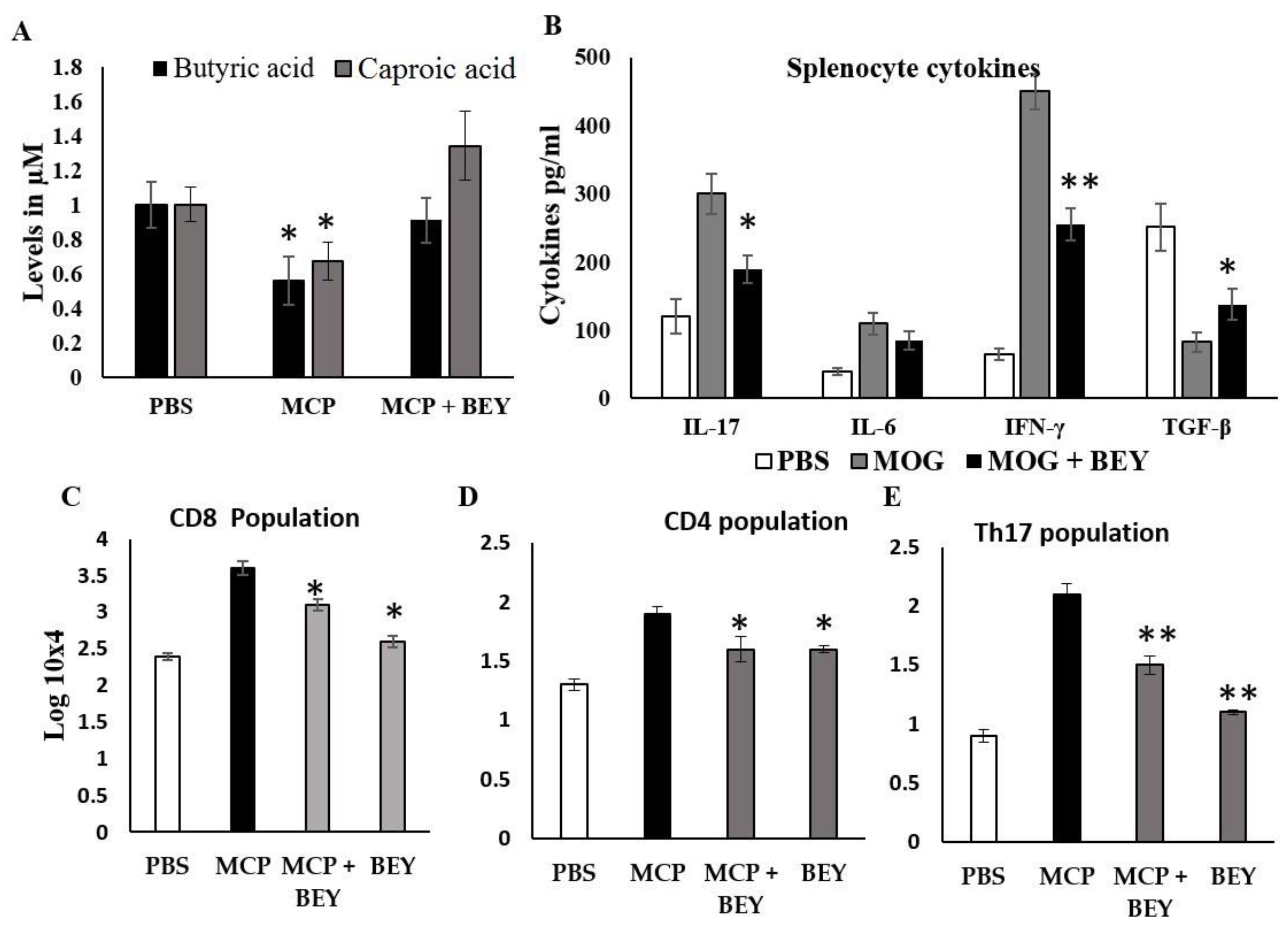
2.5. Effect of BEY on Biochemical and Inflammatory Markers in MCP-Induced EAE Mice
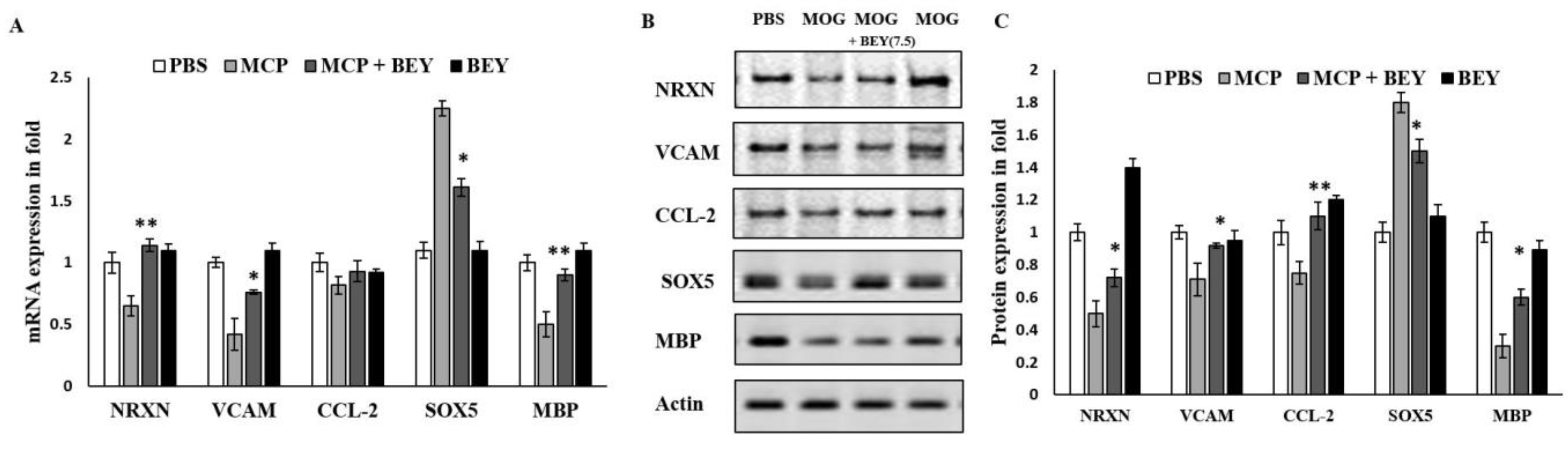
2.6. BEY Alleviates EAE Symptoms via the Regulation of the Transcriptional Factors
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. BA Growth Conditions and Yogurt Preparation
4.3. In Vitro Stimulation of Splenocytes Using MOG35–55
4.4. Quantification of Secreted Cytokines by ELISA
4.5. EAE Induction and Clinical Evaluation of Experimental Mice
4.6. Histological Analysis
4.7. Different Inflammatory Markers Assessed in the Spinal Cord
4.8. Quantification of SCFAs
4.9. RNA Extraction and Evaluation of Quantitative PCR
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Bodke, H.; Jogdand, S. Role of Probiotics in Human Health. Cureus 2022, 14, e31313. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xing, W.; Wei, S.; Gao, Q.; Wei, X.; Shi, L.; Kong, Y.; Su, Z. Semi-rational screening of probiotics from the fecal flora of healthy adults against dss-induced colitis mice by enhancing anti-inflammatory activity and modulating the gut microbiota. J. Microbiol. Biotechnol. 2019, 29, 1478–1487. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Fernández-Caballero, J.Á.; Chueca, N.; García, F.; Gómez-Llorente, C.; Sáez-Lara, M.J.; Fontana, L.; Gil, Á. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999–4015. [Google Scholar] [CrossRef]
- Jiang, J.; Chu, C.; Wu, C.; Wang, C.; Zhang, C.; Li, T.; Zhai, Q.; Yu, L.; Tian, F.; Chen, W. Efficacy of probiotics in multiple sclerosis: A systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct. 2021, 12, 2354–2377. [Google Scholar] [CrossRef]
- Alfonsetti, M.; Castelli, V.; D’angelo, M. Are we what we eat? impact of diet on the gut–brain axis in Parkinson’s disease. Nutrients 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A Systematic Review. J. Neuroinflamm. 2019, 16, 231. [Google Scholar] [CrossRef]
- Salehipour, Z.; Haghmorad, D.; Sankian, M.; Rastin, M.; Nosratabadi, R.; Soltan Dallal, M.M.; Tabasi, N.; Khazaee, M.; Nasiraii, L.R.; Mahmoudi, M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering cd4+ t cell subset balance. Biomed. Pharmacother. 2017, 95, 1535–1548. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A Meta Analysis and Systematic Review. J. Cell Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Hosseinifard, E.S.; Morshedi, M.; Bavafa-Valenlia, K.; Saghafi-Asl, M. The novel insight into anti-inflammatory and anxiolytic effects of psychobiotics in diabetic rats: Possible link between gut microbiota and brain regions. Eur. J. Nutr. 2019, 58, 3361–3375. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Khalifa, A.; Sheikh, A.; Ibrahim, H.I.M. Bacillus amyloliquefaciens enriched camel milk attenuated colitis symptoms in mice model. Nutrients 2022, 14, 1967. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Khaskheli, G.B.; Shah, A.H.; Marghazani, I.B.; Barham, G.S.; Shah, Q.A.; Khand, F.M.; Buzdar, J.A.; Soomro, F.; Fazlani, S.A. Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in human and animals: A comprehensive review. Anim. Biotechnol. 2022, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Eid, A.H.; Omar, H.A.; Arafa, E.S.A.; Maghrabi, I.A. Camel’s milk ameliorates tnbs-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2014, 69, 294–302. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Awad, S. Milk bioactive peptides: Antioxidant, antimicrobial and anti-diabetic activities. Adv. Biochem. 2019, 7, 22–33. [Google Scholar] [CrossRef]
- Rahimlou, M.; Nematollahi, S.; Husain, D.; Banaei-Jahromi, N.; Majdinasab, N.; Hosseini, S.A. Corrigendum: Probiotic supplementation and systemic inflammation in relapsing-remitting multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Front. Neurosci. 2022, 16, 1085572. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Lemus, H.N.; Warrington, A.E.; Rodriguez, M. Multiple sclerosis: Mechanisms of disease and strategies for myelin and axonal repair. Neurol. Clin. 2018, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Kantarci, O.H. Progressive forms of multiple sclerosis: Distinct entity or age-dependent phenomena. Neurol. Clin. 2018, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Chang, A.; Fox, R.J.; Tkach, J.A.; Svarovsky, T.; Nakamura, K.; Rudick, R.A.; Trapp, B.D. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann. Neurol. 2007, 62, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Gou, H.-Z.; Zhang, Y.-L.; Song, X.-J.; Zhang, L. Role of intestinal flora in primary sclerosing cholangitis and its potential therapeutic value. World J. Gastroenterol. 2022, 28, 6213. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against cns demyelinating disease. Mucosal. Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; Yang, C.; Song, G.H.; Wong, J.; Ho, K.Y. Melatonin regulation as a possible mechanism for probiotic (vsl#3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig. Dis. Sci. 2015, 60, 170–178. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J. Dietary modulation of intestinal microbiota: Future opportunities in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Microbiol. 2019, 10, 740. [Google Scholar] [CrossRef]
- Erny, D.; de Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.N.; Shahi, S.K.; Mangalam, A.K. The “Gut Feeling”: Breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 2018, 15, 109–125. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Wang, B.; Zhang, F.; Shao, Y. Influence of bactrian camel milk on the gut microbiota. J. Dairy Sci. 2018, 101, 5758–5769. [Google Scholar] [CrossRef]
- Naghavian, R.; Ghaedi, K.; Kiani-Esfahani, A.; Ganjalikhani-Hakemi, M.; Etemadifar, M.; Nasr-Esfahani, M.H. MiR-141 and MiR-200a, Revelation of new possible players in modulation of th17/treg differentiation and pathogenesis of multiple sclerosis. PLoS ONE 2015, 10, e0124555. [Google Scholar] [CrossRef]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse microbiota models: Comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect. Med. 2018, 8, a029017. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Valan Arasu, M.; Vijayaraghavan, P.; Esmail, G.A.; Duraipandiyan, V.; Kim, Y.O.; Kim, H.; Kim, H.-J. Probiotic and antioxidant potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms 2020, 8, 1461. [Google Scholar] [CrossRef]
- Saravanan, S.; Hairul Islam, V.I.; David, H.A.; Lakshmi Sundaram, R.; Chellappandian, M.; Balakrishna, K.; Rajendran, R.; Vijayaraghavan, P.; Gabriel Paulraj, M.; Ignacimuthu, S. Bioassay guided fractionation and identification of active anti-inflammatory constituent from Delonix elata flowers using RAW 264.7 cells. Pharm Biol. 2015, 53, 174–184. [Google Scholar] [CrossRef] [PubMed]
- de Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated players in the pathogenesis of multiple sclerosis (Ms). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef]
- Peters, A.; Pitcher, L.A.; Sullivan, J.M.; Mitsdoerffer, M.; Acton, S.E.; Franz, B.; Wucherpfennig, K.; Turley, S.; Carroll, M.C.; Sobel, R.A.; et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011, 35, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Campos, A.; Alaminos, M.; Raimondo, S.; Geuna, S. Staining methods for normal and regenerative myelin in the nervous system. In Histochemistry of Single Molecules; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1560. [Google Scholar]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Ibrahim, H.I.M. A molecular insight into the synergistic mechanism of nigella sativa (black cumin) with β-lactam antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. Appl. Sci. 2021, 11, 3206. [Google Scholar] [CrossRef]
- Kaliyamoorthy, V.; Jacop, J.P.; Thirugnanasambantham, K.; Ibrahim, H.I.M.; Kandhasamy, S. The synergic impact of lignin and Lactobacillus Plantarum on DSS-induced colitis model via regulating CD44 and MiR 199a alliance. World J. Microbiol. Biotechnol. 2022, 38, 233. [Google Scholar] [CrossRef]
- Simpson, M.R.; Brede, G.; Johansen, J.; Johnsen, R.; Storrø, O.; Sætrom, P.; Øien, T. Human Breast Milk MiRNA, Maternal Probiotic Supplementation and Atopic Dermatitis in Offspring. PLoS ONE 2015, 10, e0143496. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, K.M.; Hassan, M.F.Y.; Al-Qaba, S.F. Protective effect of fermented camel milk containing Bifidobacterium longum BB536 on blood lipid profile in hypercholesterolemic rats. J. Nutr. Metab. 2021, 2021, 1557945. [Google Scholar] [CrossRef] [PubMed]
- Suto, A.; Tanaka, S.; Nakajima, H. Sox5 and Th17 cell differentiation. Oncotarget 2015, 6, 19952–19953. [Google Scholar] [CrossRef]
- Cui, X.; Ye, Z.; Wang, D.; Yang, Y.; Jiao, C.; Ma, J.; Tang, N.; Zhang, H. Aryl hydrocarbon receptor activation ameliorates experimental colitis by modulating the tolerogenic dendritic and regulatory t cell formation. Cell Biosci. 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, W.J.; Laman, J.D.; ’t Hart, B.A. Modulation of multiple sclerosis and its animal model experimental autoimmune encephalomyelitis by food and gut microbiota. Front. Immunol. 2017, 8, 1081. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Saluk, J. Probiotics and commensal gut microbiota as the effective alternative therapy for multiple sclerosis patients treatment. Int. J. Mol. Sci. 2022, 23, 14478. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Mowry, E.M. Emerging concepts on the gut microbiome and multiple sclerosis. J. Interferon Cytokine Res. 2016, 36, 347–357. [Google Scholar] [CrossRef]
- Salvi, V.; Gianello, V.; Tiberio, L.; Sozzani, S.; Bosisio, D. Cytokine Targeting by MiRNAs in Autoimmune Diseases. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Pashangzadeh, S.; Motallebnezhad, M.; Vafashoar, F.; Khalvandi, A.; Mojtabavi, N. Implications the Role of MiR-155 in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 669382. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A Randomized, Double-Blind, Controlled Trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- He, J.; Guo, K.; Chen, Q.; Wang, Y. Jirimutu Camel milk modulates the gut microbiota and has anti-inflammatory effects in a mouse model of colitis. J. Dairy Sci. 2022, 105, 3782–3793. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Eixarch, H.; Ponce-Alonso, M.; Castillo, M.; Lebrón-Galán, R.; Mestre, L.; Guaza, C.; Clemente, D.; del Campo, R.; Montalban, X.; et al. A commercial probiotic induces tolerogenic and reduces pathogenic responses in experimental autoimmune encephalomyelitis. Cells 2020, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- DeMaio, A.; Mehrotra, S.; Sambamurti, K.; Husain, S. The role of the adaptive immune system and T cell dysfunction in neurodegenerative Diseases. J. Neuroinflammation 2022, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Axisa, P.-P.; Yoshida, T.M.; Lucca, L.E.; Kasler, H.G.; Lincoln, M.R.; Pham, G.H.; del Priore, D.; Carpier, J.-M.; Lucas, C.L.; Verdin, E.; et al. A multiple sclerosis–protective coding variant reveals an essential role for HDAC7 in regulatory T cells. Sci. Transl. Med. 2022, 14, eabl3651. [Google Scholar] [CrossRef]
- Pokhrel, R.H.; Kang, B.; Timilshina, M.; Chang, J.-H. AMPK Amplifies IL2–STAT5 signaling to maintain stability of regulatory t cells in aged mice. Int. J. Mol. Sci. 2022, 23, 12384. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M. Multiple sclerosis: New insights and trends. Asian Pac. J. Trop. Biomed. 2016, 6, 429–440. [Google Scholar] [CrossRef]
- Evans, E.; Piccio, L.; Cross, A.H. Use of vitamins and dietary supplements by patients with multiple sclerosis a review. JAMA Neurol. 2018, 75, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.M.; Goncalves, B.D.C.; Gomez, M.V.; Vieira, L.B.; Ribeiro, F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Umbrello, G.; Esposito, S. Microbiota and neurologic diseases: Potential effects of probiotics. J. Transl. Med. 2016, 14, 298. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]



| Name of the Primer | Forward Primer | Reverse Primer | PCR Product Size (bp) |
|---|---|---|---|
| MBP | ATTCACCGAGGAGAGGCTGGAA | TGTGTGCTTGGAGTCTGTCACC | 245 |
| CCL2 | GCTACAAGAGGATCACCAGCAG | GTCTGGACCCATTCCTTCTTGG | 122 |
| SOX5 | CGCCAGATGAAAGAGCAACTCAG | TGAGTCAGGCTCTCCAGTGTTG | 147 |
| NRXN | AGGACATTGACCCCTGTGAG | CCTTCATCCCGGTTTCTGTA | 241 |
| VCAM | TGA CGA TG CGT GTG CCA GT | GCT GTC GGT TCC CAT TGT CT | 228 |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA | 188 |
| miR218-5p | CGAGTGCATTTGTGCTTG ATCTA | TGGTGTCGTGGAGTCG | 89 |
| U6 | CTC GCTTCGGCAGCACA′ | AACGCTTCACGAATT TGCGT- | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.; Ibrahim, H.I.M.; Sheikh, A.; Khalil, H.E. Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models. Pharmaceuticals 2023, 16, 357. https://doi.org/10.3390/ph16030357
Khalifa A, Ibrahim HIM, Sheikh A, Khalil HE. Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models. Pharmaceuticals. 2023; 16(3):357. https://doi.org/10.3390/ph16030357
Chicago/Turabian StyleKhalifa, Ashraf, Hairul Islam Mohamed Ibrahim, Abdullah Sheikh, and Hany Ezzat Khalil. 2023. "Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models" Pharmaceuticals 16, no. 3: 357. https://doi.org/10.3390/ph16030357
APA StyleKhalifa, A., Ibrahim, H. I. M., Sheikh, A., & Khalil, H. E. (2023). Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models. Pharmaceuticals, 16(3), 357. https://doi.org/10.3390/ph16030357







