Abstract
A new method for simultaneous determination of puerarin, daidzin, daidzein and genistein in Radix puerariae by ultra-high performance liquid chromatography was established. The target analytes were extracted from Radix puerariae by 70% ethylene glycol with the assistance of ultrasonication, purified by the absorption of N-propyl ethylenediamine (PSA), and separated on a Supersil ODS column (4.6 mm × 250 mm × 2.5 μm). Gradient elution in 12 min was performed with the mobile phase 0.1% formic acid(A)–acetonitrile(B). The column temperature was 25 °C and the flow rate was 1 mL/min. The detection wavelength of the four target analytes was 250 nm. The limits of detection (LODs) of puerarin, daidzin, daidzein and genistein were 0.086 mg/L, 0.020 mg/L, 0.027 mg/L and 0.037 mg/L, respectively, and limits of quantitation (LOQs) were 0.29 mg/L, 0.065 mg/L, 0.090 mg/L and 0.12 mg/L, respectively. The recovery of the four substances ranged from 90.5% to 109.6%, and the relative standard deviation (n = 6) was less than 7.7%. With the established methods, puerarin, daidzin, daidzein and genistein in Radix puerariae from 11 origins were determined. The contents of the four compounds varied with the origin and variety. It provides basic data and technical means for quality control and regulation of Radix puerariae.
1. Introduction
Radix puerariae is the tuber root of the leguminous plant Puerariae, which was originally recorded in Shennong Bencao Jing (an ancient Chinese medical book) in ancient China [1]. It is a twining perennial woody herb found in China, Korea, Japan, India and the United States. It is also known as the “invasive species” by Americans, and the US government spends billions of dollars to eliminate it every year. Radix puerariae is mainly found in Yunnan, Anhui, Sichuan, Jiangxi, Guangxi, Guangdong, Hainan provinces and other places in China. As a recognized nutritive plant, Radix puerariae is used not only in food but also in medicine in China. As many as 90 prescriptions for Radix puerariae are listed in the 2020 edition of the Chinese Pharmacopoeia, and modern pharmaceutical companies have developed numerous products. In terms of food, Radix puerariae has been developed into some products such as noodles, freshly squeezed drinks and bread due to its high starch content and unique taste [2]. With the wide range of applications of Radix puerariae, its safety has been of great concern. Wang et al. reported that Radix puerariae extract may be hepatotoxic [3]. Studies have shown that puerarin in Radix puerariae can cause acute renal failure, hepatotoxicity and hemolytic reactions [3,4]. The developmental and reproductive toxicity of isoflavones in Radix puerariae has also been reported [5].
In addition, Radix puerariae contains isoflavones, triterpenes, coumarins, glycosides and other components [6]. Of these, isoflavones are the main active components, with relatively high levels of puerarin, daidzein, daidzein and genistein [7], which are commonly used as active components in the quality control of Radix puerariae [6,8,9]. The isoflavones in Radix puerariae are phenolic phytoestrogens, and due to their structural similarity to estradiol, they may interfere with the endocrine system, posing a health risk to humans [10,11]. As a result, the safety of Radix puerariae has received widespread attention.
At present, the contents of isoflavones in Radix puerariae are determined by high-performance liquid chromatography (HPLC) with a UV, fluorescence or mass spectrometric detector [12,13,14]. Due to the complexity of Radix puerariae matrix, there are still many interfering substances in the extract [15], which not only affect the detection of the target analytes but also shorten the service life of the chromatographic column [16,17]. Extracts of Radix puerariae can be purified by some adsorbents prior to injection for analysis. Purification adsorbents such as HC-C18 [18], silica gel [19], primary secondary amine (PSA) [20], kieselguhr [21] and ZIF-8 [22] have been widely applied to remove the interference in other studies. However, there are few reports on the purification of samples using those absorbents in the determination of isoflavones in Radix puerariae.
The aim of this research was to establish a new UHPLC method for the simultaneous analysis of puerarin, daidzin, daidzein and genistein in Radix puerariae and to attempt to optimize the sample pretreatment with five adsorbents. Furthermore, four isoflavones in Radix puerariae from different origins were determined with the developed method.
2. Materials and Methods
2.1. Chemicals and Reagents
Methanol, ethylene glycol and formic acid (analytically pure) were purchased from Xilong Science Co., Ltd. (Shantou, China). Acetonitrile (HPLC grade) was purchased from TEDIA Co., Ltd. (Fairfield, OH, USA). Ethanol (chromatographic pure) was bought from Xilong Science Co., Ltd. (Shantou, China). Puerarin, daidzin, daidzein and genistein (HPLC grade) were obtained from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Puerarin, daidzin, daidzein and genistein were prepared into 1000 mg/L standard stock solution with methanol. The stock solution was diluted to prepare a series of mixed standard working solutions as required.
2.2. Sample Pretreatment
The dried Radix puerariae sample was crushed and sieved through a fine screen. Approximately 0.5 g of sample powder was accurately weighed and placed in a 50 mL centrifuge tube, then 25 mL 70% ethylene glycol aqueous solution was added, and the sample was mixed by a vortexer (Ika, Staufen, Germany). After ultrasonic extraction at 25 °C for 40 min, the centrifugation was performed at 4000 r/min for 5 min in a centrifuge (3K15, Sigma Laboratory Centrifuges, Osterode, Germany). One milliliter of the extract was added to 4 mL of 70% ethylene glycol aqueous solution and 50 mg PSA adsorbent. After shaking for 30 min, the mixture was centrifuged at 4000 r/min for 5 min. One milliliter of the supernatant was filtered through 0.22 μm organic microporous membrane and stored at 4 °C until measurement.
2.3. UPLC Condition
Ultra-high performance liquid chromatography (UHPLC) analysis was performed using an Agilent 1290 ultra-performance liquid chromatograph (Agilent, Santa Clara, CA, USA) equipped with high-pressure binary pumps and a diode array detector (DAD). The chromatographic column was Supersil ODS (4.6 mm × 250 mm × 2.5 μm). The column temperature was 25 °C. The mobile phase was 0.1% formic acid (A) and acetonitrile (B). The gradient elution procedure is shown in Table 1. The flow rate was 1 mL/min. The detection wavelength was 250 nm and the injection volume was 10 μL.

Table 1.
The gradient elution procedure.
2.4. Statistical Analysis
Microsoft Excel (v.2019, Microsoft corporation, Redmond, WA, USA) was used for the statistical processing of all data, SPSS 20.0 (IBM, Chicago, IL, USA) was used for the analysis of variance, and the LSD method was used to test the significance of difference between different means.
3. Results and Discussion
3.1. Optimization of Chromatographic Conditions
In this study, three different mobile phase combinations (including 0.3% formic acid-methanol, 0.3% formic acid-acetonitrile and 0.1% formic acid-acetonitrile) and the gradient elution procedures were optimized. The results showed that puerarin, daidzin, daidzein and genistein could be separated by gradient elution using the combination of 0.1% formic acid (A) and acetonitrile (B) as the mobile phase in 12 min. The UHPLC chromatogram of four compounds is shown in Figure 1. It was previously reported that the separation of these four compounds by HPLC required at least 40 min [23] or even longer than 80 min [24]. The condition optimized in this study is advantageous in terms of gradient time.
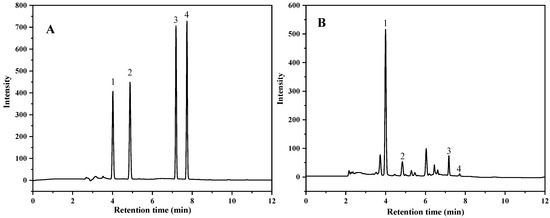
Figure 1.
UHPLC chromatogram of the standards (A) (50 mg/L) and the sample (B) (1. Puerarin; 2. Daidzin; 3. Daidzein; 4. Genistein) (sample was purified with PSA).
3.2. Optimization of Extraction Conditions
Methanol, ethanol and acetonitrile are commonly used to extract isoflavones [12,25,26,27]. Extraction using solvent alone is often inefficient. Ultrasound methods have been identified as the most cost-effective way to increase the yield of isoflavones with lower time and solvent costs at lower temperatures [28]. In this study, the extraction for testing samples was performed using four solvents with the assistance of ultrasonication, and the extraction yields were compared. Briefly, 0.5 g of Radix puerariae samples were weighed and 20 mL of 70% methanol, 70% ethanol, 70% ethylene glycol and 70% acetonitrile were selected as the extraction solvent; after being vortexed, the samples were extracted with the assistance of ultrasonication at 25 °C for 30 min, then the extracts were centrifuged at 4000 r/min for 5 min. The extraction yields are shown in Figure 2. The 70% ethylene glycol exhibited the most effectiveness in terms of extraction yield. Therefore, 70% ethylene glycol was selected as the extraction solvent in this study.
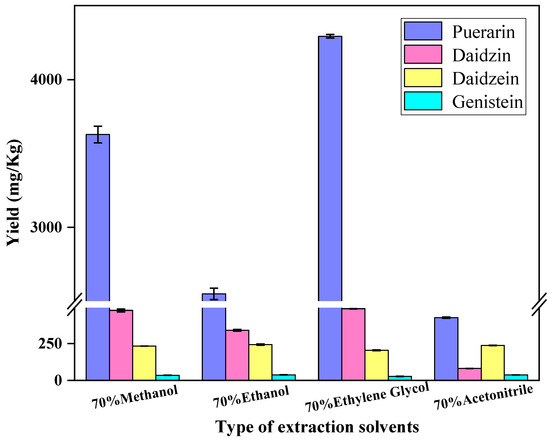
Figure 2.
Effect of the extraction solvent on the yield of puerarin, daidzin, daidzein and genistein.
The effect of the amount of ethylene glycol solution was investigated on the extraction yield of puerarin, daidzin, daidzein and genistein. The results are shown in Figure 3. The extraction effects of daidzein and genistein did not change much among different extraction solvent doses. Puerarin and daidzein demonstrated the best extraction yields when the extraction solvent dosage was 25 mL, so the extraction solvent dosage was chosen to be 25 mL.
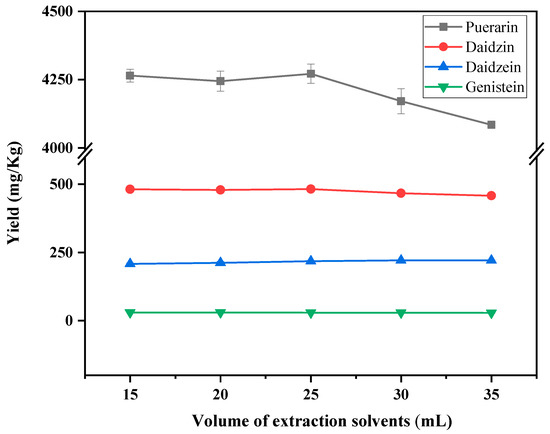
Figure 3.
Effect of ethylene glycol dosage on the yield of puerarin, daidzin, daidzein and genistein.
The effect of extraction time in the range of 10–50 min on the yield of target compounds was also investigated. The results are shown in Figure 4. Daidzein and genistein showed little change in yield within 10–50 min. The yields of puerarin and daidzein tended to be stable after 40 min of ultrasonic time. Therefore, the ultrasonic extraction time was selected to be 40 min.
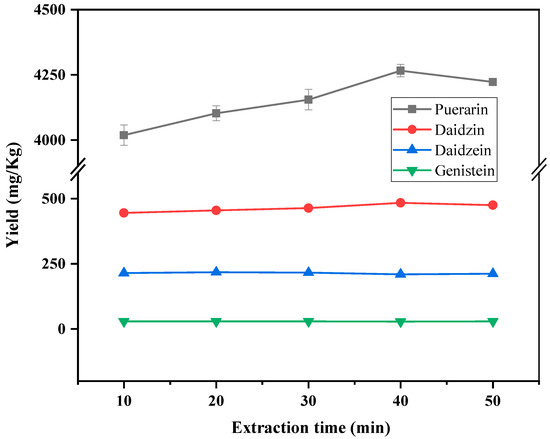
Figure 4.
Effect of the extraction time on the yield of puerarin, daidzin, daidzein and genistein.
3.3. Optimization of Purification Conditions
Considering the complexity of the extract of the Radix puerariae sample, some matrix compounds may interfere with the detection of the target analytes and shorten the service life of the column. For instance, pigments in the extract often interfere with the detection of the target analytes [29,30]. Therefore, several solid adsorbents were investigated in this experiment to remove the interfering substances in the extract. The sorbent type, dosage and adsorption time were optimized. Firstly, the purification effects of HC-C18, silica gel, PSA, diatomite and ZIF-8 on the extract were investigated. As shown in Figure 5, PSA has the best pigment removal effect compared with other adsorbents. Then the purification liquid was determined by high-performance liquid chromatography, and the results are shown in Figure 6, PSA has the best purification effect on the extract, which can remove the impurities and remain genistein compared with the other adsorbents. Furthermore, other adsorbents will have an adsorption effect on genistein. Therefore, PSA was chosen as purifying sorbent. The purification effect was investigated for PSA doses in the range of 10 to 300 mg. The results showed that the decolorization effect did not change significantly when the PSA dosage was greater than 50 mg. However, as the PSA dose was increased beyond 50 mg, the PSA showed an adsorption effect on the target analyte. Therefore, the PSA dosage was selected as 50 mg. In addition, the adsorption time in the range of 10–50 min was investigated. The optimal purification was obtained when the treatment was continued for 20 min. Therefore, the adsorption time was chosen to be 20 min in the present study.
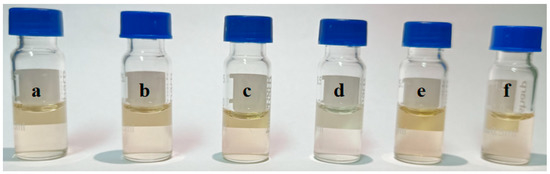
Figure 5.
Purification effect of different adsorbents on the extract: (a) no material added; (b) HC-C18; (c) diatomite; (d) PSA; (e) ZIF-8; and (f) silica gel.
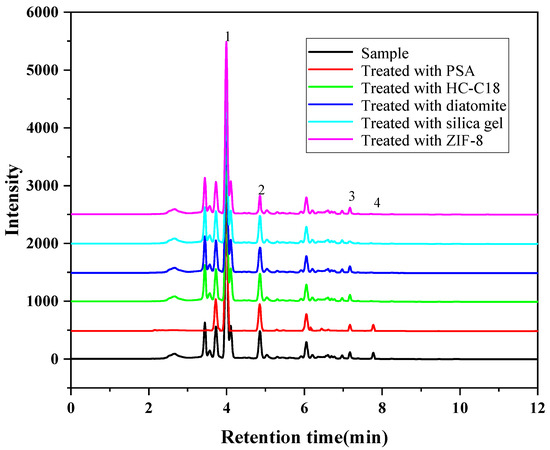
Figure 6.
Purification effect of different adsorbents on the extract (1. Puerarin; 2. Daidzin; 3. Daidzein; 4. Genistein).
3.4. Linearity, Limits of Detection and Quantification
A series of mixed standard operating solutions with different concentrations were prepared and measured under optimized experimental conditions. The peak area of each component, Y, was linearly fitted to the concentration of the analyte, X, to obtain the operating curve, and the results are presented in Table 2. The limits of detection (LODs) and limits of quantification (LOQs) values of the investigated analytes were assessed using signal-to-noise (S/N) ratios of 3 and 10, respectively; the results are shown in Table 2. The linear correlation coefficient (R2) of the analyte was better than 0.9981, indicating good linearity. Puerarin, daidzin, daidzein and genistein had LODs of 0.086 mg/L, 0.020 mg/L, 0.027 mg/L and 0.037 mg/L, respectively, and LOQs of 0.29 mg/L, 0.065 mg/L, 0.090 mg/L and 0.12 mg/L, respectively.

Table 2.
Linear equation, detection limit and quantification limit of puerarin, daidzin, daidzein and genistein.
3.5. Recovery and Precision
Three mixed standard solutions with different (Low, Medium and High) concentration levels were added to the samples, respectively, to carry out the spiked recovery experiment, and each level was measured in parallel six times. The results are shown in Table 3. The recoveries of the target analytes were 96.1–102.2% for puerarin, 97.6–109.6% for daidzin, 94.9–104.7% for daidzein, and 90.5–102.2% for genistein, respectively, and the relative standard deviation (RSD, n = 6) was less than 7.7%, indicating that this method has good precision, and can be used for the analysis of actual samples. Moreover, as shown in Figure 7, the measured concentration increases and the peak position remains the same when different standard concentrations are added. This indicates that the tested compounds are not interfered with by the matrix.

Table 3.
Recovery results of spiked puerarin, daidzin, daidzein and genistein in Radix puerariae (n = 6).
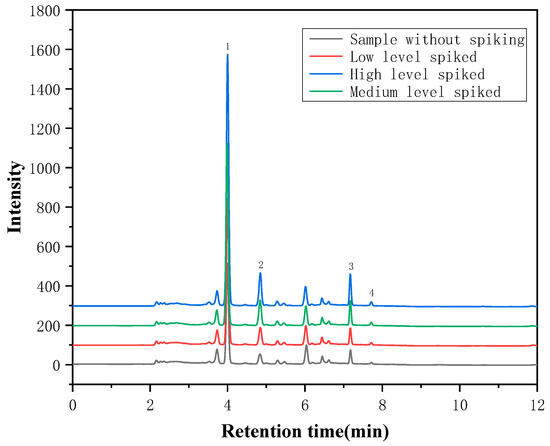
Figure 7.
The chromatogram of the samples with and without spiked standards (1. Puerarin; 2. Daidzin; 3. Daidzein; 4. Genistein).
3.6. Determination of the Sample
Four target compounds in Radix puerariae samples from 11 different regions of China were determined by the established analytical method, and the results are shown in Table 4. The levels of four compounds in the Radix puerariae samples from different regions were varied. The highest level of puerarin was detected in the sample from Chengdu, Sichuan, which was 101.9 mg/g. The lowest level of puerarin was found in the sample from Bozhou, Anhui, at only 4.14 mg/g. The highest level of daidzein was found in the sample from Linyi, Shandong, as high as 1.36 mg/g. The content differences of the compounds are attributed to the variety of Radix puerariae. Radix puerariae lobata (Wild.) Ohwi (RPL) and Radix puerariae thomsonii Benth (Fabaceae family) (RPT) are two varieties of Radix puerariae [31]. The collected samples contain both varieties of RPL and RPT (Table 4). Notably, the levels of puerarin, daidzin and daidzein in RPL are much higher than those in RPT. In the previous study, puerarin, daidzin and daidzein were confirmed to be the markers to distinguish RPL and RPT [12]. RPT is rich in starch and is commonly used as food, and contains fewer isoflavones than RPL [15,31]. RPL is commonly used as a herb in China. The highest levels of puerarin and daidzin were detected in RPL samples collected from Chengdu, Sichuan province. Among the RPT samples, those from Chuxiong, Yunnan province, were found to have the highest levels of puerarin and daidzin. On the whole, the genistein content in RPL is higher than that in RPT, except in the sample from Shennongjia, Hubei (Table 4).

Table 4.
Contents of puerarin, daidzin, daidzein and genistein in Radix puerariae from different regions (n = 3).
4. Conclusions
In the present study, a UHPLC method with the optimal pretreatment of the sample was established for the simultaneous determination of puerarin, daidzin, daidzein and genistein in Radix puerariae. PSA is the most effective absorbent for sample purification. The method is simple, rapid, accurate and suitable for the determination of puerarin, daidzin, daidzein and genistein in Radix puerariae samples. With the established methods, puerarin, daidzin, daidzein and genistein in Radix puerariae from 11 origins in China were determined. Levels of four compounds in Radix puerariae vary with the origin. The levels of puerarin, daidzein and daidzin are much higher in RPL than in RPT. Puerarin, daidzein and daidzin may be the indicators of discrimination of RPL and RPT.
Author Contributions
Conceptualization, C.L. and Y.W.; methodology, Y.G.; validation, C.L., Y.W. and M.X.: investigation, Y.G. and M.X.; data curation, Y.G. and M.X.; writing—original draft preparation, Y.G. and C.L.; writing—review and editing, C.L. and H.W.; supervision, Y.W. and C.L.; project administration, H.W. and Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Project (grant number 2019YFC1604904). Science and Technology Innovation Platform Project of Jiangxi Province (grant number 20192BCD40001) and Research Project of State Key Laboratory of Food Science and Technology, China in Nanchang University (grant number SKLF-ZZB-202127).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Zhang, S.; Wang, J.; Zhao, H.; Luo, Y. Effects of three flavonoids from an ancient traditional Chinese medicine Radix puerariae on geriatric diseases. Brain Circ. 2018, 4, 174. [Google Scholar] [PubMed]
- Liu, J.; Zhang, J.; Liao, T.; Zhou, L.; Zou, L.; Liu, Y.; Zhang, L.; Liu, W. Thermal inactivation kinetics of kudzu (Puerariae lobata) polyphenol oxidase and the influence of food constituents. Foods 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qiu, L.; Wu, X.; Wei, H.; Xu, F. Evaluation of kudzu root extract-induced hepatotoxicity. J. Ethnopharmacol. 2015, 176, 321–326. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, B.; Ma, M. Pathogenesis of acute hemolysis and renal failure induced by puerarin. Chin. J. New Med. Clin. Med. 2003, 22, 699–702. [Google Scholar]
- Guan, L.; Chen, Z. Possible developmental and reproductive toxicity of isoflavones in soybean and Kudzu root. Asia Pac. J. Clin. Nutr. 2005, 14, S105. [Google Scholar]
- Yang, L.; Yang, C.; Li, C.; Zhao, Q.; Liu, L.; Fang, X.; Chen, X.-Y. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016, 61, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Mukhlynina, E.A.; Moroz, G.A.; Gette, I.F.; Danilova, I.G.; Kovaleva, E.G. Anti-diabetic effect of isoflavone rich kudzu root extract in experimentally induced diabetic rats. J. Funct. Foods 2020, 68, 103922. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H.; Dong, J.; Wang, H.; Hashi, Y.; Chen, S. Identification of isoflavonoids in Radix puerariae for quality control using on-line high performance liquid chromatography-diode array detector-electrospray ionization-mass spectrometry coupled with post-column derivatization. Food Res. Int. 2012, 48, 528–537. [Google Scholar] [CrossRef]
- Chen, T.R.; Chen, L.A.; Wei, Q.K. Evaluation of quality of Radix puerariae herbal medicine by isoflavonoids. J. Pharm. Pharmacol. 2010, 62, 644–650. [Google Scholar] [CrossRef]
- Boué, S.M.; Wiese, T.E.; Nehls, S.; Burow, M.E.; Elliott, S.; Carter-Wientjes, C.H.; Shih, B.Y.; McLachlan, J.A.; Cleveland, T.E. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J. Agric. Food Chem. 2003, 51, 2193–2199. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, E.; Guan, Y.; Xu, P.; Shen, Q.; Liu, Z.; Zhu, W.; Chen, L.; Liu, H.; Dong, H. Safety of high-dose Puerariae Lobatae Radix in adolescent rats based on metabolomics. Food Sci. Nutr. 2021, 9, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhao, H.; Zhang, Q.; Li, G.-H.; Yang, F.; Wang, Y.; Li, Y.; Wang, Y. A rapid method for simultaneous determination of 14 phenolic compounds in Radix puerariae using microwave-assisted extraction and ultra high performance liquid chromatography coupled with diode array detection and time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhao, H.; Song, Y.; Zhang, Q.; Wang, Y. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography–triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Zhang, C.; Wu, W.; Liang, X. Analysis of the estrogenic components in kudzu root by bioassay and high performance liquid chromatography. J. Steroid Biochem. Mol. Biol. 2005, 94, 375–381. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Zhang, F.; Liu, J.; Ren, Z.; Xu, Y.; Liu, T.; Zhou, W.; Li, H.; Zhang, C. An analytical strategy for accurate, rapid and sensitive quantitative analysis of isoflavones in traditional Chinese medicines using ultra-high performance supercritical fluid chromatography: Take Radix puerariae as an example. J. Chromatogr. A 2019, 1606, 460385. [Google Scholar] [CrossRef]
- Fan, J.-P.; Mao, D.-Y.; Zhang, X.-H.; Qi, G.-X.; Liao, D.-D.; Chen, H.-P.; Huang, K. Preparation and characterization of a novel freestanding flexible reduced graphene oxide composite membrane for adsorption of isoflavone in Radix puerariae Lobatae. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124911. [Google Scholar] [CrossRef]
- Fan, J.-P.; Cheng, Y.-T.; Zhang, X.-H.; Xiao, Z.-P.; Liao, D.-D.; Chen, H.-P.; Huang, K.; Peng, H.-L. Preparation of a novel mixed non-covalent and semi-covalent molecularly imprinted membrane with hierarchical pores for separation of genistein in Radix puerariae Lobatae. React. Funct. Polym. 2020, 146, 104439. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Y.; Xu, Y.; Guo, X.; Guo, L.; Tan, T.; Liu, F.; Wan, Y. Study of the contents of analogues of aristolochic acid in Houttuynia cordata by ultra-high performance liquid chromatography tandem mass spectrometry. Foods 2022, 11, 302. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Q.; Zhang, G.; Zhao, W.; Lian, H.; Zhang, S. Solid phase extraction and high performance liquid chromatography with tetraaza [2] arene [2] triazine-bonded silica gel adsorbent for determination of nitrophenols and diethylstilbestrol in river water. Chin. J. Chromatogr. 2020, 38, 143–148. [Google Scholar]
- Tuzimski, T.; Szubartowski, S. Method development for selected bisphenols analysis in sweetened condensed milk from a can and breast milk samples by HPLC–DAD and HPLC-QqQ-MS: Comparison of sorbents (Z-SEP, Z-SEP Plus, PSA, C18, Chitin and EMR-Lipid) for clean-up of QuEChERS extract. Molecules 2019, 24, 2093. [Google Scholar] [CrossRef]
- Kirschner, N.; Dias, A.N.; Budziak, D.; da Silveira, C.B.; Merib, J.; Carasek, E. Novel approach to high-throughput determination of endocrine disruptors using recycled diatomaceous earth as a green sorbent phase for thin-film solid-phase microextraction combined with 96-well plate system. Anal. Chim. Acta 2017, 996, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, M.; Zheng, Z.; Wan, Y.; Wu, S.; Li, C. Preparation of ZIF-8 and Its Application in Determination of Pyridoxine and Pyridoxal in Ginkgo Seeds by Ultra-Performance Liquid Chromatography. Foods 2022, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Song, K.; Zhang, Q.; Guo, J.; Huang, J. Simultaneous determination of six isoflavones from puerariae lobatae radix by cpe-hplc and effect of puerarin on tyrosinase activity. Molecules 2020, 25, 344. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Xing, J.; Wang, C.; Song, J.; Li, C.; Yang, X.; Yang, G. Simultaneous analysis and retention behavior of major isoflavonoids in Radix puerariae lobatae and Radix puerariae thomsonii by high performance liquid chromatography with cyclodextrins as a mobile phase modifier. Anal. Chim. Acta 2012, 712, 145–151. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Fan, E. Optimisation of ultrasound-assisted extraction of puerarin and total isoflavones from Puerariae Lobatae Radix (Puerariae lobata (Wild.) Ohwi) with response surface methodology. Phytochem. Anal. 2012, 23, 513–519. [Google Scholar] [CrossRef]
- He, J.; Zhao, Z.; Shi, Z.; Zhao, M.; Li, Y.; Chang, W. Analysis of isoflavone daidzein in Puerariae radix with micelle-mediated extraction and preconcentration. J. Agric. Food Chem. 2005, 53, 518–523. [Google Scholar] [CrossRef]
- Lin, F.; Giusti, M.M. Effects of solvent polarity and acidity on the extraction efficiency of isoflavones from soybeans (Glycine max). J. Agric. Food Chem. 2005, 53, 3795–3800. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, C.-C. Comparison of techniques for extraction of isoflavones from the root of Radix puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E. Application of quechers method for the determination of phenylurea herbicides in beetroot by hplc with uv-vis detection. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 145–147. [Google Scholar] [CrossRef]
- Wu, X.; Ding, Z. Evaluation of matrix effects for pesticide residue analysis by QuEChERs coupled with UHPLC-MS/MS in complex herbal matrix. Food Chem. 2022, 405, 134755. [Google Scholar] [CrossRef]
- Chen, S.-B.; Liu, H.-P.; Tian, R.-T.; Yang, D.-J.; Chen, S.-L.; Xu, H.-X.; Chan, A.S.; Xie, P.-S. High-performance thin-layer chromatographic fingerprints of isoflavonoids for distinguishing between Radix puerariae Lobate and Radix puerariae Thomsonii. J. Chromatogr. A 2006, 1121, 114–119. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).