Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
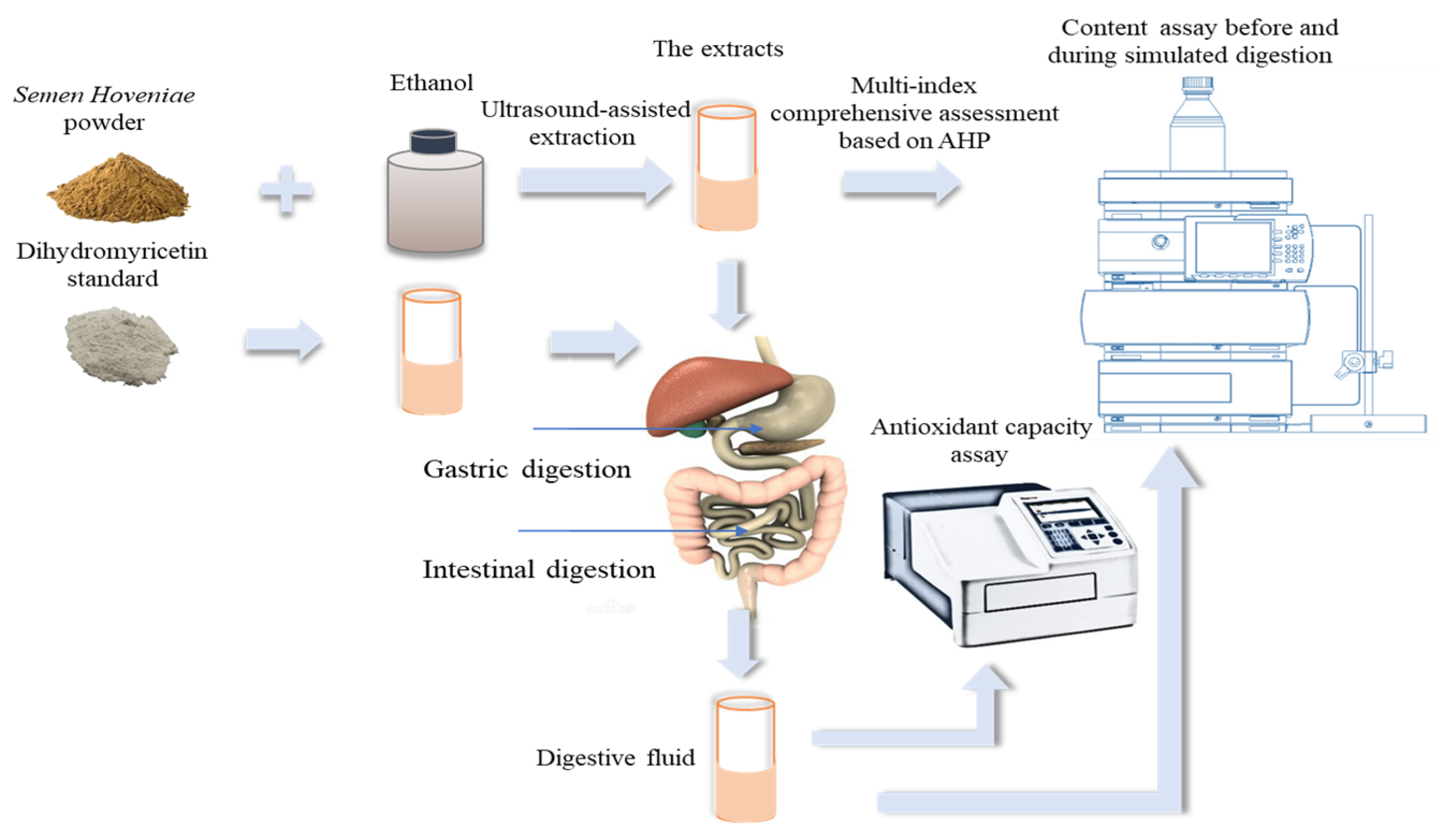
2.2. Extraction of Flavonoids Compounds
2.3. Experimental Design
2.4. Multi-Index Grading Method
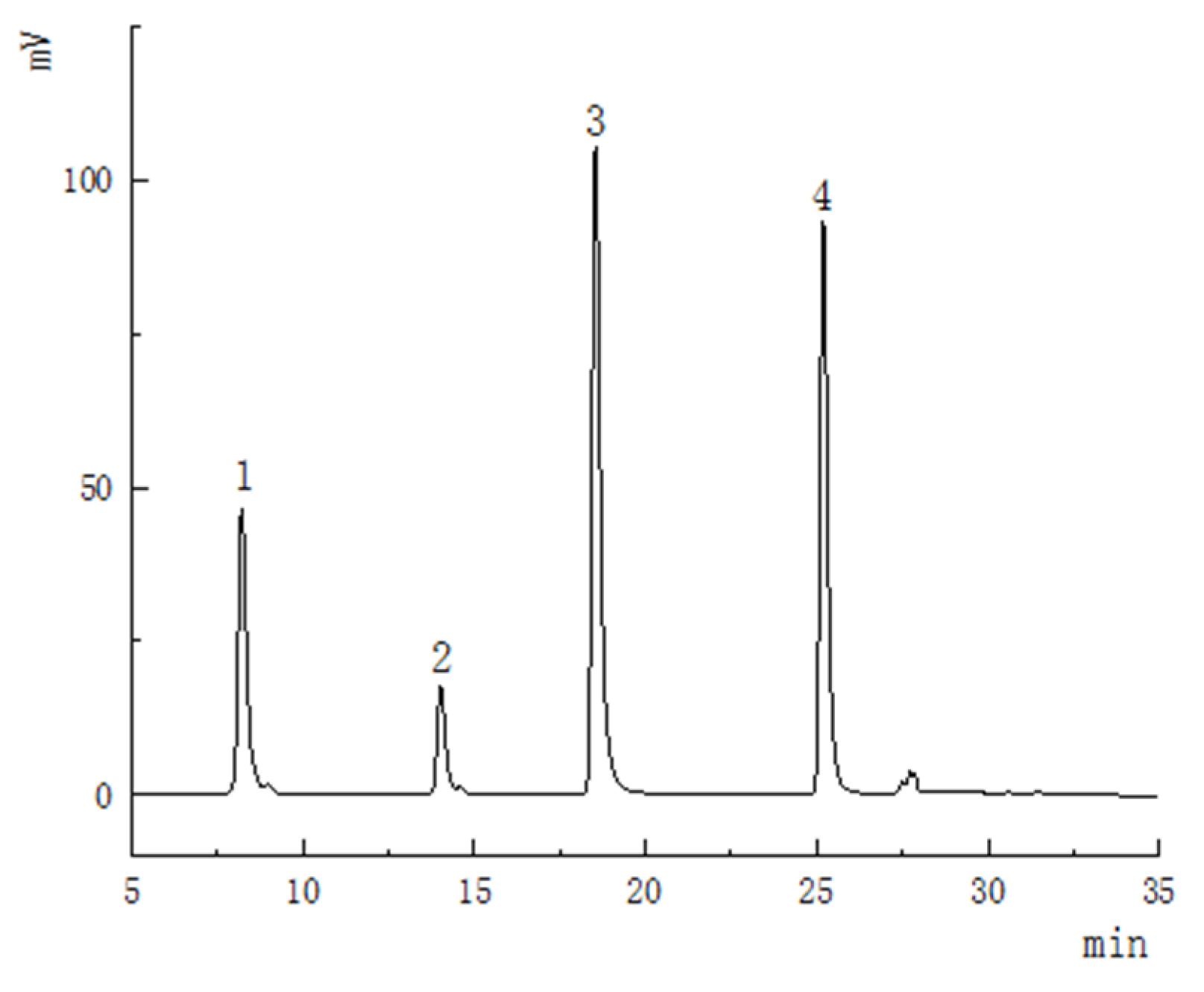
2.5. HPLC Analysis of Flavonoids Compounds in Optimum Conditions
2.6. Analysis of Total Phenolic and Flavonoids Content
2.7. In Vitro Simulated Digestion
2.8. Antioxidant Capacity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Multi-index Grading Method with AHP
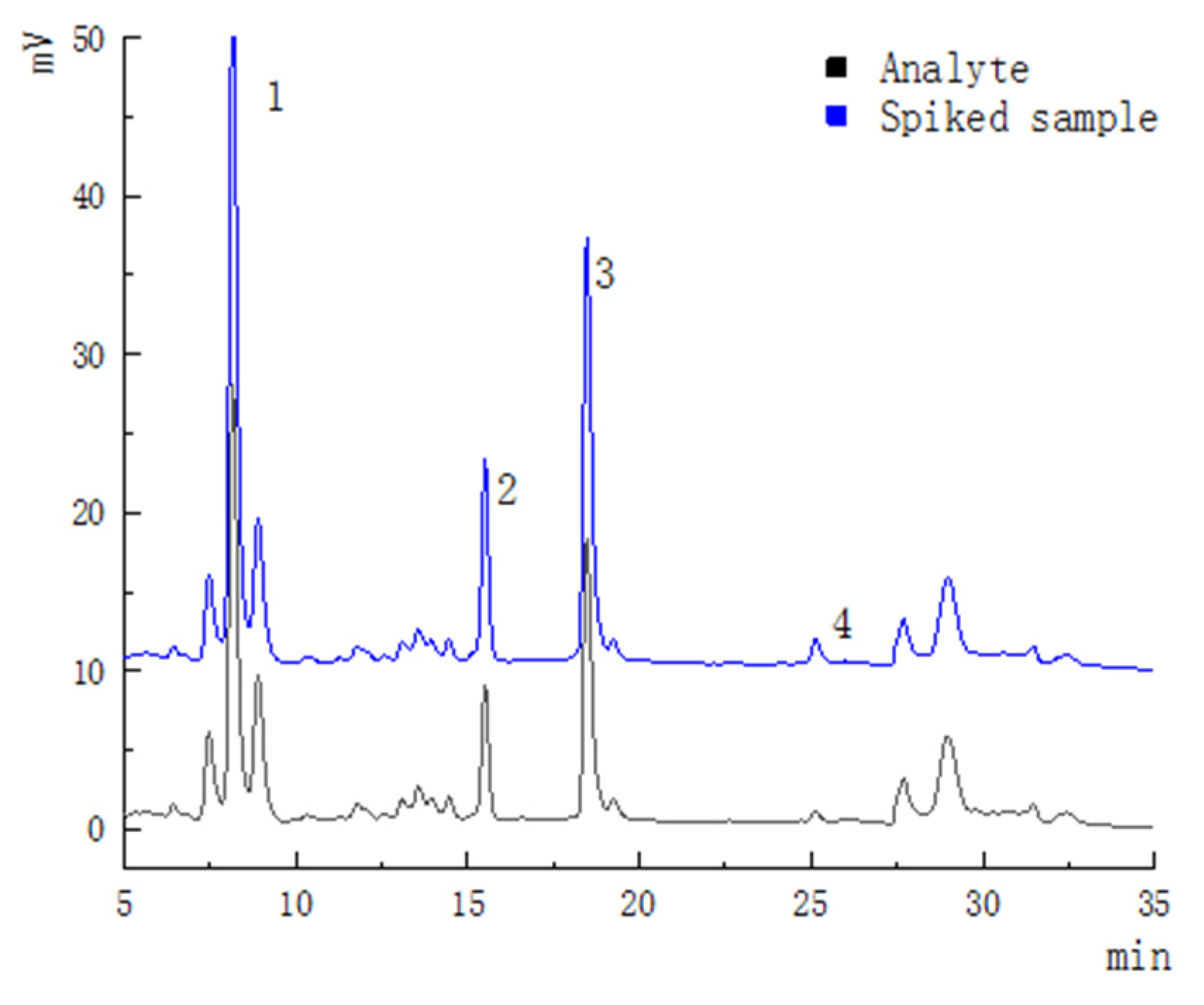
3.2. Methodology Validation for HPLC Analysis
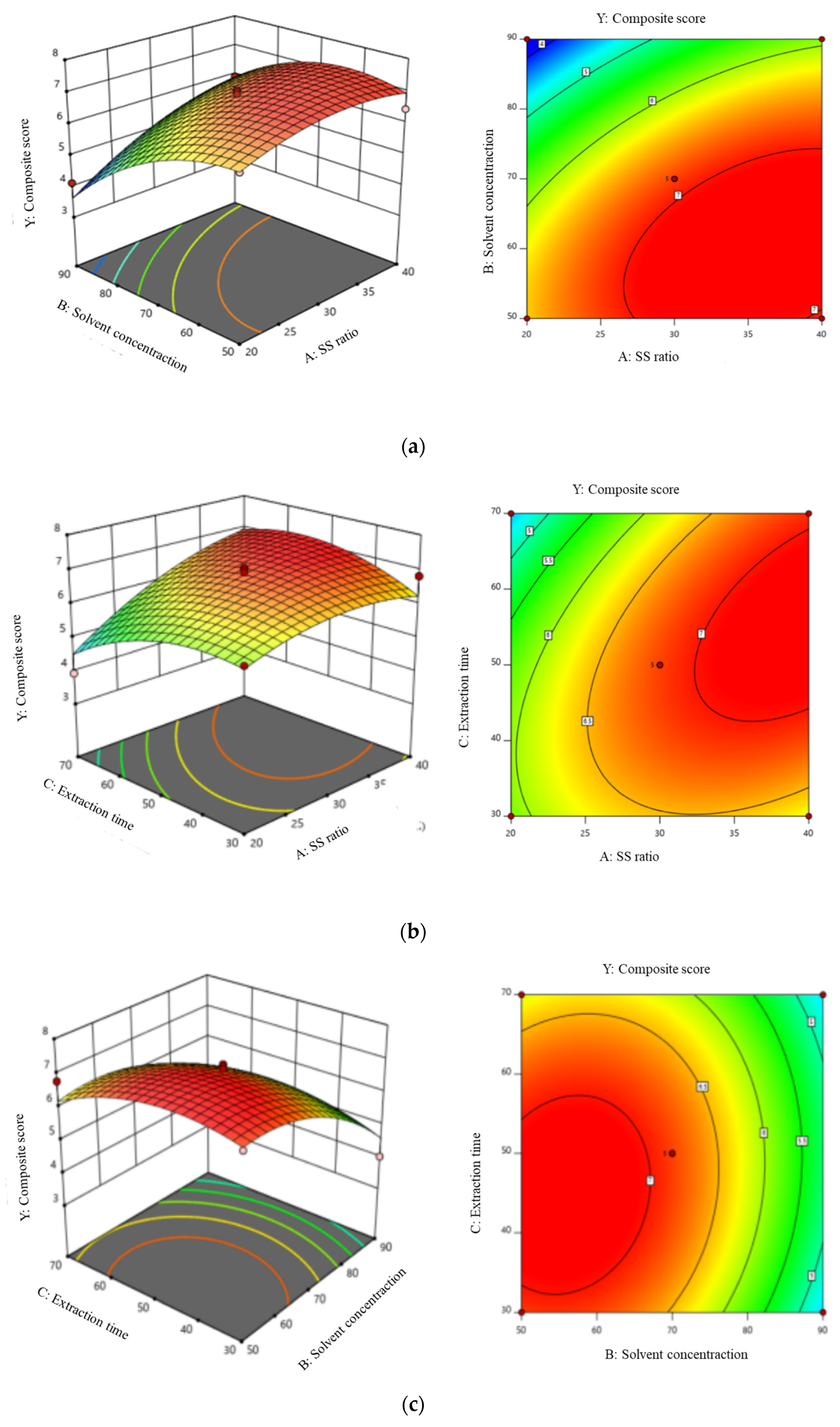
3.3. Optimization of Semen Hoveniae Flavonoids Extraction by BBD
3.4. Fitting the Model
3.5. Verification of Predictive Models
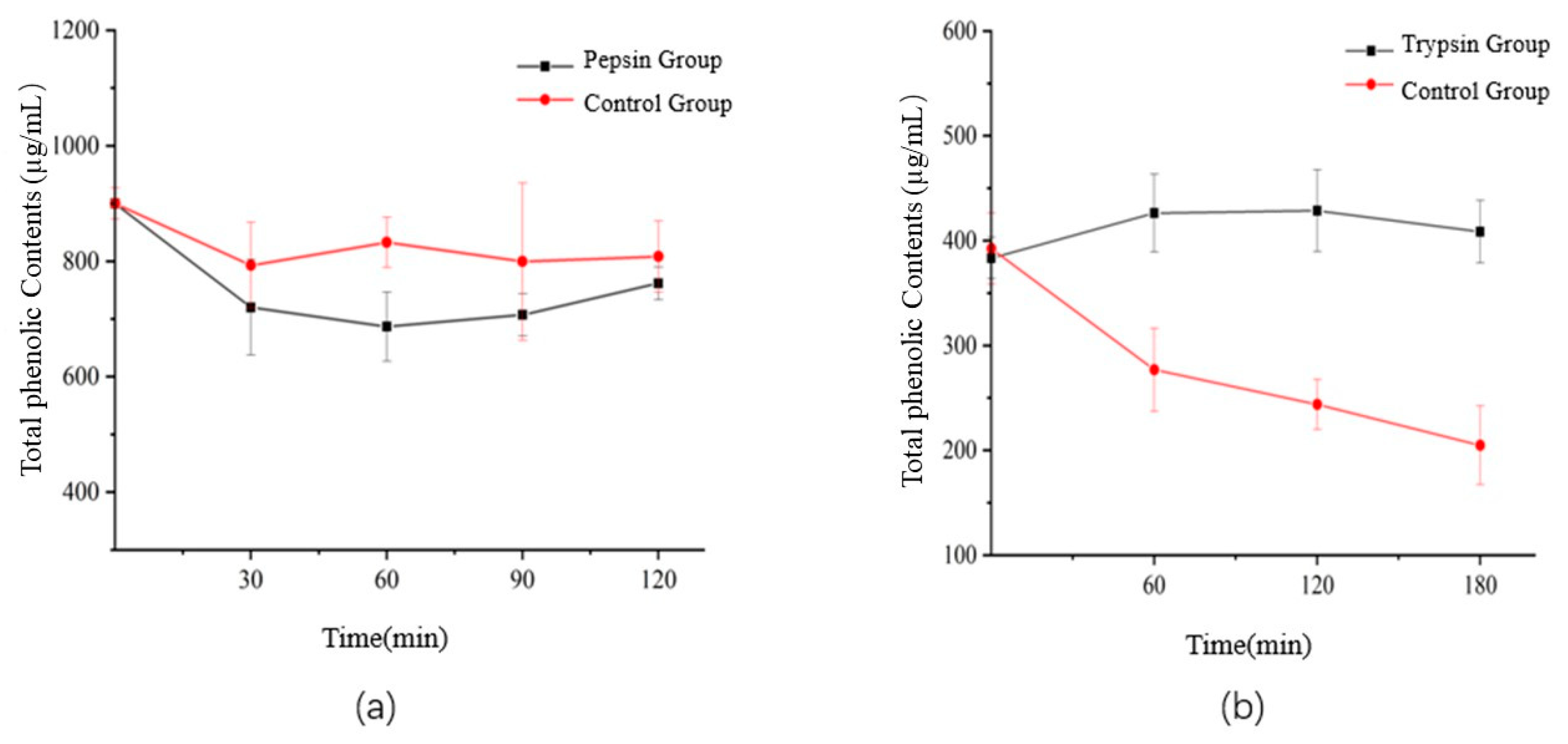
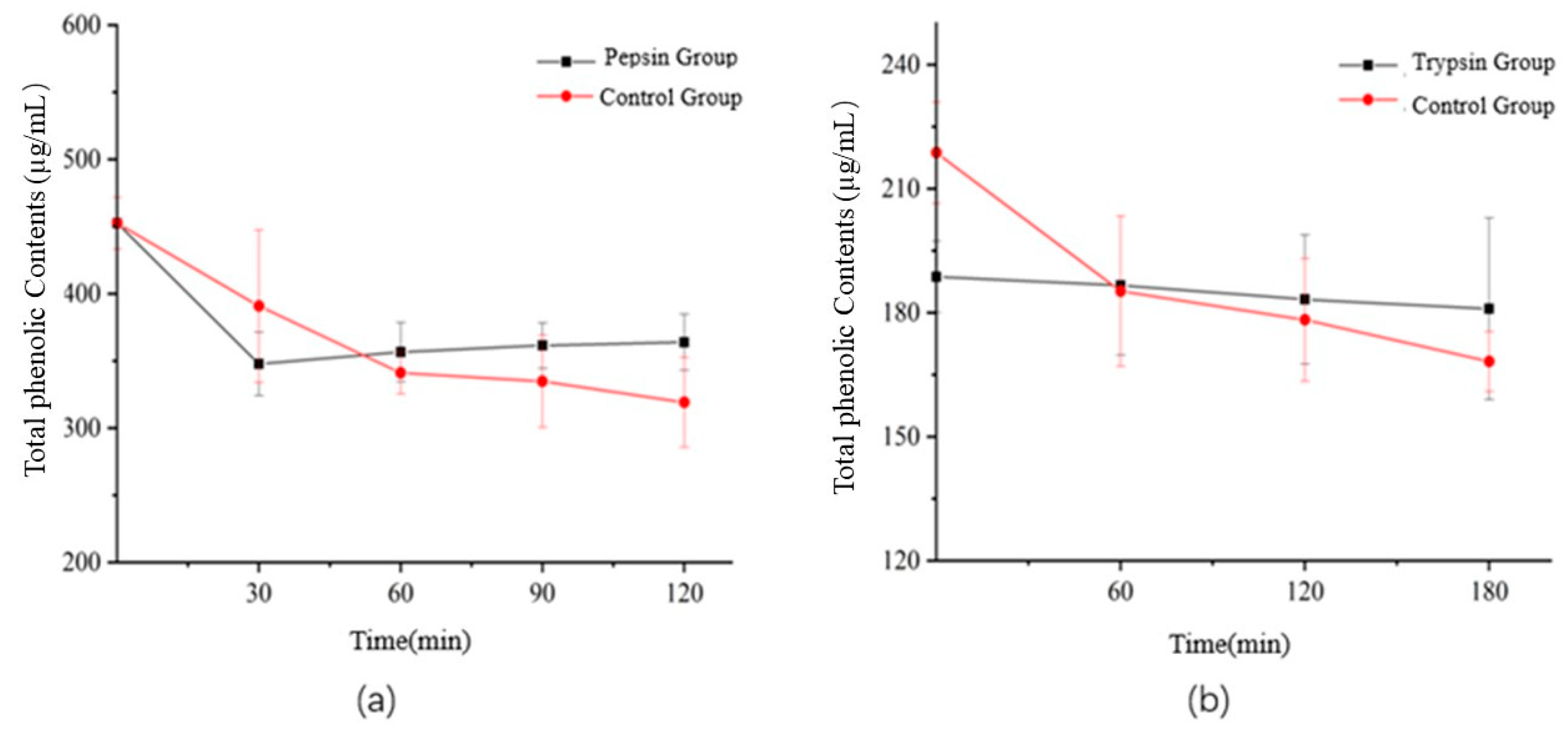
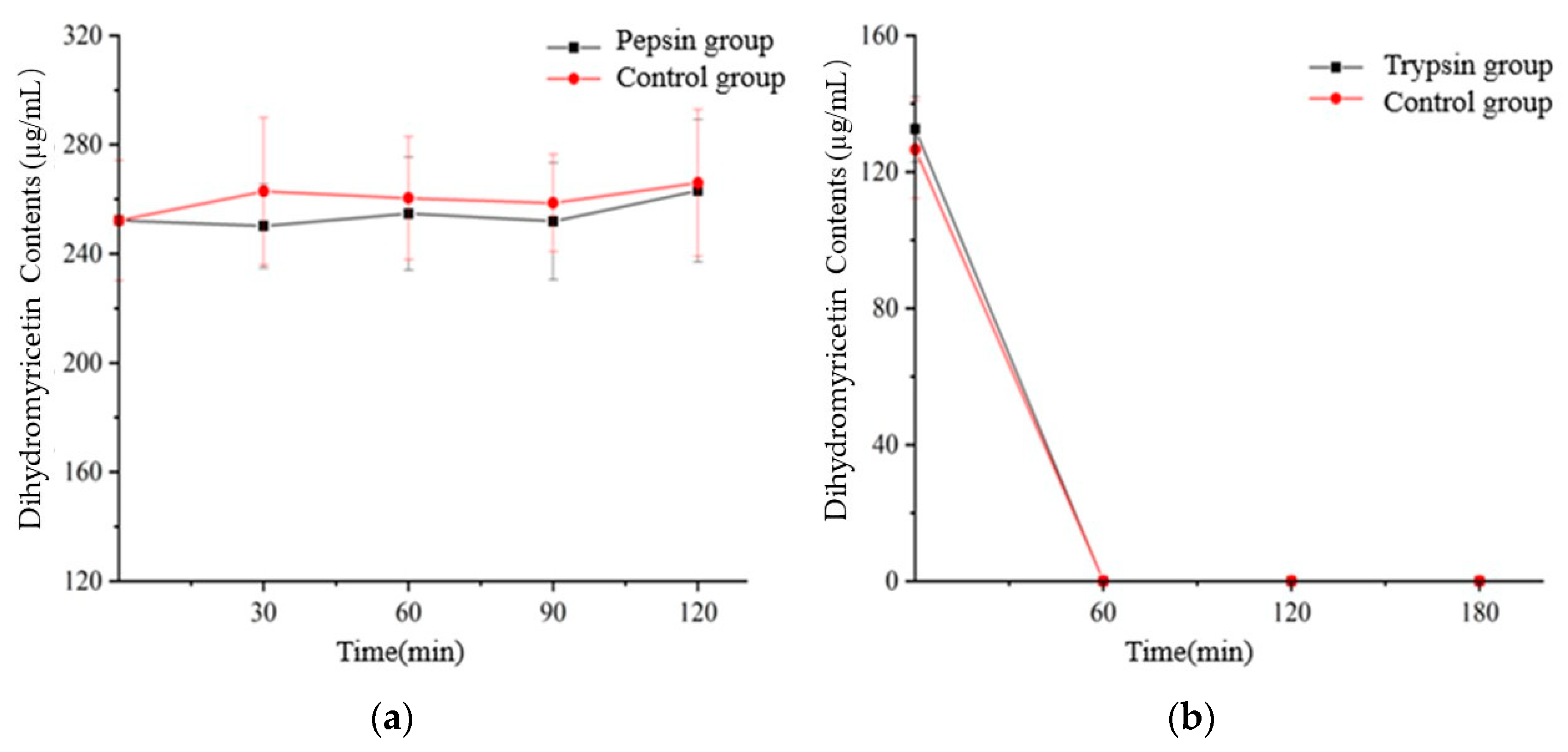
3.6. Effect of Digestion on the Flavonoids and Total Phenolic Contents
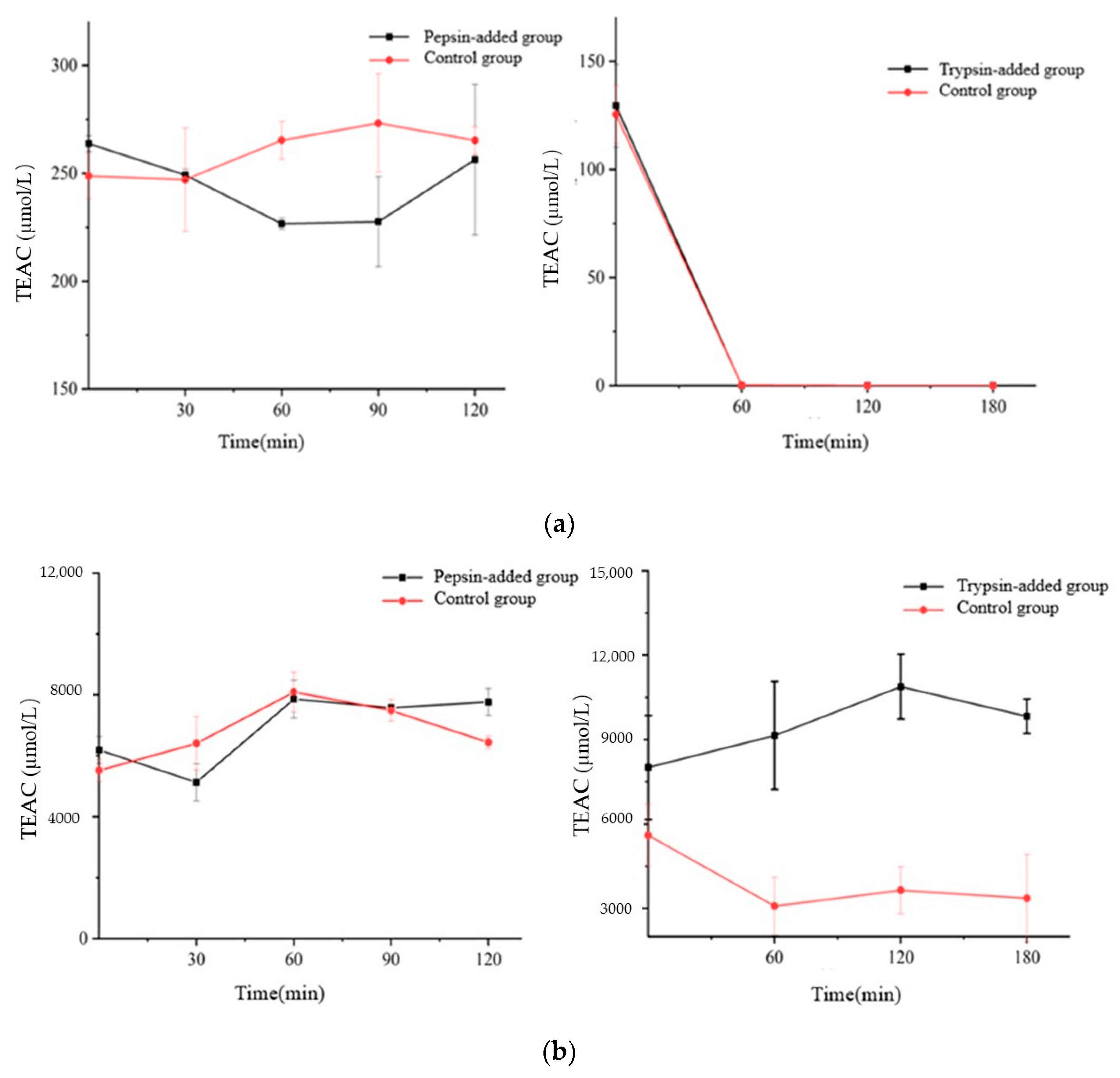
3.7. Effect of Digestion on the Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.P.; Ye, G.Y. Analysis and Evaluation of Nutritional Components and Total Flavonoids in Hovenia dulcis Thunb. Food Res. Dev. 2016, 37, 4. [Google Scholar]
- Li, M.-C.; Xie, C.-J.; Meng, C.-W.; Zhang, Y.-Q.; Gao, J.-G.; Wang, W.-H.; Liu, J.Y.; Xu, Y.N. Chemical constituents from Hovenia dulcis Thunb. And their chemotaxonomic significance. Biochem. Syst. Ecol. 2021, 94, 104204. [Google Scholar]
- Wang, Y.P.; Yuan, G.Y.; Chen, Y.Y.; Cao, Y.; Xie, K.Y.; Han, E.F.; Sun, R.L.; Gu, Y. Analysis and Evaluation of Nutrition Composition and Total Flavonoids on Hovenia Seed. Food Res. Dev. 2016, 37, 21–24. [Google Scholar]
- Qiu, P.; Dong, Y.; Zhu, T.; Luo, Y.Y.; Kang, X.J.; Pang, M.X.; Li, H.Z.; Xu, H.; Gu, C.; Pan, S.H.; et al. Semen hoveniae extract ameliorates alcohol-induced chronic liver damage in rats via modulation of the abnormalities of gut-liver axis. Phytomed. Int. J. Phytother. Phytopharm. 2019, 52, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Cho, S.S.; Yoon, I.S. Hot-water extract of the branches of Hovenia dulcis Thunb. (Rhamnaceae) ameliorates low-fiber diet-induced constipation in rats. Drug Des. Dev. Ther. 2018, 12, 695–703. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Hoveniae Semen Seu Fructus Ethanol Extract Exhibits Anti-Inflammatory Activity via MAPK, AP-1, and STAT Signaling Pathways in LPS-Stimulated RAW 264.7 and Mouse Peritoneal Macrophages. Mediat. Inflamm. 2019, 2019, 9184769. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueda, T.; Yoshizumi, S.; Ninomiya, K.; Murakami, N.; Matsuda, H.; Saito, M.; Fujii, W.; Tanaka, T.; et al. [Bioactive constituents of Chinese natural medicines. III. Absolute stereostructures of new dihydroflavonols, hovenitins I, II, and III, isolated from hoveniae semen seu fructus, the seed and fruit of Hovenia dulcis THUNB. (Rhamnaceae): Inhibitory effect on alcohol-induced muscular relaxation and hepatoprotective activity]. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1997, 117, 108–118. [Google Scholar] [CrossRef]
- Gong, X.; Ji, M.; Xu, J.; Zhang, C.; Li, M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020, 60, 2303–2326. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Y.L. Research progress on hypoglycemic effect of bioactive components of chinese herbal medicine. Clin. J. Chin. Med. 2020, 12, 57–59. [Google Scholar]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J.; et al. Antidiabetic Potential of Flavonoids from Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2020, 120, 159–235. [Google Scholar] [CrossRef] [PubMed]
- Tao, D. Study on the Renal Protective Effect and Mechanism of Dihydroquercetin on Diabetes Nephropathy. Master’s Thesis, Naval Medical University, Shanghai, China, 2019. [Google Scholar]
- Xie, S.D.; Chen, H.Q.; Zhang, Y. Optimization of Extraction Process and Antioxidant Activity of Total Flavonoids from Hovenia dulcis Thunb. Storage Process 2021, 21, 56–63. [Google Scholar]
- Wang, Y.H.; Jiang, X.Y.; Sun, R.L. Research Progress on Extraction Methods and Efficacy of Flavonoids from Hovenia dulcis Thunb. Henan Agric. 2022, 3, 50–54. [Google Scholar]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.H.; Moreira, T.F.M.; de Oliveira, A.; Bracht, L.; Ineu, R.P.; Leimann, F.V. Antioxidant Activity of Encapsulated Extracts and Bioactives from Natural Sources. Curr. Pharm. Des. 2020, 26, 3847–3861. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Xian, M.L.; Du, G.; Liu, X.; He, J.J.; Wang, P.; Qin, W.; Zhao, L. Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities. Foods 2020, 9, 456. [Google Scholar] [CrossRef]
- Li, G.; Weiru, L.; Yanlin, d. Bibliometric Analysis of Intervention Research on Drug and Food Homologous Ingredients in Type 2 diabetes Based on CiteSpace. Fujian J. Tradit. Chin. Med. 2021, 52, 4. [Google Scholar]
- Yu, X.Z.; Wang, L.; Du, S.K.; Li, Z.X.; Zhang, J.Y. Optimization of Ultrasonic Extraction of Total Flavonoids from Hovenia dulcis Thunb by Response Surface Methodology. Food Sci. 2011, 32, 5. [Google Scholar]
- Hou, J.L.; Mao, G.N.; Hu, Y.; He, Y.J.; Yang, W.J.; Kong, Y. Study on the Antioxidant Activity of Gegen and Hovenia dulcis Thunb in Vitro. Prog. Vet. Med. 2020, 41, 7. [Google Scholar]
- Tian, Z.Y.; Chai, Z.; Xu, Y.F. Optimization of Extraction Process of Total Flavonoids from Hovenia dulcis Thunb by Orthogonal Design. J. Henan Univ. 2015, 34, 5. [Google Scholar]
- Wang, L. Study on Processing and Functional Characteristics of Hovenia dulcis Thunb. Master’s Thesis, Northwest A&F University, Xian, China, 2010. [Google Scholar]
- Zhang, H.X.; Yang, S.J.; Zhang, J. Extraction Technology of Total Flavonoids from Hovenia dulcis Thunb and Its Antialcoholic Effect. J. Food Biotechnol. 2006, 25, 4. [Google Scholar]
- Cai, X.J.; Qu, Y.P.; Wang, Y.T.; Li, Z.; Jiang, G.Z. Optimization of Extraction Process of Hovenia dulcis Thunb by Response Surface Methodology. West. Chin. Med. 2018, 31, 5. [Google Scholar]
- Sun, Y.F.; Guan, M.; Shi, J. Extraction process optimization of Jinmai Gargle based on analytic hierarchy process and multi-index orthogonal design. China Pharm. 2022, 25, 4. [Google Scholar]
- Han, Y.F.; Tang, X.; Shi, Y.; Pu, L.L.; Pan, L.; Lai, X.R.; Zhang, F.W. Optimization of Extraction Process for Yangyin Runmu Granules by Box-Behnken Design Based on Entropy Weight Method-analytic Hierarchy Process Method. Chin. J. Mod. Appl. Pharm. 2022, 39, 896–903. [Google Scholar]
- Lan, J.L.; Ruan, Y.P.; Qiu, L.Q. [Multi-index optimization of extraction process of Fengyin Decoction based on BAS-GA-BP neural network combined with entropy weight method]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2020, 45, 5686–5693. [Google Scholar] [CrossRef]
- Xia, L.; Ho, C.; Lin, X. Evaluation of the elderly health examination app based on the comprehensive evaluation method of AHP-fuzzy theory. Math. Biosci. Eng. MBE 2021, 18, 4731–4742. [Google Scholar] [CrossRef] [PubMed]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Braimah, M.N.; Anozie, A.N.; Odejobi, O.J. Utilization of Response Surface Methodology (RSM) in the Optimization of Crude Oil Refinery Process; New Port-Harcourt Refinery: Alesa Eleme, Nigeria, 2016. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, D.J.; Zhang, G.F.; Lu, B.X. Advances in the study of in vitro simulated digestion. Food Mach. 2021, 37, 201–206. [Google Scholar]
- Zhu, R.W.; Dun, H.Y.; Liu, T.; Lu, J. Effects of in vitro simulated digestion on polyphenol, flavonoid anthocyanin and synergistic antioxidant activity of composite black rice porridge. Food Ferment. Ind. 2021, 47, 133–140. [Google Scholar]
- Liu, Y. Improving Phenolic Bioactivity in Double-Strain Fermented oats And their Characteristics In Vitro Digestion. Master’s Thesis, South China University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Liu, S.L.; Yu, M.M.; Pan, Q.Q.; Shen, R.L.; Li, H.; Xiang, Q.S. Changes of total phenols and antioxidant activity of rosemary extract during simulated gastrointestinal digestion. Sci. Technol. Food Ind. 2019, 40, 5. [Google Scholar]
- Fan, L.J.; Song, A.K.; Wang, Z.P.; Wang, J.H.; Li, X.W.; Wang, W. Changes of Antioxidant Components and Antioxidant Activity of Grape Seed Polyphenol Extracts During in Vitro Simulated Digestion. Food Sci. Technol. 2022, 47, 235–242. [Google Scholar]
- Xia, W.; Lin, Y.; Gong, E.; Li, T.; Lian, F.; Zheng, B.; Liu, R. Wild pink bayberry fruit: The effect of in vitro gastrointestinal digestion on phytochemical profiles, and antioxidant and antiproliferative activities. Food Funct. 2021, 12, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Mei, Q.; Lu, Y.M.; Ji, M. AHP-Analysis and Research on Fuzzy Comprehensive Evaluation Method. China Saf. Sci. J. 2004, 14, 4. [Google Scholar]
- Yu, J.Y.; Wang, J.; Quan, Y.J.; Wang, Y. Study on Ultrasonic Assisted Extraction of Proanthocyanidins from White Lotus Seeds in Chuzhou Based on AHP Analysis. Food Saf. Guide 2021, 31, 117–121. [Google Scholar]
- Park, J.S.; Kim, I.S.; Shaheed Ur, R.; Na, C.S.; Yoo, H.H. HPLC Determination of Bioactive Flavonoids in Hovenia dulcis Fruit Extracts. J. Chromatogr. Sci. 2016, 54, 130–135. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Stecher, G.; Bonn, G.K. Determination of total polyphenolic compounds and flavonoids in Matricaria chamomella flowers. Pak. J. Pharm. Sci. 2019, 32, 2163–2165. [Google Scholar]
- Fernandes, A.J.; Ferreira, M.R.; Randau, K.P.; de Souza, T.P.; Soares, L.A. Total flavonoids content in the raw material and aqueous extractives from Bauhinia monandra Kurz (Caesalpiniaceae). Sci. World J. 2012, 2012, 923462. [Google Scholar] [CrossRef]
- Ma, H.; Hou, A.; Tang, J.; Zhong, A.; Li, K.; Xiao, Y.; Li, Z. Antioxidant Activity of Vitis davidii Foex Seed and Its Effects on Gut Microbiota during Colonic Fermentation after In Vitro Simulated Digestion. Foods 2022, 11, 2615. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Martínez-Monzó, J.; García-Segovia, P.; Becze, A.; Török, A.I.; Cadar, O.; Coldea, T.E.; Igual, M. In Vitro Digestibility of Minerals and B Group Vitamins from Different Brewers’ Spent Grains. Nutrients 2022, 14, 3512. [Google Scholar] [CrossRef] [PubMed]
- Ouerfelli, M.; Bettaieb Ben Kâab, L.; Almajano, M.P. Radical Scavenging and Antioxidant Activity of Anthyllis Vulneraria Leaves and Flowers. Molecules 2018, 23, 1657. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- GB/T 27404-2008; Specification for Laboratory Quality Control Food Physical and Chemical Testing. Standards Press of China: Beijing, China, 2009.
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Yang, I.; Jayaprakasha, G.K.; Patil, B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct. 2018, 9, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.L.; Peng, M.X.; Liu, D.; Sun, H.Y.; Tang, X.W. Changes in Total Polyphenols, Total Flavonoids and Antioxidant Activity of Citrus during in Vitro Gastrointestinal Digestion Process. Food Sci. 2016, 37, 8. [Google Scholar]
- Tischer Seraglio, S.K.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Oliveira Costa, A.C. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Cedola, A.; Palermo, C.; Centonze, D.; Del Nobile, M.A.; Conte, A. Characterization and Bio-Accessibility Evaluation of Olive Leaf Extract-Enriched “Taralli”. Foods 2020, 9, 1268. [Google Scholar] [CrossRef]
- Ge, H.; Chen, Y.; Chen, J.; Tian, J.; Liang, X.; Chen, L. Evaluation of antioxidant activities of ethanol extract from Ligusticum subjected to in-vitro gastrointestinal digestion. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 119, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.M.; Hu, G.P.; Cai, X.; Shi, X.P.; Ye, C.; Wang, F.; Hu, L.C.; Wang, Y.W. Stability and antioxidant activity of phenolic compounds during in vitro digestion in mulberry yoghurt. Acta Agric. Jiangxi 2022, 34, 178–185. [Google Scholar]
| Run | Factors | |||
|---|---|---|---|---|
| A | B | C | Composite Score | |
| 1 | −1 | −1 | 0 | 6.42 |
| 2 | +1 | −1 | 0 | 6.44 |
| 3 | −1 | +1 | 0 | 4.13 |
| 4 | +1 | +1 | 0 | 5.92 |
| 5 | −1 | 0 | −1 | 5.96 |
| 6 | +1 | 0 | −1 | 6.82 |
| 7 | −1 | 0 | +1 | 3.94 |
| 8 | +1 | 0 | +1 | 6.83 |
| 9 | 0 | −1 | −1 | 6.80 |
| 10 | 0 | +1 | −1 | 4.02 |
| 11 | 0 | −1 | +1 | 6.78 |
| 12 | 0 | +1 | +1 | 4.71 |
| 13 | 0 | 0 | 0 | 7.05 |
| 14 | 0 | 0 | 0 | 6.26 |
| 15 | 0 | 0 | 0 | 7.06 |
| 16 | 0 | 0 | 0 | 7.00 |
| 17 | 0 | 0 | 0 | 6.93 |
| True values | ||||
| −1 | 1:20 | 50 | 30 | |
| 0 | 1:30 | 70 | 50 | |
| +1 | 1:40 | 90 | 70 | |
| Judgment Scale | Definition | Reciprocal |
|---|---|---|
| 1 | The factor i is equally important than the factor j. | If the ratio of importance of factor i to factor j is dij, then the ratio of importance of factor j to factor i is dij = 1/dij |
| 3 | The factor i is more important than the factor j | |
| 5 | The factor i is obviously more important than the factor j | |
| 7 | The factor i is significantly more important than the factor j | |
| 9 | The factor i is extremely important than the factor j | |
| 2,4,6,8 | In the middle of the above two adjacent judgment scales |
| Project | A1 | A2 | A3 | A4 | W’ | W |
|---|---|---|---|---|---|---|
| A1 | 1 | 2 | 3 | 4 | 2.2134 | 0.4668 |
| A2 | 1/2 | 1 | 2 | 3 | 1.3161 | 0.2776 |
| A3 | 1/3 | 1/2 | 1 | 2 | 0.7598 | 0.1603 |
| A4 | 1/4 | 1/3 | 1/2 | 1 | 0.4518 | 0.0953 |
| Consistency Check | CR = 0.0072 < 0.1 | Pass | ||||
| Four Flavonoids | Regression Equation | Linear Range (μg/mL) | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Dihydromyricetin | Y = 0.5650X − 25.0060 | 10~2000 | 0.9919 | 0.3 | 0.8 |
| Taxifolin | Y = 0.82380X + 4.2838 | 10~1000 | 0.9992 | 0.3 | 0.6 |
| Myricetin | Y = 31.1250X − 233.3900 | 10~1000 | 0.9989 | 0.2 | 0.5 |
| Quercetin | Y = 33.6970X − 271.1300 | 1~250 | 0.9903 | 0.2 | 0.5 |
| Analyte | Nominal Concentration (μg/mL) | Observed Concentration (μg/mL, n = 6) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Dihydromyricetin | 50 | 47.87 ± 0.89 | 95.74 | 3.55 |
| 200 | 184.33 ± 0.47 | 92.16 | 1.39 | |
| 800 | 756.9 ± 0.25 | 94.61 | 3.91 | |
| Taxifolin | 10 | 11.56 ± 0.15 | 110.6 | 4.67 |
| 40 | 36.46 ± 0.09 | 91.15 | 1.04 | |
| 160 | 140.1 ± 0.17 | 87.5 | 2.13 | |
| Myricetin | 10 | 10.36 ± 0.13 | 103.6 | 0.97 |
| 40 | 42.59 ± 0.15 | 106.5 | 2.53 | |
| 160 | 134.57 ± 0.23 | 84.11 | 1.55 | |
| Quercetin | 5 | 4.17 ± 0.06 | 83.4 | 1.22 |
| 20 | 19.03 ± 0.12 | 95.15 | 3.31 | |
| 80 | 77.87 ± 0.14 | 97.34 | 0.57 |
| Source | SS | df | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 18.08 | 9 | 2.01 | 5.99 | 0.0139 * |
| A-Solid-liquid ratio | 3.87 | 1 | 3.87 | 11.54 | 0.0115 * |
| B-Solvent concentration | 7.36 | 1 | 7.36 | 21.93 | 0.0023 ** |
| C-extration time | 0.23 | 1 | 0.23 | 0.67 | 0.4387 |
| AB | 0.78 | 1 | 0.78 | 2.33 | 0.1709 |
| AC | 1.04 | 1 | 1.04 | 3.1 | 0.1217 |
| BC | 0.13 | 1 | 0.13 | 0.38 | 0.5575 |
| A2 | 0.71 | 1 | 0.78 | 2.11 | 0.1896 |
| B2 | 2.18 | 1 | 2.18 | 6.49 | 0.0382 * |
| C2 | 1.33 | 1 | 1.33 | 3.95 | 0.0871 |
| Residual | 2.35 | 7 | 0.34 | ||
| Lack of Fit | 1.88 | 3 | 0.63 | 5.38 | 0.0688 |
| Pure Error | 0.47 | 4 | 0.12 | ||
| Cor Total | 20.43 | 16 |
| Flavonoids Compounds | Group | Undigested (%) | Gastric Phase (%) | Intestinal Phase (%) |
|---|---|---|---|---|
| Dihydromyricetin | Enzyme-added | 100 | 94.39 a | 0 b |
| Eontrol | 100 | 101.45 a | 0 c | |
| Taxifolin | Enzyme-added | 100 | 84.18 a | 34.87 b |
| Control | 100 | 77.57 b | 44.67 c | |
| Myricetin | Enzyme-added | 100 | 75.84 b | 0 b |
| Control | 100 | 77.99 b | 0 c | |
| Quercetin | Enzyme-added | 100 | 67.75 b | 0 c |
| Control | 100 | 72.94 b | 0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Tan, Y.; Shi, M.; Zeng, C.; Qin, S. Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation. Foods 2023, 12, 773. https://doi.org/10.3390/foods12040773
Fu X, Tan Y, Shi M, Zeng C, Qin S. Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation. Foods. 2023; 12(4):773. https://doi.org/10.3390/foods12040773
Chicago/Turabian StyleFu, Xiaomei, Yan Tan, Meng Shi, Chaoxi Zeng, and Si Qin. 2023. "Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation" Foods 12, no. 4: 773. https://doi.org/10.3390/foods12040773
APA StyleFu, X., Tan, Y., Shi, M., Zeng, C., & Qin, S. (2023). Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation. Foods, 12(4), 773. https://doi.org/10.3390/foods12040773








