Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect
Abstract
1. Introduction
2. Results
2.1. HPLC Analysis
2.2. Antiviral Activity
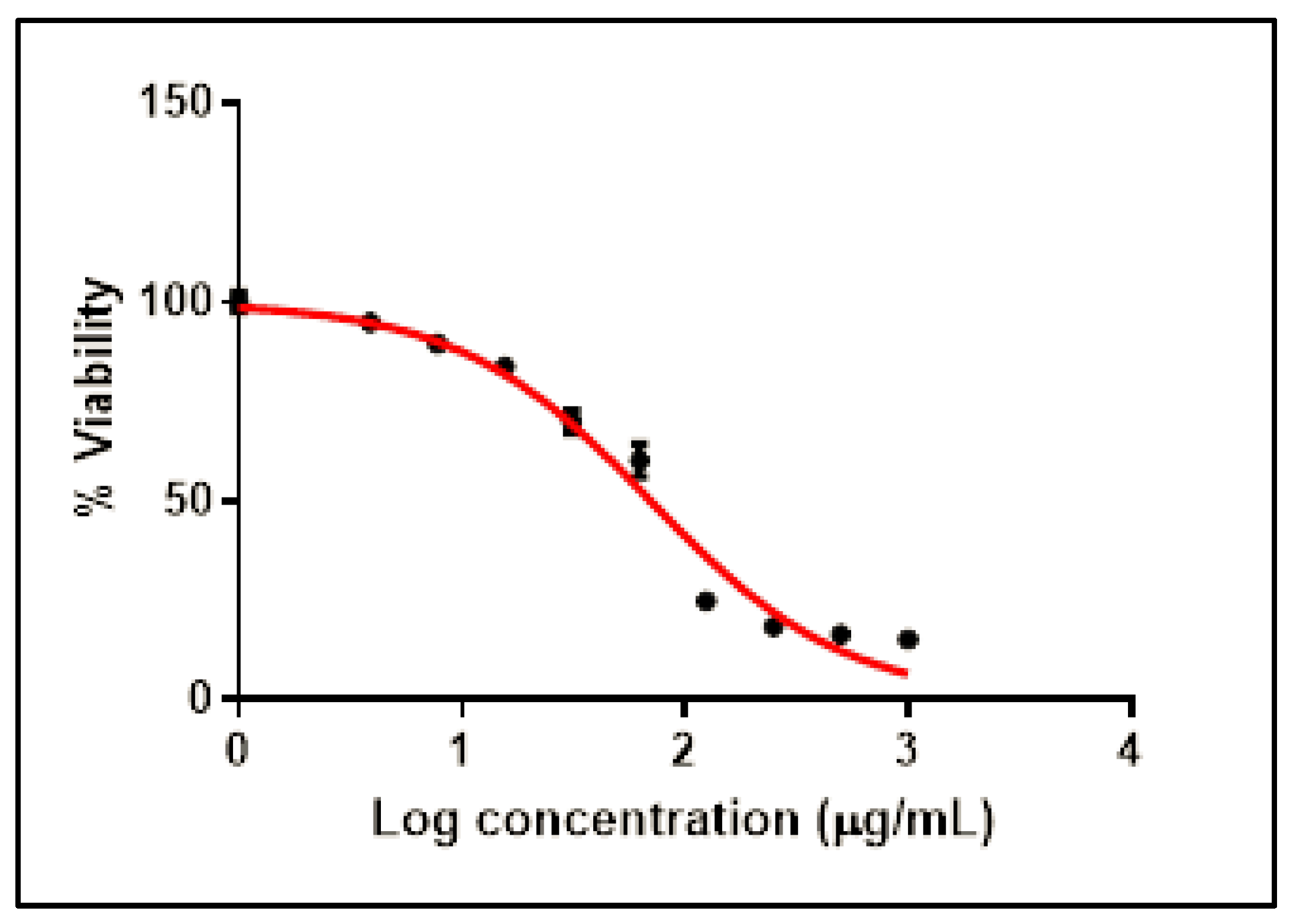
2.2.1. Cytotoxicity of SCRE on Vero-E6 Cells
2.2.2. Antiviral Activity of SCRE
2.3. In Vivo Lung Protection
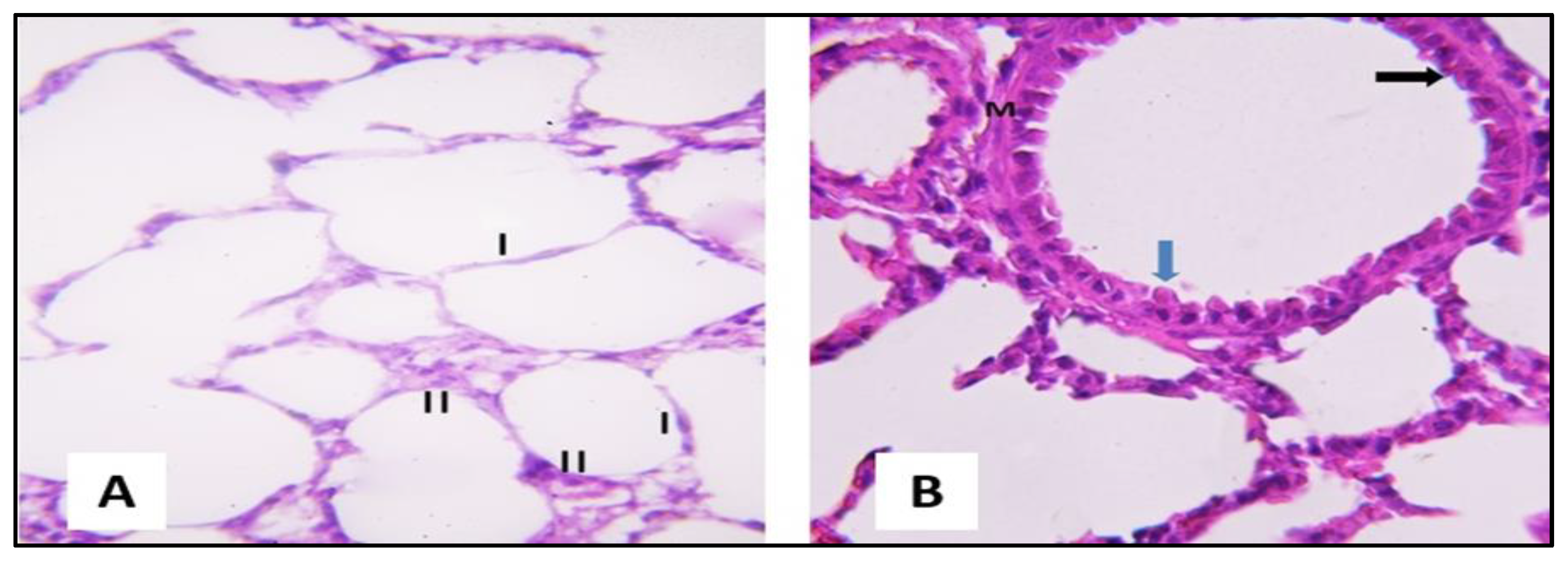
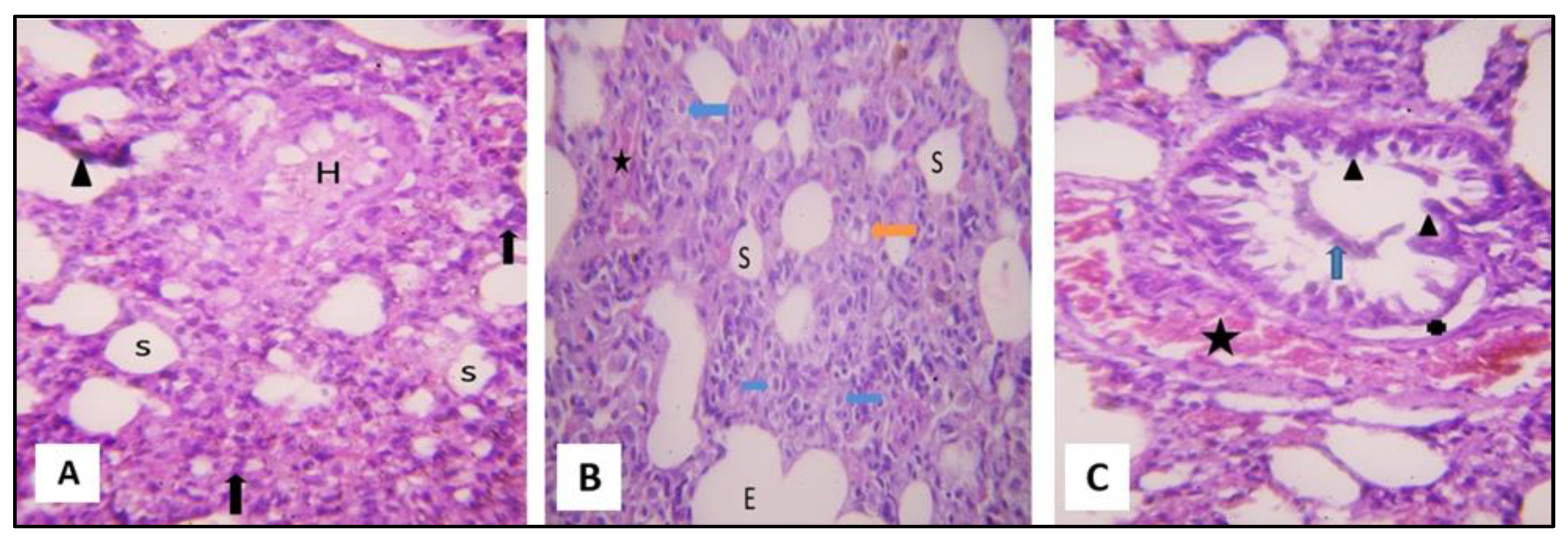
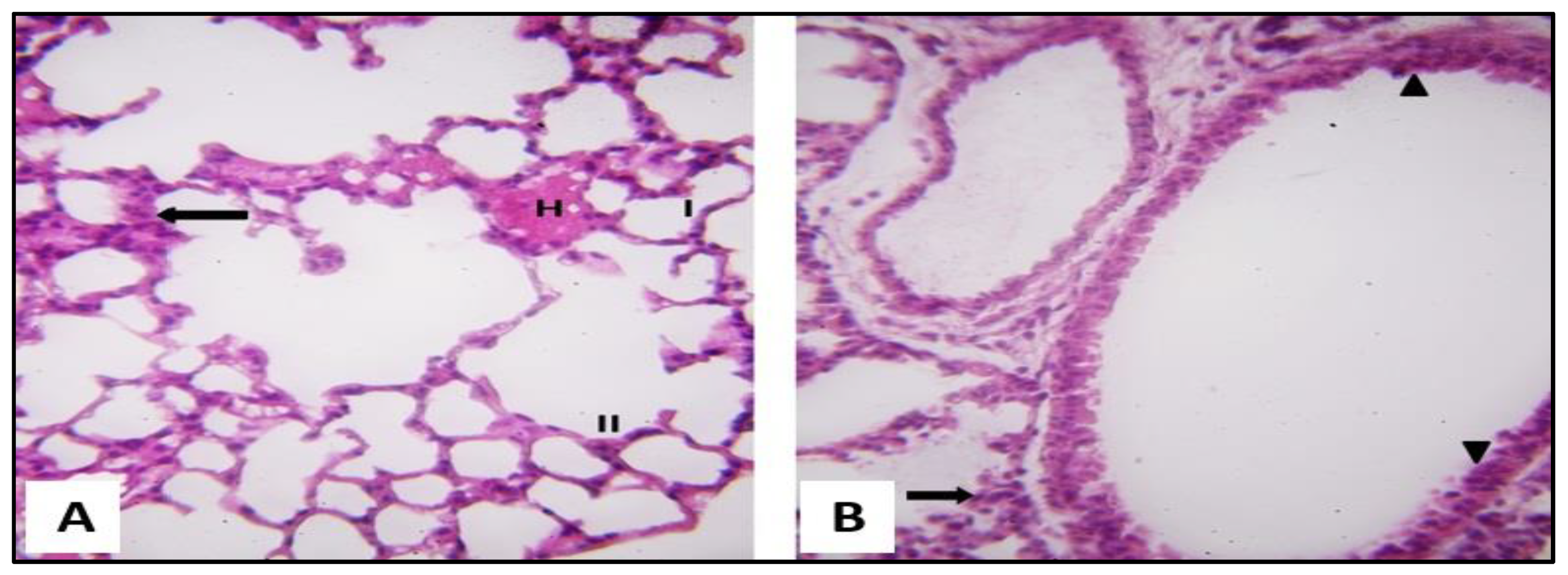
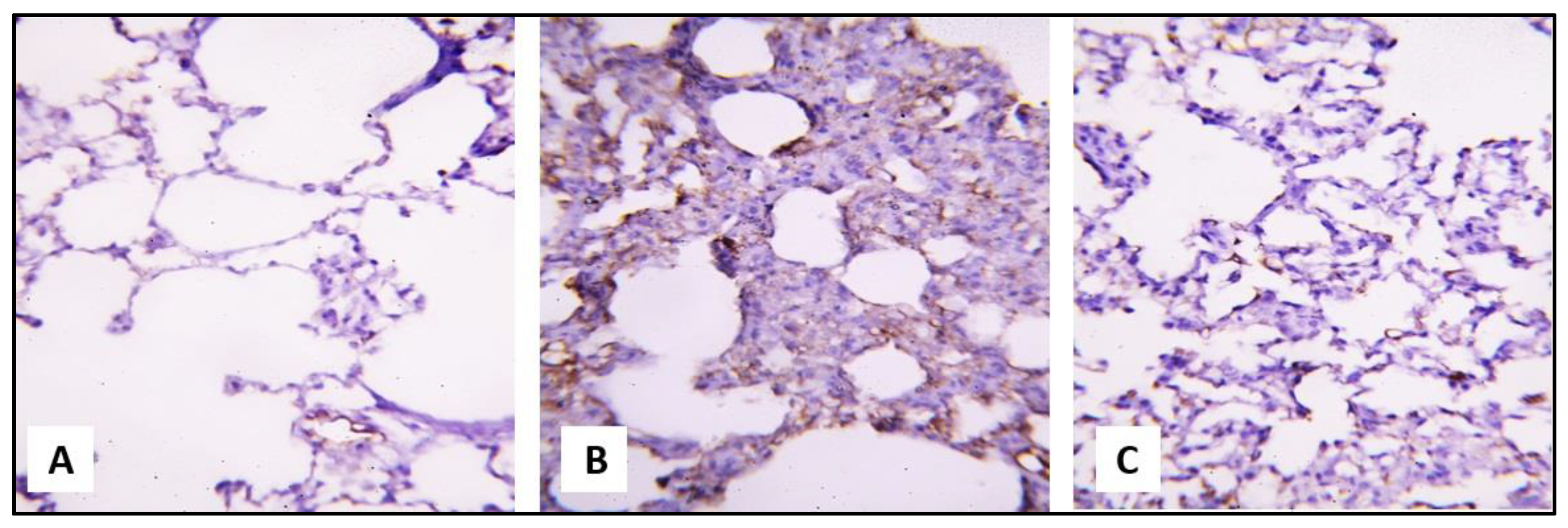
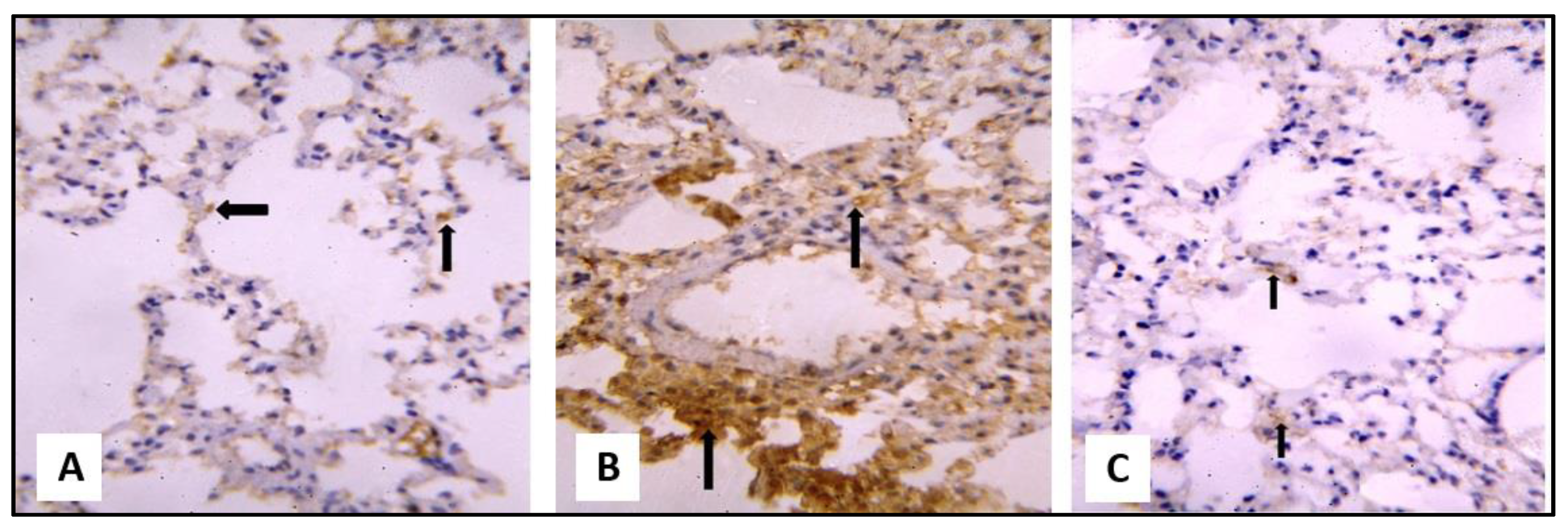
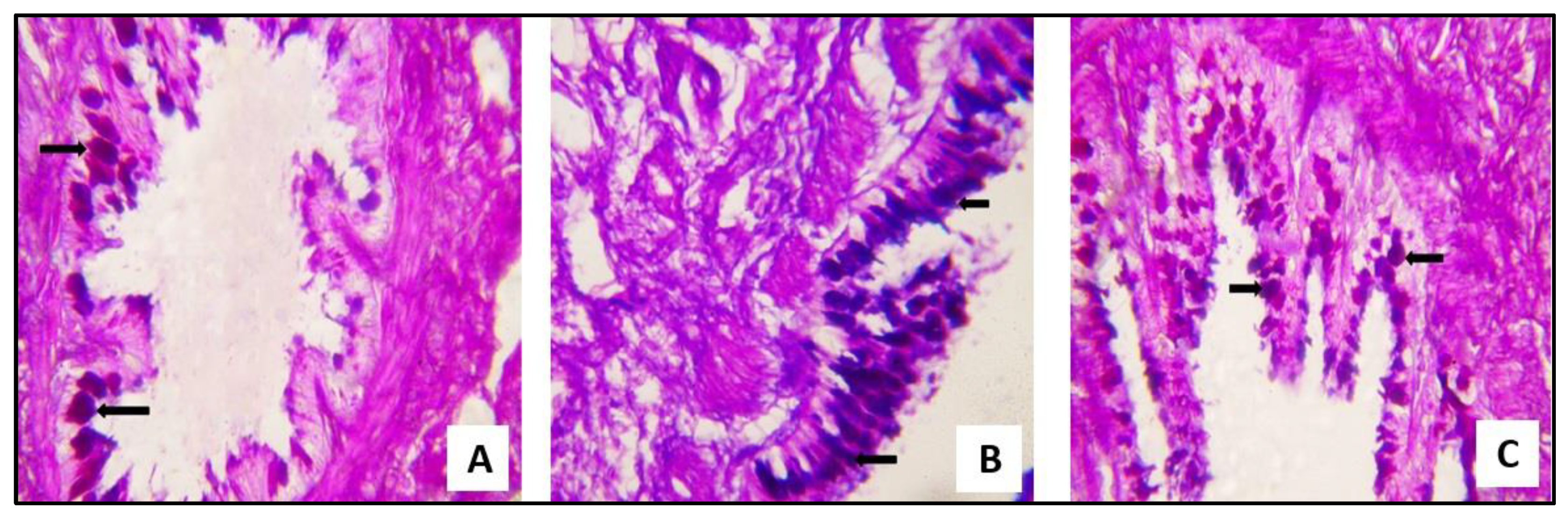
2.3.1. Microscopical Results
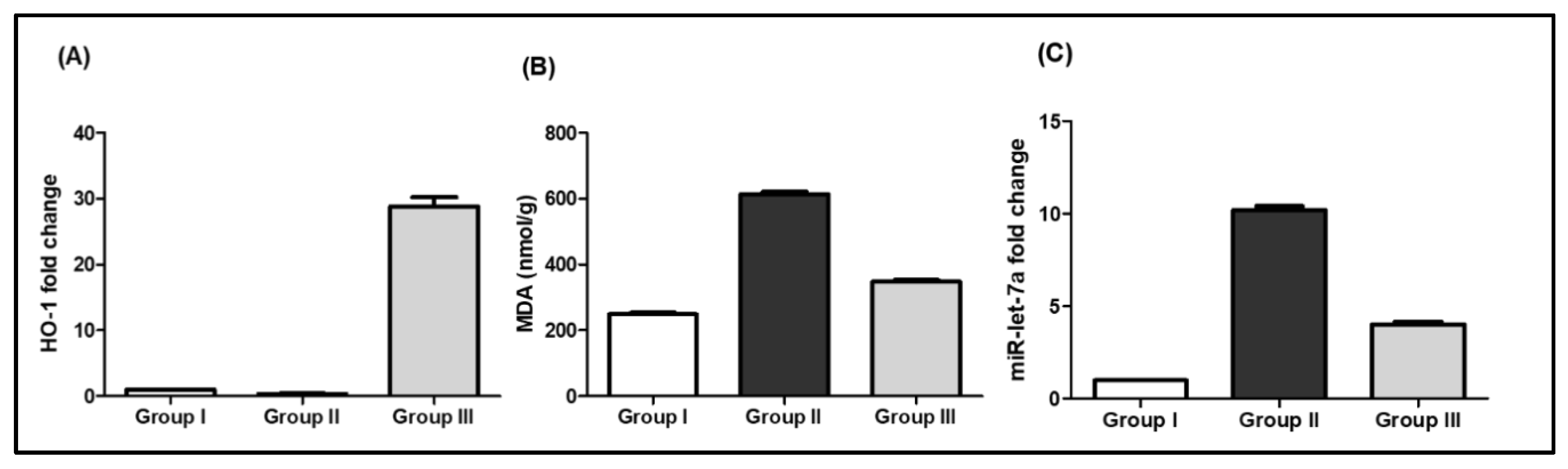
2.3.2. Biochemical Results
SCRE Reduced Lipid Peroxidation and Oxidative Stress in CP-Treated Rats
miR-let-7a Expression in Response to CP and SCRE
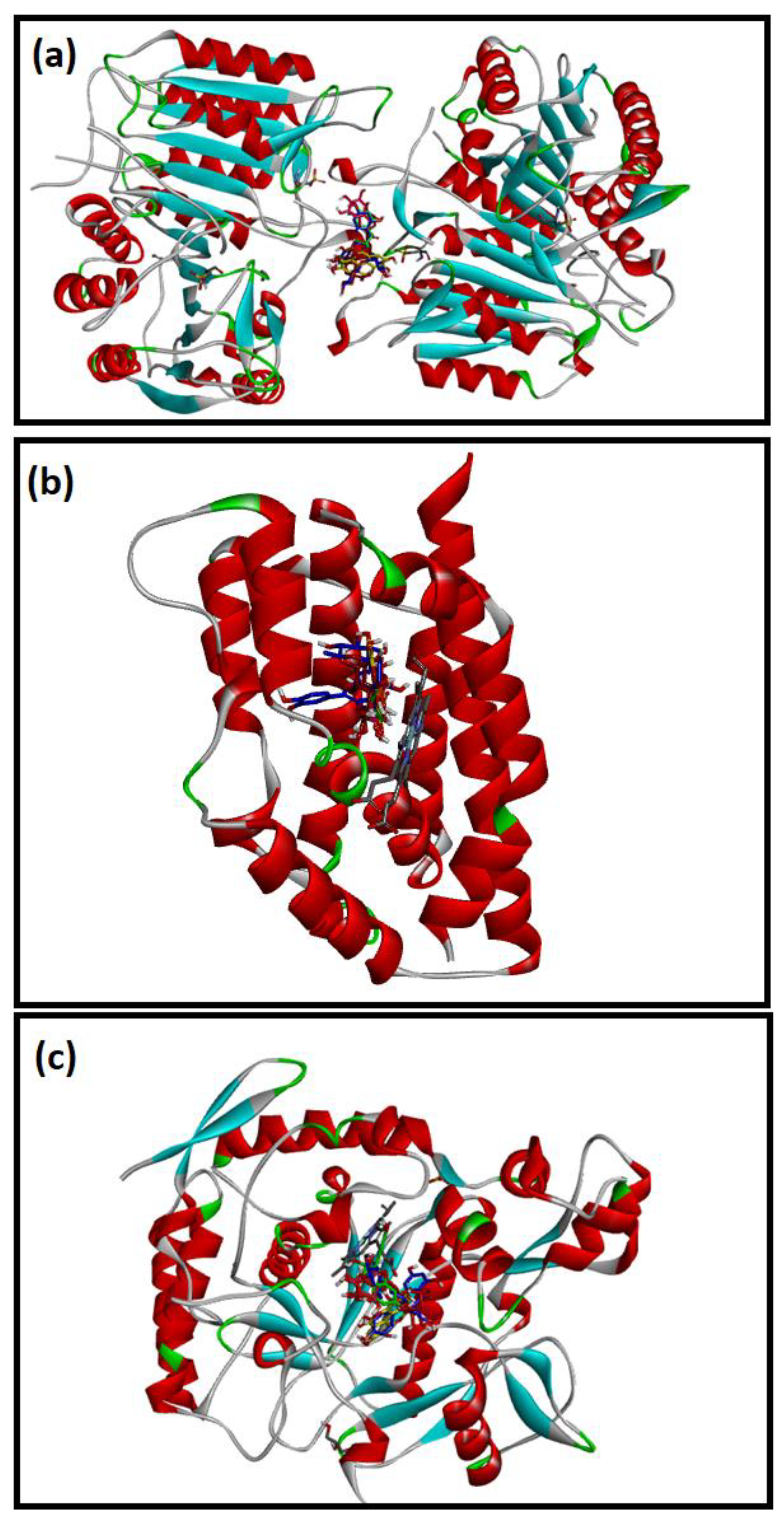
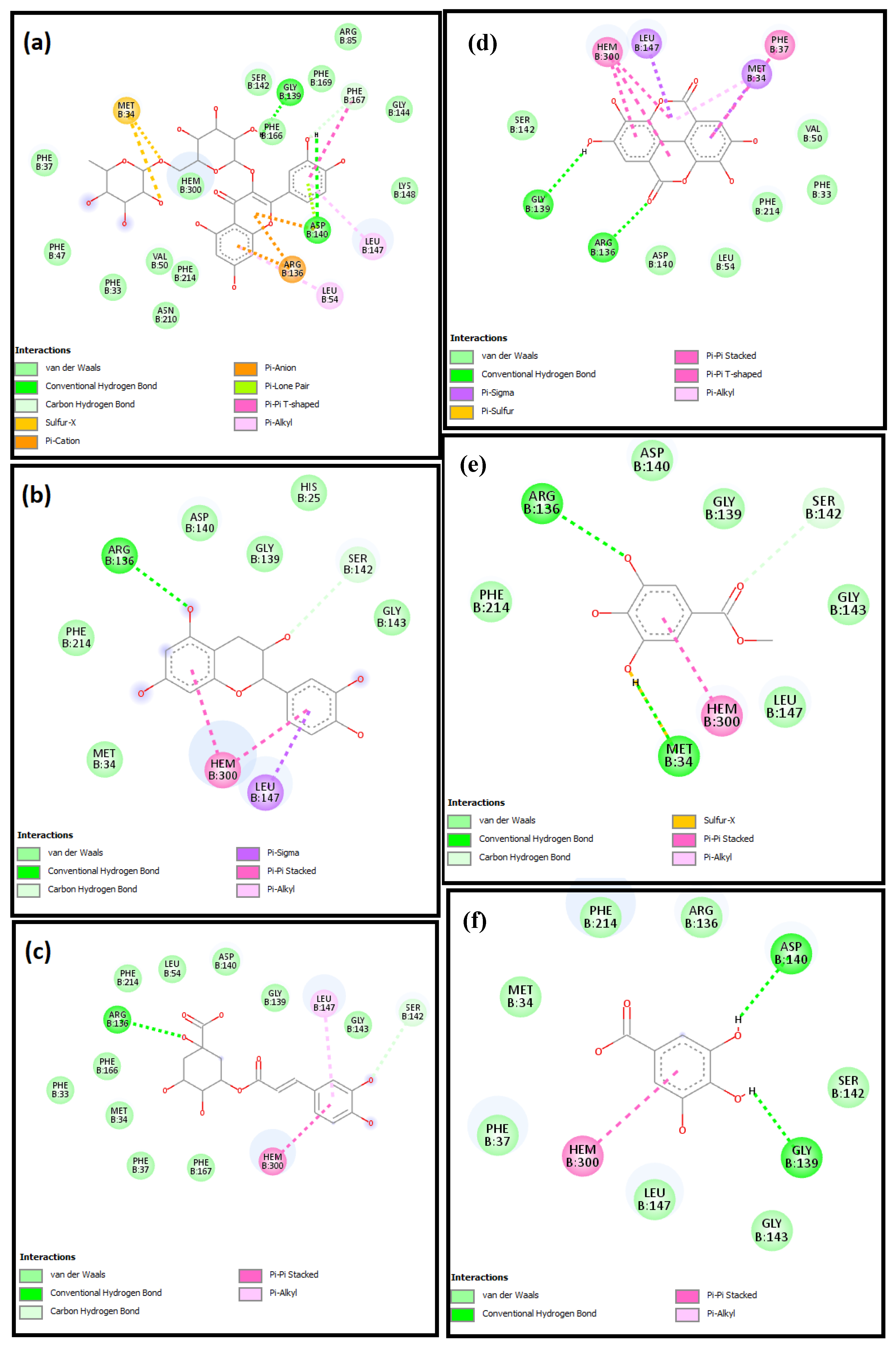
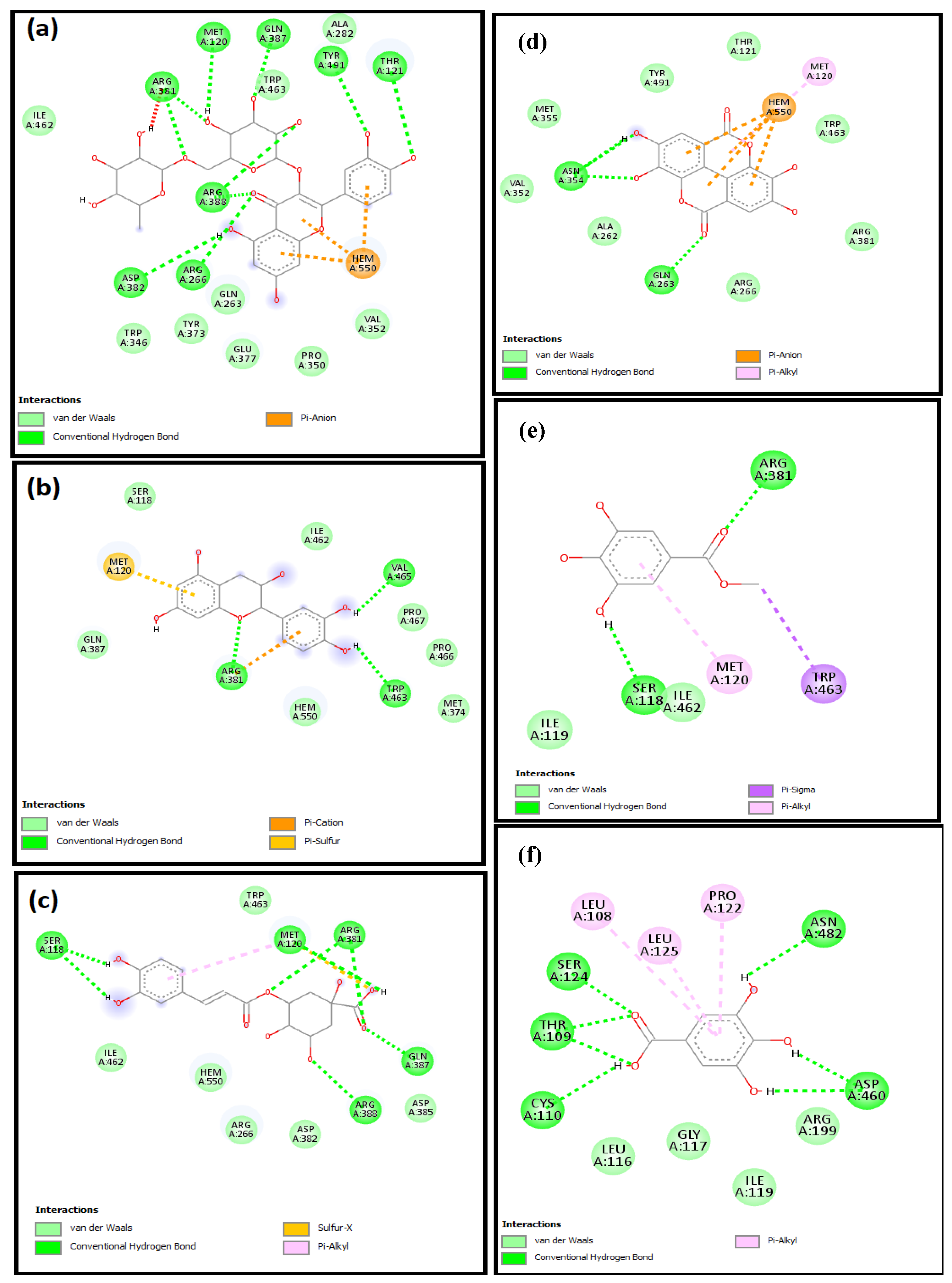
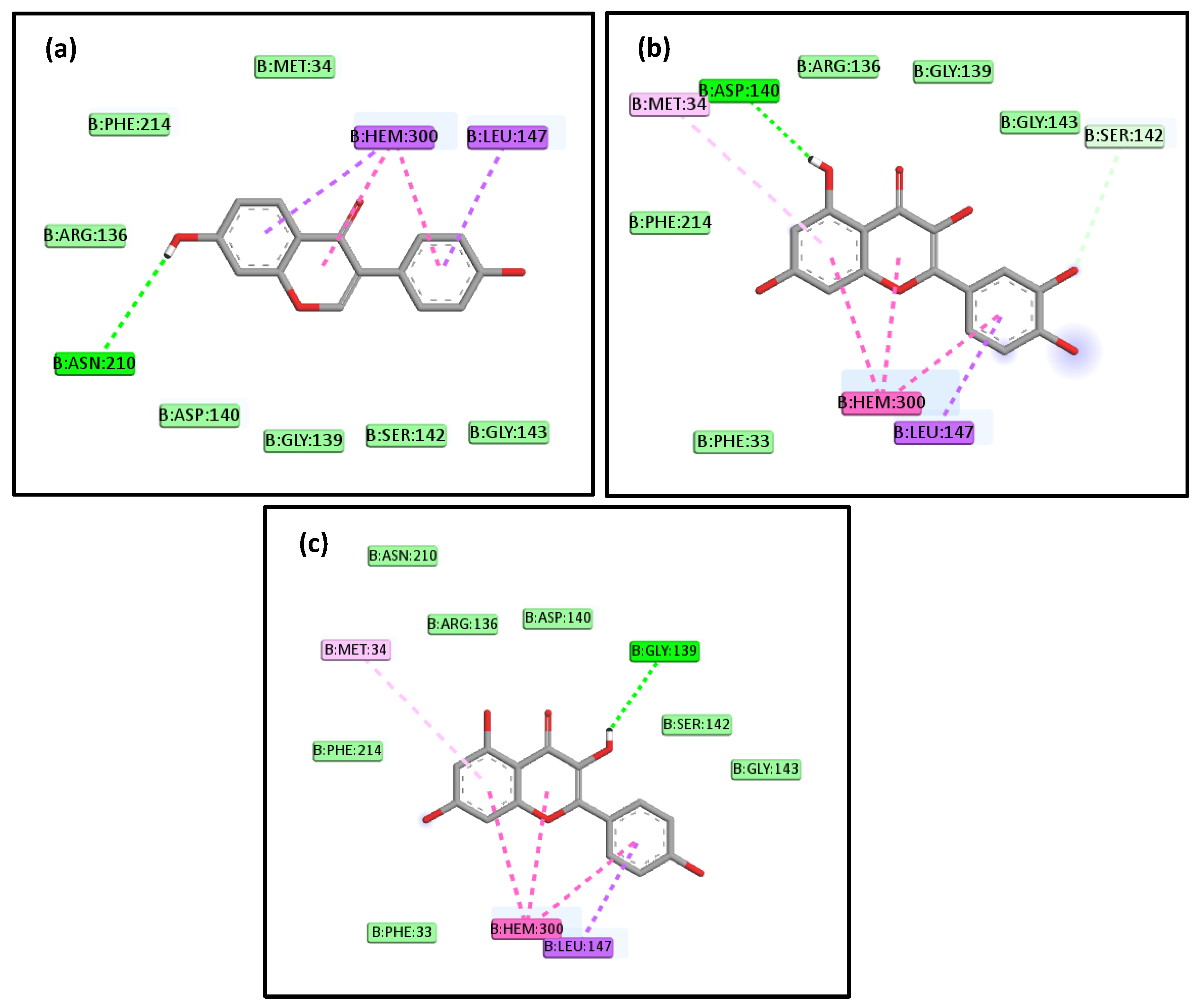
2.4. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Plant Preparation and High-Performance Liquid Chromatography (HPLC) Analysis
4.2. Chemicals and Solvents
4.3. In Vitro Antiviral Activity
4.3.1. Viruses and Cells
4.3.2. MTT Cytotoxicity Test
4.3.3. Plaque Assay
4.4. In Vivo SCRE Lung Protection
4.4.1. Animals
4.4.2. Experimental Protocol
- -
- Group I (control) was given 0.5 % carboxymethyl cellulose (CMC) orally for 10 days and only one intraperitoneal (IP) saline injection on the seventh day.
- -
- Group II (CP-treated) was given 0.5 % CMC for 10 days and a single IP CP injection (200 mg/kg) on the seventh day [29].
- -
4.4.3. Histological and Immunohistochemical Studies
4.4.4. Biochemical Studies
Detection of MDA Level
Detection of the Relative Gene Expression of miR-Let7a and HO-1
4.5. In Silico Lung Protective Potential of SCRE
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Shahbaz, H.M.; Holley, R.A. Knowledge and information sources about COVID-19 among university students in Jordan: A cross-sectional study. Front. Public Health 2020, 8, 254. [Google Scholar] [CrossRef]
- Elekhnawy, E.A.; Sonbol, F.I.; Elbanna, T.E.; Abdelaziz, A.A. Evaluation of the impact of adaptation of Klebsiella pneumoniae clinical isolates to benzalkonium chloride on biofilm formation. Egypt. J. Med. Hum. Genet. 2021, 22, 51. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front. Immunol. 2020, 11, 2309. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014, 22, 183–191. [Google Scholar] [CrossRef]
- Hegazy, A.; Mostafa, I.; Elshaier, Y.A.; Mahmoud, S.H.; Abo Shama, N.M.; Shehata, M.; Yahya, G.; Nasr, N.F.; El-Halawany, A.M.; Ali, M.A. Robust Antiviral Activity of Santonica Flower Extract (Artemisia cina) against Avian and Human Influenza A Viruses: In Vitro and Chemoinformatic Studies. ACS Omega 2022, 7, 41212–41223. [Google Scholar] [CrossRef]
- Mills, K.A.; Chess-Williams, R.; McDermott, C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: Chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch. Toxicol. 2019, 93, 3291–3303. [Google Scholar] [CrossRef]
- El Gharabawy, G.S.; Abd Allah, E.E.-D.E.; Amr, I.M.; Elmitwalli, M. Histological and Immunohistochemical Study of The Effect of Cyclophosphamide on Testis of Male Adult Albino Rats and The Possible Protective Role of Vitamin E. Egypt. J. Hosp. Med. 2019, 77, 5930–5946. [Google Scholar] [CrossRef]
- Dan, D.C.; Fischer, R.; Adler, S.; Förger, F.; Villiger, P. Cyclophosphamide: As bad as its reputation? Long-term single centre experience of cyclophosphamide side effects in the treatment of systemic autoimmune diseases. Swiss Med. Wkly. 2014, 144, w14030. [Google Scholar] [CrossRef]
- Li, X.; Peng, H.; Wu, J.; Xu, Y. Brain natriuretic peptide-regulated expression of inflammatory cytokines in lipopolysaccharide (LPS)-activated macrophages via NF-κB and mitogen activated protein kinase (MAPK) pathways. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3119. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Groot, M.; Sharma, L.; Cruz, C.S.D.; Jin, Y. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax 2019, 74, 865–874. [Google Scholar] [CrossRef]
- Elliot, S.; Periera-Simon, S.; Xia, X.; Catanuto, P.; Rubio, G.; Shahzeidi, S.; El Salem, F.; Shapiro, J.; Briegel, K.; Korach, K.S. MicroRNA let-7 downregulates ligand-independent estrogen receptor–mediated male-predominant pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Zhang, L.; Hu, Y. MicroRNA-let-7a inhibition inhibits LPS-induced inflammatory injury of chondrocytes by targeting IL6R. Mol. Med. Rep. 2019, 20, 2633–2640. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elseady, W.S.; Saleh, A.; Alotaibi, K.N.; El-Sherbeni, S.A. Antibacterial, Immunomodulatory, and Lung Protective Effects of Boswelliadalzielii Oleoresin Ethanol Extract in Pulmonary Diseases: In Vitro and In Vivo Studies. Antibiotics 2021, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme oxygenases in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, D.-S.; Li, B.; Choi, Y.H.; Lee, S.-H.; Kim, Y.-C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279. [Google Scholar] [CrossRef]
- D’amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Alotaibi, B.; Mokhtar, F.A.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A. Antimicrobial activity of Brassica rapa L. flowers extract on gastrointestinal tract infections and antiulcer potential against indomethacin-induced gastric ulcer in rats supported by metabolomics profiling. J. Inflamm. Res. 2021, 14, 7411. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lekarskie 2004, 57, 453–455. [Google Scholar]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 3137. [Google Scholar] [CrossRef] [PubMed]
- Mammate, N.; El Oumari, F.E.; Imtara, H.; Belchkar, S.; Lahrichi, A.; Alqahtani, A.S.; Noman, O.M.; Tarayrah, M.; Houssaini, T.S. Antioxidant and Anti-Urolithiatic Activity of Aqueous and Ethanolic Extracts from Saussurea costus (Falc) Lispich Using Scanning Electron Microscopy. Life 2022, 12, 1026. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F.; Al Hamoud, G.A.; Bukhari, S.I.; Moubayed, N.M. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS ONE 2020, 15, e0237567. [Google Scholar] [CrossRef]
- Ács, N.; Gambino, M.; Brøndsted, L. Bacteriophage enumeration and detection methods. Front. Microbiol. 2020, 11, 2662. [Google Scholar] [CrossRef]
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2. Curr. Protoc. Microbiol. 2020, 57, cpmc105. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; Elekhnawy, E.; Negm, W.A.; El-Masry, T.A.; Almukainzi, M.; Zayed, A.; Ulber, R. Insight into Fucoidan-Based PEGylated PLGA Nanoparticles Encapsulating Methyl Anthranilic Acid: In Vitro Evaluation and In Vivo Anti-Inflammatory Study. Mar. Drugs 2022, 20, 694. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Ahmadi, A.; Chabra, A.; Naghshvar, F.; Salehi, F.; Habibi, E.; Haghi-Aminjan, H. An ethanol extract of Origanum vulgare attenuates cyclophosphamide-induced pulmonary injury and oxidative lung damage in mice. Pharm. Biol. 2014, 52, 1229–1236. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Bhattacharya, S. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol. Cell. Biochem. 2015, 405, 243–256. [Google Scholar] [CrossRef]
- Abdel-Latif, G.A.; Elwahab, A.H.A.; Hasan, R.A.; ElMongy, N.F.; Ramzy, M.M.; Louka, M.L.; Schaalan, M.F. A novel protective role of sacubitril/valsartan in cyclophosphamide induced lung injury in rats: Impact of miRNA-150-3p on NF-κB/MAPK signaling trajectories. Sci. Rep. 2020, 10, 13045. [Google Scholar] [CrossRef]
- Speyer, C.L.; Neff, T.A.; Warner, R.L.; Guo, R.-F.; Sarma, J.V.; Riedemann, N.C.; Murphy, M.E.; Murphy, H.S.; Ward, P.A. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am. J. Pathol. 2003, 163, 2319–2328. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atheroscler. J. 2010, 2, 189–192. [Google Scholar]
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407. [Google Scholar] [CrossRef]
- Zahara, K.; Tabassum, S.; Sabir, S.; Arshad, M.; Qureshi, R.; Amjad, M.S.; Chaudhari, S.K. A review of therapeutic potential of Saussurea lappa-An endangered plant from Himalaya. Asian Pac. J. Trop. Med. 2014, 7, S60–S69. [Google Scholar] [CrossRef]
- Lee, G.-I.; Ha, J.Y.; Min, K.R.; Nakagawa, H.; Tsurufuji, S.; Chang, I.-M.; Kim, Y. Inhibitory effects of oriental herbal medicines on IL-8 induction in lipopolysaccharide-activated rat macrophages. Planta Med. 1995, 61, 26–30. [Google Scholar] [CrossRef]
- Choi, D.-H.; Kim, J.-Y.; An, J.-H.; Sung, S.-H.; Kong, H.-S. Effects of Saussurea costus on apoptosis imbalance and inflammation in benign prostatic hyperplasia. J. Ethnopharmacol. 2021, 279, 114349. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lin, H.-Y.; Su, K.-Y.; Chen, C.-H.; Yu, Y.-L.; Lin, C.-C.; Yu, S.-L.; Yan, H.-Y.; Su, K.-J.; Chen, Y.-L.S. Rutin, a flavonoid that is a main component of Saussurea involucrata, attenuates the senescence effect in D-galactose aging mouse model. Evid.-Based Complement. Altern. Med. 2012, 2012, 980276. [Google Scholar] [CrossRef]
- Polikepahad, S.; Knight, J.M.; Naghavi, A.O.; Oplt, T.; Creighton, C.J.; Shaw, C.; Benham, A.L.; Kim, J.; Soibam, B.; Harris, R.A. Proinflammatory role for let-7 microRNAS in experimental asthma. J. Biol. Chem. 2010, 285, 30139–30149. [Google Scholar] [CrossRef]
- Seliem, E.M.; Azab, M.E.; Ismail, R.S.; Nafeaa, A.A.; Alotaibi, B.S.; Negm, W.A. Green Coffee Bean Extract Normalize Obesity-Induced Alterations of Metabolic Parameters in Rats by Upregulating Adiponectin and GLUT4 Levels and Reducing RBP-4 and HOMA-IR. Life 2022, 12, 693. [Google Scholar] [CrossRef]
- Attallah, N.G.; El-Kadem, A.H.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Alanazi, A.S.; Al-Hamoud, G.A.; Ragab, A.E. Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study. Pharmaceuticals 2021, 14, 1313. [Google Scholar] [CrossRef]
- Olama, N.K.; Taha, M.; Rady, H.Y. The potential protective role of coenzyme q10 on the cyclophosphamide induced lung toxicity in adult male albino rats: A histological and ultrastructural study. Int. J. Sci. Rep. 2018, 4, 225–234. [Google Scholar] [CrossRef]
- Taslimi, P.; Kandemir, F.M.; Demir, Y.; İleritürk, M.; Temel, Y.; Caglayan, C.; Gulçin, İ. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: Pharmacological evaluation of some metabolic enzyme activities. J. Biochem. Mol. Toxicol. 2019, 33, e22313. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M.; Bancroft, O. Theory and Practice of Histological Techniques, 5th ed.; Churchill-Livingstone: New York, NY, USA, 2002. [Google Scholar]
- Hsu, S.; Raine, L.; Fanger, H. Use of biotin-avidin-peroxi dase conplex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody techniques. Am. J. Clin. Pathol 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Viñas, J.L.; Sola, A.; Genescà, M.; Alfaro, V.; Pí, F.; Hotter, G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic. Biol. Med. 2006, 40, 992–1003. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Attallah, N.G.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- Du, J.-Q.; Wu, J.; Zhang, H.-J.; Zhang, Y.-H.; Qiu, B.-Y.; Wu, F.; Chen, Y.-H.; Li, J.-Y.; Nan, F.-J.; Ding, J.-P.; et al. Isoquinoline-1,3,4-trione Derivatives Inactivate Caspase-3 by Generation of Reactive Oxygen Species. J. Biol. Chem. 2008, 283, 30205–30215. [Google Scholar] [CrossRef]
- Rahman, M.N.; Vlahakis, J.Z.; Szarek, W.A.; Nakatsu, K.; Jia, Z. X-ray Crystal Structure of Human Heme Oxygenase-1 in Complex with 1-(Adamantan-1-yl)-2-(1H-imidazol-1-yl)ethanone: A Common Binding Mode for Imidazole-Based Heme Oxygenase-1 Inhibitors. J. Med. Chem. 2008, 51, 5943–5952. [Google Scholar] [CrossRef]
- Li, H.; Raman, C.S.; Glaser, C.B.; Blasko, E.; Young, T.A.; Parkinson, J.F.; Whitlow, M.; Poulos, T.L. Crystal Structures of Zinc-free and -bound Heme Domain of Human Inducible Nitric-oxide Synthase: Implications for dimer stability and comparison with endothelial nitric-oxide synthase. J. Biol. Chem. 1999, 274, 21276–21284. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- MarvinSketch, Version 22.2; ChemAxon: Budapest, Hungary, 2022. Available online: https://chemaxon.com/products/marvin (accessed on 5 February 2022).
- BIOVIA. Dassault Systèmes. Discovery Studio Visualizer, V16.1.0; Dassault Systèmes: San Diego, CA, USA, 2016.
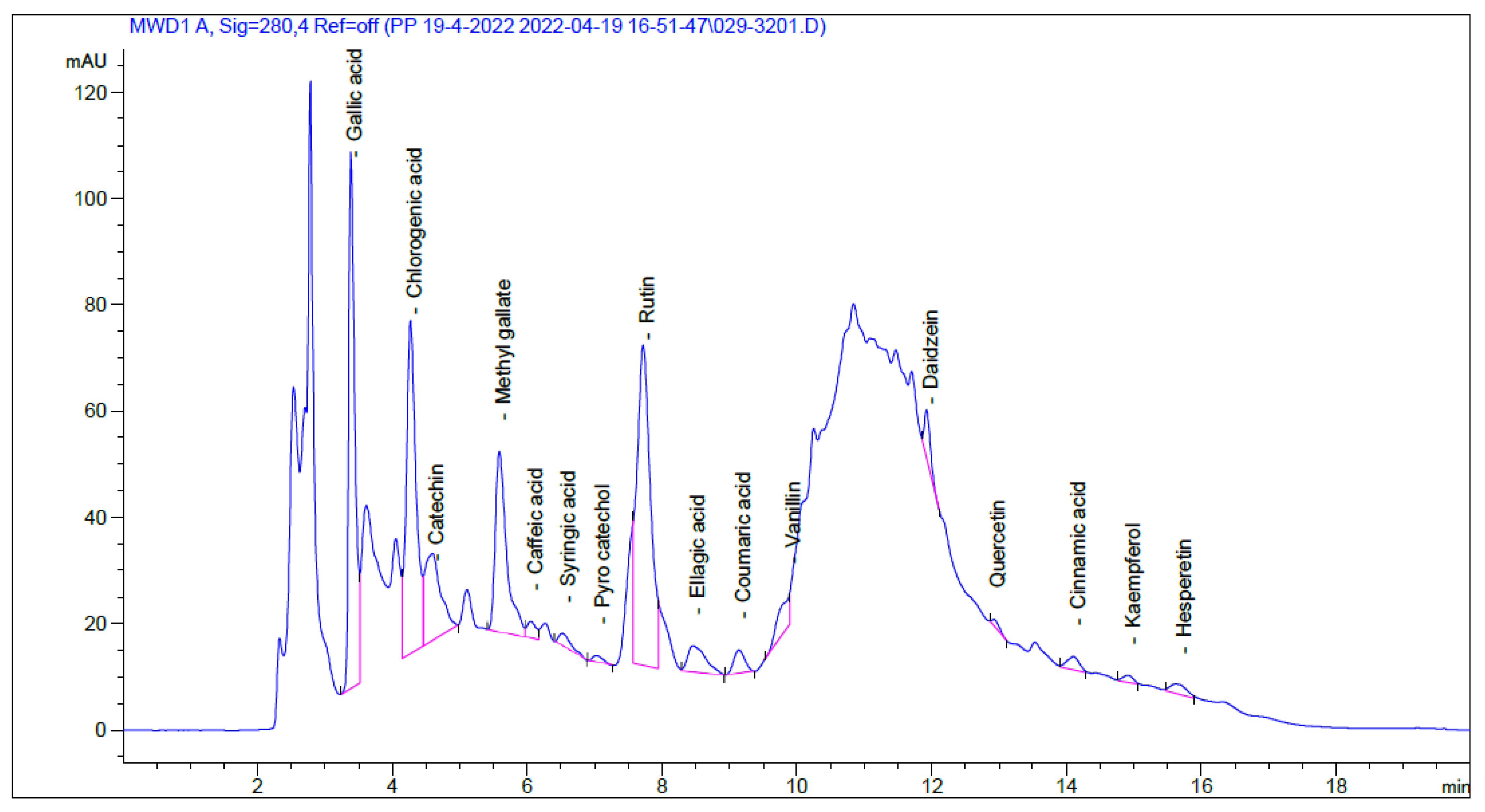



| Identified Compound | RT (Min) | Area (mAU*s) | Conc (µg/mL) | Conc (µg/g) |
|---|---|---|---|---|
| Gallic acid | 3.385 | 636.15 | 51.56 | 865.05 |
| Chlorogenic acid | 4.269 | 625.20 | 90.85 | 1524.30 |
| Catechin | 4.596 | 268.25 | 74.60 | 1251.74 |
| Methyl gallate | 5.589 | 397.33 | 25.92 | 434.83 |
| Caffeic acid | 6.055 | 27.85 | 2.14 | 35.84 |
| Syringic acid | 6.522 | 32.05 | 2.63 | 44.10 |
| Pyro catechol | 7.028 | 13.91 | 1.98 | 33.15 |
| Rutin | 7.722 | 832.21 | 61.23 | 1027.28 |
| Ellagic acid | 8.464 | 92.95 | 32.27 | 541.44 |
| Coumaric acid | 9.144 | 49.85 | 1.64 | 27.44 |
| Vanillin | 9.891 | 70.48 | 2.69 | 45.20 |
| Ferulic acid | 10.237 | 0.00 | 0.00 | 0.00 |
| Naringenin | 10.490 | 0.00 | 0.00 | 0.00 |
| Daidzein | 11.927 | 54.06 | 3.74 | 62.69 |
| Quercetin | 12.925 | 10.20 | 1.25 | 21.02 |
| Cinnamic acid | 14.111 | 30.51 | 0.65 | 10.86 |
| Apigenin | 14.496 | 0.00 | 0.00 | 0.00 |
| Kaempferol | 14.924 | 14.03 | 1.49 | 25.04 |
| Hesperetin | 15.684 | 30.45 | 1.78 | 29.93 |
| Receptor | Grid Box (x, y, z) | Affinity (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| Center | Size | RUT | GAL | CHL | ELL | GAT | CAT | |
| Caspase 3 | −46.6, 15.4, −22.4 | 19.7, 15.6, 18.1 | −8.9 | −5.0 | −7.4 | −7.5 | −5.0 | −7.8 |
| HO-1 | 27.0, 16.2, −37.6 | 21.7, 21.4, 20.0 | −7.5 | −5.9 | −8.0 | −7.0 | −6.0 | −8.5 |
| iNOS | 12.5, 59.9, 20.9 | 30.5, 22.9, 23.5 | −10.6 | −6.9 | −8.0 | −7.1 | −6.7 | −7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attallah, N.G.M.; Kabbash, A.; Negm, W.A.; Elekhnawy, E.; Binsuwaidan, R.; Al-Fakhrany, O.M.; Shaldam, M.A.; Moglad, E.; Tarek, M.; Samir, N.; et al. Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect. Pharmaceuticals 2023, 16, 318. https://doi.org/10.3390/ph16020318
Attallah NGM, Kabbash A, Negm WA, Elekhnawy E, Binsuwaidan R, Al-Fakhrany OM, Shaldam MA, Moglad E, Tarek M, Samir N, et al. Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect. Pharmaceuticals. 2023; 16(2):318. https://doi.org/10.3390/ph16020318
Chicago/Turabian StyleAttallah, Nashwah G. M., Amal Kabbash, Walaa A. Negm, Engy Elekhnawy, Reem Binsuwaidan, Omnia Momtaz Al-Fakhrany, Moataz A. Shaldam, Ehssan Moglad, Marwa Tarek, Nehal Samir, and et al. 2023. "Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect" Pharmaceuticals 16, no. 2: 318. https://doi.org/10.3390/ph16020318
APA StyleAttallah, N. G. M., Kabbash, A., Negm, W. A., Elekhnawy, E., Binsuwaidan, R., Al-Fakhrany, O. M., Shaldam, M. A., Moglad, E., Tarek, M., Samir, N., & Fawzy, H. M. (2023). Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect. Pharmaceuticals, 16(2), 318. https://doi.org/10.3390/ph16020318









