Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration
Abstract
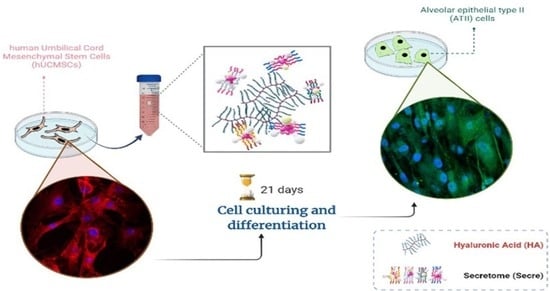
1. Introduction
2. Results
2.1. Immunofluorescence Anti-SPC and Anti-CD73
2.2. Surfactant Proteins Expression
2.3. Cell Viability
2.4. Cell Migration Study
2.5. In Vitro Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. HA/Secretome Solution Preparation
4.3. Cell Culture
4.4. Immunofluorescence
4.5. Surfactant Proteins Expression
4.6. Cell Viability
4.7. Cell Migration Study
4.8. In Vitro Inflammatory Response
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgoyne, R.A.; Fisher, A.J.; Borthwick, L.A. The Role of Epithelial Damage in the Pulmonary Immune Response. Cells 2021, 10, 2763. [Google Scholar] [CrossRef]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Puig, F.; Camprubí-Rimblas, M.; Herrero, R.; Serrano-Mollar, A.; Gómez, M.N.; Tijero, J.; Matthay, M.A.; Blanch, L.; Artigas, A. Intratracheal instillation of alveolar type II cells enhances recovery from acute lung injury in rats. J. Heart Lung Transplant. 2018, 37, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; e Silva, J.R.L.; Rocco, P.R. Mesenchymal stromal cell therapy in COPD: From bench to bedside. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3017. [Google Scholar] [CrossRef]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC based therapies—New perspectives for the injured lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.y.; Zhou, T.y.; Zhang, Z.d.; Liu, H.y.; Zheng, Z.y.; Xie, H.q. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm 2021, 2, 351–380. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, G.; Silvestro, S.; Avanzini, M.A.; Zuccotti, G.; Mazzon, E.; Calcaterra, V. Mesenchymal Stromal Cells for the Treatment of Interstitial Lung Disease in Children: A Look from Pediatric and Pediatric Surgeon Viewpoints. Cells 2021, 10, 3270. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, P.; Larijani, B.; Alavi-Moghadam, S.; Tayanloo-Beik, A.; Mohamadi-Jahani, F.; Ranjbaran, N.; Payab, M.; Falahzadeh, K.; Mousavi, M.; Arjmand, B. Mesenchymal stem cells-derived exosomes for wound regeneration. Cell Biol. Transl. Med. 2018, 4, 119–131. [Google Scholar]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.L.; Corsico, A.G. Mesenchymal stromal cell secretome for severe COVID-19 infections: Premises for the therapeutic use. Cells 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, F.; di Gennaro, M.; Lista, G.; Messina, F.; Ambrosio, L.; Borzacchiello, A. Effect of Hyaluronic acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes. Polymers 2021, 13, 2928. [Google Scholar] [CrossRef]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-coated nanoparticles for active tumor targeting: Influence of polysaccharide molecular weight on cell uptake. Colloids Surf. B Biointerfaces 2022, 210, 112240. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, Y.; Qiu, D. Design of selective cell migration biomaterials and their applications for tissue regeneration. J. Mater. Sci. 2021, 56, 4080–4096. [Google Scholar] [CrossRef]
- Fuchs, S.; Hollins, A.; Laue, M.; Schaefer, U.; Roemer, K.; Gumbleton, M.; Lehr, C.-M. Differentiation of human alveolar epithelial cells in primary culture: Morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003, 311, 31–45. [Google Scholar] [CrossRef]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.; Kim, J.M.; Kim, J.R.; Kim, K.J.; Kim, Y.J.; Park, S.I.; Jeong, J.H.; Moon, Y.m.; Lim, H.S. Systemic transplantation of human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. J. Cell. Mol. Med. 2011, 15, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.-g.; Gudapati, V.; Lim, H.; Lee, J.W. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin. Biol. Ther. 2016, 16, 859–871. [Google Scholar] [CrossRef]
- Hendijani, F.; Javanmard, S.H.; Rafiee, L.; Sadeghi-Aliabadi, H. Effect of human Wharton’s jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res. Pharm. Sci. 2015, 10, 134. [Google Scholar]
- Dostert, G.; Mesure, B.; Menu, P.; Velot, É. How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front. Cell Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. Hyaluronic acid-based wound dressing with antimicrobial properties for wound healing application. Appl. Sci. 2022, 12, 3091. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Petroniene, J.; Morkvenaite-Vilkonciene, I.; Miksiunas, R.; Bironaite, D.; Ramanaviciene, A.; Rucinskas, K.; Janusauskas, V.; Ramanavicius, A. Scanning electrochemical microscopy for the investigation of redox potential of human myocardium-derived mesenchymal stem cells grown at 2D and 3D conditions. Electrochim. Acta 2020, 360, 136956. [Google Scholar] [CrossRef]
- Petroniene, J.; Morkvenaite-Vilkonciene, I.; Miksiunas, R.; Bironaite, D.; Ramanaviciene, A.; Mikoliunaite, L.; Kisieliute, A.; Rucinskas, K.; Janusauskas, V.; Plikusiene, I. Evaluation of redox activity of human myocardium-derived mesenchymal stem cells by scanning electrochemical microscopy. Electroanalysis 2020, 32, 1337–1345. [Google Scholar] [CrossRef]
- Yang, H.; Cheam, N.M.J.; Cao, H.; Lee, M.K.H.; Sze, S.K.; Tan, N.S.; Tay, C.Y. Materials stiffness-dependent redox metabolic reprogramming of mesenchymal stem cells for secretome-based therapeutic angiogenesis. Adv. Healthc. Mater. 2019, 8, 1900929. [Google Scholar] [CrossRef] [PubMed]
- Chouw, A.; Milanda, T.; Sartika, C.R.; Kirana, M.N.; Halim, D.; Faried, A. Potency of mesenchymal stem cell and its secretome in treating COVID-19. Regen. Eng. Transl. Med. 2021, 8, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kudinov, V.A.; Artyushev, R.I.; Zurina, I.M.; Zorina, E.S.; Lapshin, R.D.; Snopova, L.B.; Mukhina, I.V.; Saburina, I.N. Inhaled Placental Mesenchymal Stromal Cell Secretome from Two-and Three-Dimensional Cell Cultures Promotes Survival and Regeneration in Acute Lung Injury Model in Mice. Int. J. Mol. Sci. 2022, 23, 3417. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural formulations provide antioxidant complement to hyaluronic acid-based topical applications used in wound healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Makvandi, P.; Della Sala, F.; di Gennaro, M.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. A Hyaluronic acid-Based Formulation with Simultaneous Local Drug Delivery and Antioxidant Ability for Active Viscosupplementation. ACS Omega 2022, 7, 10039–10048. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Sala, F.; Longobardo, G.; Lista, G.; Messina, F.; Borzacchiello, A. Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration. Int. J. Mol. Sci. 2023, 24, 3642. https://doi.org/10.3390/ijms24043642
Della Sala F, Longobardo G, Lista G, Messina F, Borzacchiello A. Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration. International Journal of Molecular Sciences. 2023; 24(4):3642. https://doi.org/10.3390/ijms24043642
Chicago/Turabian StyleDella Sala, Francesca, Gennaro Longobardo, Gianluca Lista, Francesco Messina, and Assunta Borzacchiello. 2023. "Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration" International Journal of Molecular Sciences 24, no. 4: 3642. https://doi.org/10.3390/ijms24043642
APA StyleDella Sala, F., Longobardo, G., Lista, G., Messina, F., & Borzacchiello, A. (2023). Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration. International Journal of Molecular Sciences, 24(4), 3642. https://doi.org/10.3390/ijms24043642