New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenolic Content (TPC), Total Flavonoidic Content (TFC), and Total Caffeic Acid Derivatives Content (TCADC)
2.2. HPLC-UV-MS Analysis
2.3. Antioxidant Activity
2.4. Pharmacological Studies
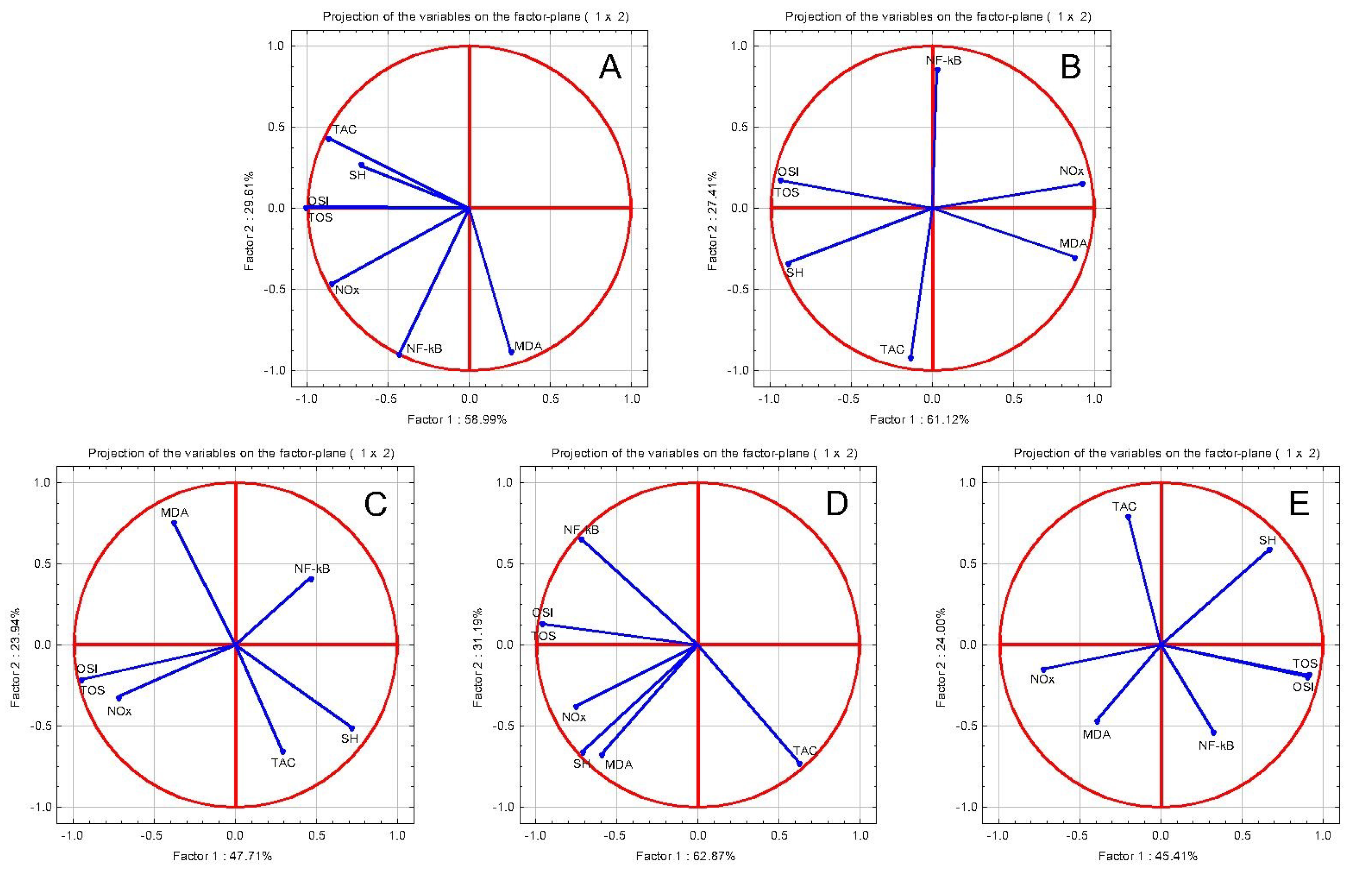
2.4.1. The Evaluation of In Vivo Anti-Inflammatory Effects
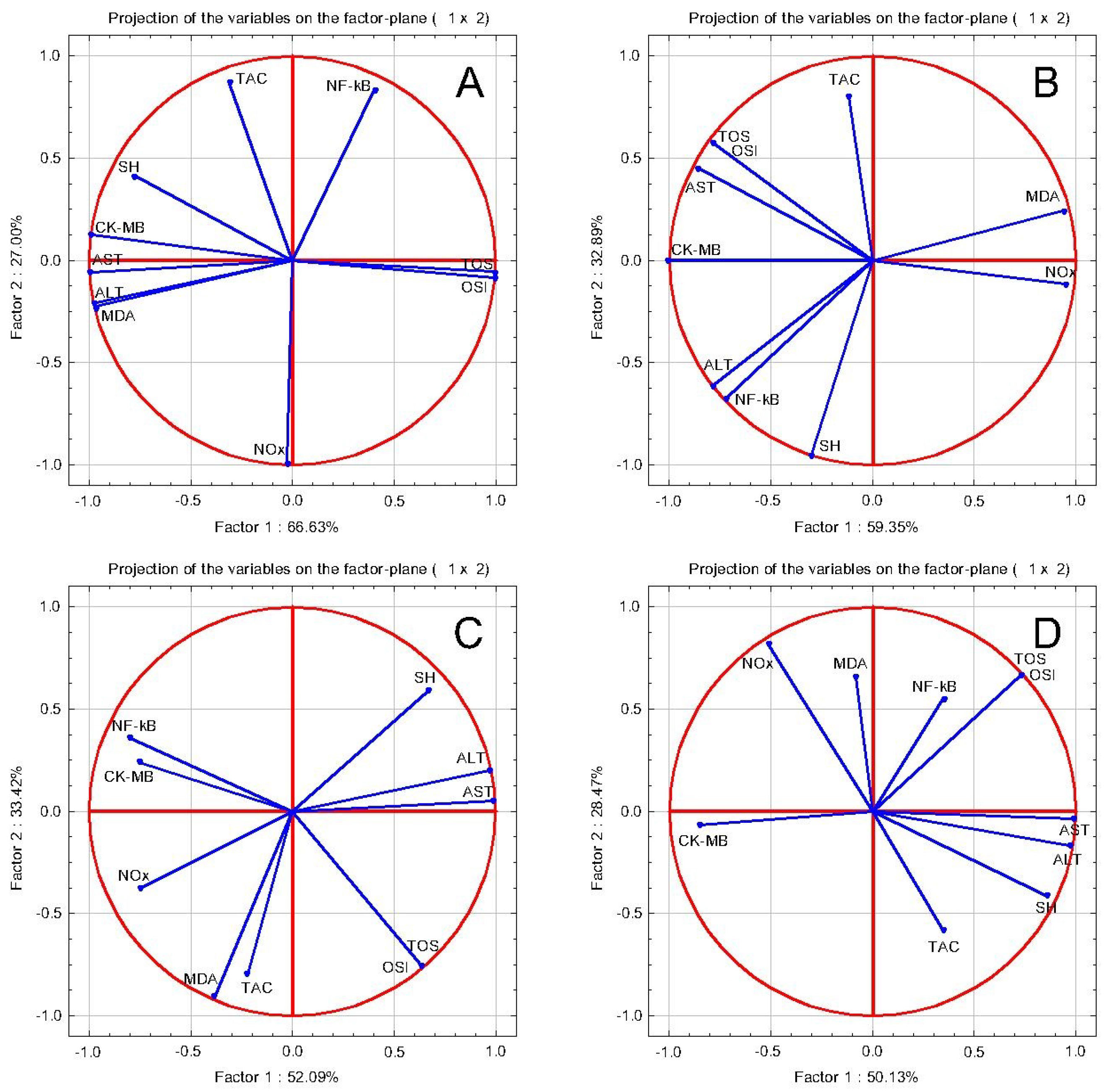
2.4.2. The Evaluation of In Vivo Cardioprotective Effects
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Extraction Procedure
3.3. Total Polyhenols Content Determination
3.4. Total Flavonoids Content Determination
3.5. Total Caffeic Acid Derivates Content Determination
3.6. Evaluation of the In Vitro Antioxidant Capacity
3.6.1. DPPH Radical Scavenging Activity
3.6.2. Ferric-Reducing Antioxidant Power Assay
3.7. HPLC-UV-MS Separation
3.8. Pharmacological Evaluation
3.8.1. Experimental Animals
3.8.2. Protocols
The Evaluation of In Vivo Anti-Inflammatory Effects
The Evaluation of In Vivo Cardioprotective Effects
The Evaluation of Oxidative Stress Parameters
3.8.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.A.; Hussain, M.; Hussain, N.; Alqahtani, A.M.; Alqahtani, T. Cardioprotective Effect of Rumex vesicarius Linn. Leaf Extract against Catecholamine-Induced Cardiotoxicity. Molecules 2022, 27, 3383. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic Fractions from Dandelion Leaves and Petals as Modulators of the Antioxidant Status and Lipid Profile in an In Vivo Study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef]
- Wang, T.; Wu, S.; Ibrahim, I.A.A.; Fan, L. Cardioprotective Role of Swertiamarin, a Plant Glycoside Against Experimentally Induced Myocardial Infarction via Antioxidant and Anti-inflammatory Functions. Appl. Biochem. Biotechnol. 2022, 194, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Malhotra, S.; Subban, R. Dandelion (Taraxacum officinale)—Hepatoprotective Herb with Therapeutic Potential. Pharmacogn. Rev. 2008, 2, 163–167. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. Int. J. Funct. Nutr. 2004, 134 (Suppl. S12), 3479–3485. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Moldoch, J.; Stochmal, A.; Olas, B. The anti-oxidative and hemostasis-related multifunctionality of L-chicoric acid, the main component of dandelion: An in vitro study of its cellular safety, antioxidant and anti-platelet properties, and effect on coagulation. J. Funct. Foods 2019, 62, 103524. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. J. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Olas, B. New Perspectives on the Effect of Dandelion, Its Food Products and Other Preparations on the Cardiovascular System and Its Diseases. Nutrients 2022, 14, 1350. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.-J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.; Vlase, L.; Benedec, D.; Hanganu, D.; Vlase, A.M.; Oniga, I. Polyphenolic compounds, antioxidant activity and nephroprotective properties of Romanian Taraxacum officinale. Farmacia 2022, 70, 47–53. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid as a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef]
- Ivanov, I. Polyphenols Content and Antioxidant Activities of Taraxacum officinale F.H. Wigg (Dandelion) Leaves. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 889–893. [Google Scholar]
- Nowak, A.; Duchnik, W.; Zielonka-Brzezicka, J.; Muzykiewicz, A.; Florkowska, K.; Klimowicz, A.; Kucharski, Ł.; Wysocka, D.; Dziedzic, A. The antioxidant activity of ethanolic and aqueous extracts of dandelion (Taraxacum officinale L.). Pomeranian J. Life Sci. 2019, 65, 83–88. [Google Scholar] [CrossRef]
- Pavel, I.; Parvu, A.E.; Dehelean, C.; Vlase, L.; Csuk, R.; Muntean, D. Assessment of the antioxidant effect of a maslinic acid derivative in an experimental model of acute inflammation. Farmacia 2017, 65, 390–395. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Kim, S.J.; Kim, H.S. Anti-inflammatory evaluation of the methanolic extract of Taraxacum officinale in LPS-stimulated human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2017, 17, 508. [Google Scholar] [CrossRef]
- Seo, S.W.; Koo, H.N.; An, H.J.; Kwon, K.B.; Lim, B.C.; Seo, E.A.; Ryu, D.G.; Moon, G.; Kim, H.Y.; Kim, H.M.; et al. Taraxacum officinale protects against cholecystokinin-induced acute pancreatitis in rats. World J. Gastroenterol. 2005, 11, 597–599. [Google Scholar] [CrossRef]
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007, 101, 74–81. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic Compounds, Antioxidant, and Cardioprotective Effects of Pomace Extracts from Fetească Neagră Cultivar. Oxidative Med. Cell. Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef]
- Wu, H.; Luo, D.; Li, C.; Zhang, H.; Shunxian, A.; Zhang, Y.; Sun, C. Chicoric Acid Improves Heart and Blood Responses to Hypobaric Hypoxia in Tibetan Yaks. Am. J. Chin. Med. 2018, 46, 339–355. [Google Scholar] [CrossRef]
- Tsai, K.L.; Kao, C.L.; Hung, C.H.; Cheng, Y.H.; Lin, H.C.; Chu, P.M. Chicoric acid is a potent anti-atherosclerotic ingredient by anti-oxidant action and anti-inflammation capacity. Oncotarget 2017, 8, 29600–29612. [Google Scholar] [CrossRef]
- Baniahmad, B.; Safaeian, L.; Vaseghi, G.; Rabbani, M.; Mohammadi, B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res. Pharm. Sci. 2020, 15, 87–96. [Google Scholar] [CrossRef]
- Manjunatha, S.; Shaik, A.H.; Maruthi Prasad, E.; Al Omar, S.Y.; Mohammad, A.; Kodidhela, L.D. Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci. Rep. 2020, 10, 3426. [Google Scholar] [CrossRef]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. J. Evid. Based Complement. Altern. Med. 2020, 2020, 5926381. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.-M.; Toma, V.A.; Oniga, I. Phytochemical Profile, Antioxidant, Cardioprotective and Nephroprotective Activity of Romanian Chicory Extract. Plants 2021, 10, 64. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.M.; Moldovan, C.; Oniga, I. Phytochemical Composition, Antioxidant, Antimicrobial and in Vivo Anti-inflammatory Activity of Traditionally Used Romanian Ajuga laxmannii (Murray) Benth. (“Nobleman’s Beard”—Barba Împăratului). Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ștefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic compounds and biological effects of edible Rumex scutatus and Pseudosempervivum sempervivum: Potential sources of natural agents with health benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Lorena, F.; Oniga, I.; Brindusa, T.; Olah, N.-K.; Gheldiu, A.-M.; Raita, O.; Vlase, L. Chemical, antioxidant and antibacterial studies of Romanian Heracleum sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In Vitro Evaluation of Biological Activities of Canes and Pomace Extracts from Several Varieties of Vitis vinifera L. for Inclusion in Freeze-Drying Mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Mocan, A.; Hanganu, D.; Benedec, D.; Gheldiu, A.-M.; Crişan, G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Dig. J. Nanomater. Biostructures 2014, 9, 1085–1094. [Google Scholar]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef]
- Keul, A.; Vlase, L.; Crăciunaş, C. Clonal propagation and production of cichoric acid in three species of Echinaceae. In Vitro Cell. Dev. Biol. Plant 2012, 48, 249–258. [Google Scholar] [CrossRef]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J. Physiol. Pharmacol. 2014, 65, 309–315. [Google Scholar]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Balea, Ş.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Andreicuț, A.; Pârvu, M. Phytochemical profiling, antioxidant and cardioprotective properties of pinot noir cultivar pomace extracts. Farmacia 2018, 66, 432–441. [Google Scholar] [CrossRef]
- Sarac, F.; Yeniocak, S.; Erbin, A.; Yucetas, E.; Altundal, K.; Ucpinar, B.; Saygili, A.; Koldas, M. Ischemia Modified Albumin and D-dimer in the Diagnosis of Testicular Torsion: An Experimental Model. Urol. J. 2019, 16, 567–571. [Google Scholar]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- da Costa, C.M.; dos Santos, R.C.C.; Lima, S.E. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. 2006, 42, 345–350. [Google Scholar] [CrossRef]
| Extract | TPC (mg GAE/g d.w.) | TFC (mg RE/g d.w.) | TCADC (mg CAE/g d.w.) |
|---|---|---|---|
| TOT | 26.75 ± 0.73 | 6.28 ± 0.32 | 16.74 ± 0.80 |
| Polyphenols | [M-H]− | Retention Time (min) Rt ± SD | TOT (µg/g d.w.) |
|---|---|---|---|
| Protocatechuic acid | 153 | 2.80 ± 0.05 | 9.20 ± 0.09 |
| Vanillic acid | 167 | 6.70 ± 0.07 | 2.00 ± 0.02 |
| Syringic acid | 197 | 8.40 ± 0.09 | 0.90 ± 0.01 |
| Ferulic acid | 193 | 12.80 ± 0.10 | 53.60 ± 0.37 |
| Cichoric acid | 473 | 1.12 ± 0.01 * | 12,124.89 ± 76.38 |
| Rutin | 609 | 20.20 ± 0.15 | 14.51 ± 0.10 |
| Quercitrin | 447 | 23.64 ± 0.13 | 26.08 ± 0.22 |
| Luteolin | 285 | 29.10 ± 0.19 | 44.08 ± 0.30 |
| Apigenin | 269 | 33.10 ± 0.15 | 5.79 ± 0.05 |
| Sample | DPPH· EC50 (µg/mL) | FRAP (µM TE/g) |
|---|---|---|
| TOT | 165.93 ± 6.94 | 52.49 ± 1.57 |
| GROUPS | TOS (µM H2O2 E/L) | OSI | TAC (mM TE/L) | NOx (µM/L) | MDA (nM/L) | SH (mM/L) | NF-κB (ng/mL) |
|---|---|---|---|---|---|---|---|
| CONTROL | 5.13 ± 0.84 | 4.70 ± 0.77 | 1.0901 ± 0.001 | 32.67 ± 2.38 | 1.91 ± 0.19 | 0.52 ± 0.05 | 2.2 ± 0.22 |
| INFLAMM | 8.55 b ± 0.73 | 8.54 b ± 0.66 | 1.0873 ± 0.001 | 45.34 b ± 3.53 | 3.00 b ±0.21 | 0.25 b ± 0.02 | 4.17 a ± 0.99 |
| DICLOFENAC | 7.84 ± 0.35 | 7.84 ± 0.32 | 1.0870 ± 0.000 | 41.48 ± 2.11 | 2.94 ± 0.39 | 0.31 ± 0.04 | 2.41 f ± 0.32 |
| TOT 100 | 4.92 e ± 0.24 | 4.92 e ± 0.22 | 1.0871 ± 0.001 | 37.59 g ± 5.43 | 3.20 g ± 0.66 | 0.20 d ± 0.02 | 2.92 cg ± 0.60 |
| TOT 50 | 5.15 e ± 0.72 | 5.15 e ± 0.67 | 1.0878 ± 0.001 | 37.00 g ± 3.89 | 2.91 g ± 0.39 | 0.24 ± 0.02 | 3.64 ± 0.51 |
| TOT 25 | 4.63 e ± 0.30 | 4.63 e ± 0.27 | 1.0886 ± 0.000 | 30.32 d ± 7.13 | 3.06 g ± 0.72 | 0.27 g ± 0.11 | 3.52 ± 0.37 |
| GROUPS | TOS (µM H2O2 E/L) | OSI | TAC (mM TE/L) | NOx (µM/L) | MDA (nM/L) | SH (mM/L) | NF-κB (ng/mL) |
|---|---|---|---|---|---|---|---|
| CONTROL | 5.13 ± 0.84 | 4.70 ± 0.77 | 1.0901 ± 0.001 | 32.67 ± 2.38 | 1.91 ± 0.19 | 0.52 ± 0.05 | 2.2 ± 0.22 |
| ISO | 7.43 b ± 0.11 | 6.83 b ± 0.10 | 1.0876 ± 0.00 | 45.51 b ± 0.37 | 3.41 b ± 024 | 0.39 b ± 0.01 | 3.42 a ± 0.59 |
| TOT 100 | 4.50 e ± 0.12 | 4.13 e ± 0.11 | 1.0886 ± 0.00 | 36.49 ± 5.90 | 2.70 ± 0.20 | 0.26 d ± 0.02 | 2.24 ± 0.50 |
| TOT 50 | 4.40 e ±0.12 | 4.05 e ± 0.11 | 1.0873 ± 0.00 | 34.14 c ± 3.56 | 2.38 d ± 0.14 | 0.29 d ± 0.02 | 1.35 c ± 0.27 |
| TOT 25 | 4.19 e ± 0.13 | 3.85 e ± 0.12 | 1.0880 ± 0.00 | 30.55 e ± 3.28 | 3.26 ± 0.14 | 0.29 d ± 0.02 | 1.05 c ± 0.18 |
| GROUPS | AST (UI/L) | ALT (UI/L) | CK-MB (UI/L) |
|---|---|---|---|
| CONTROL | 35.32 ± 4.89 | 29.10 ± 4.12 | 7.26 ± 1.02 |
| ISO | 30.94 ± 8.35 | 40.04 a ± 7.29 | 12.11 b ± 1.08 |
| TOT 100 | 26.45 c ± 1.08 | 24.77 ± 0.55 | 8.11 ± 1.51 |
| TOT 50 | 32.52 ± 2.65 | 26.31 ± 1.22 | 7.92 c ± 1.16 |
| TOT 25 | 30.32 ± 3.53 | 26.36 ± 2.97 | 8.47 ± 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Oniga, O.; Vlase, A.-M.; Ielciu, I.; Toiu, A.; Oniga, I. New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture. Pharmaceuticals 2023, 16, 358. https://doi.org/10.3390/ph16030358
Epure A, Pârvu AE, Vlase L, Benedec D, Hanganu D, Oniga O, Vlase A-M, Ielciu I, Toiu A, Oniga I. New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture. Pharmaceuticals. 2023; 16(3):358. https://doi.org/10.3390/ph16030358
Chicago/Turabian StyleEpure, Alexandra, Alina E. Pârvu, Laurian Vlase, Daniela Benedec, Daniela Hanganu, Ovidiu Oniga, Ana-Maria Vlase, Irina Ielciu, Anca Toiu, and Ilioara Oniga. 2023. "New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture" Pharmaceuticals 16, no. 3: 358. https://doi.org/10.3390/ph16030358
APA StyleEpure, A., Pârvu, A. E., Vlase, L., Benedec, D., Hanganu, D., Oniga, O., Vlase, A.-M., Ielciu, I., Toiu, A., & Oniga, I. (2023). New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture. Pharmaceuticals, 16(3), 358. https://doi.org/10.3390/ph16030358











