Catching the Drift of Marine Invertebrate Diversity through Digital Repositories—A Case Study of the Mangroves and Seagrasses of Maputo Bay, Mozambique
Abstract
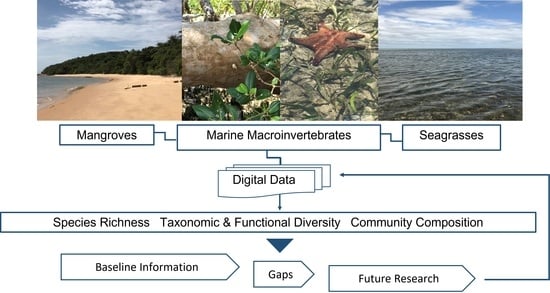
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Biodiversity Estimates and Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lefcheck, J.S.; Marion, S.R.; Lombana, A.V.; Orth, R.J. Faunal Communities Are Invariant to Fragmentation in Experimental Seagrass Landscapes. PLoS ONE 2016, 11, e0156550. [Google Scholar] [CrossRef] [PubMed]
- Carugati, L.; Gatto, B.; Rastelli, E.; Lo Martire, M.; Coral, C.; Greco, S.; Danovaro, R. Impact of Mangrove Forests Degradation on Biodiversity and Ecosystem Functioning. Sci. Rep. 2018, 8, 13298. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, S.; Gullström, M.; Balidy, H.; Samussone, D.; Cossa, D. Seagrass Meadows in Maputo Bay. In The Maputo Bay Ecosystem; Paula, J., Bandeira, S., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 147–170. ISBN 9789987955930. [Google Scholar]
- Paula, J.; Macamo, C.; Bandeira, S. Mangroves of Maputo Bay. In The Maputo Bay Ecosystem; Paula, J., Bandeira, S., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 109–126. [Google Scholar]
- Alfaro, A.C. Benthic Macro-Invertebrate Community Composition within a Mangrove/Seagrass Estuary in Northern New Zealand. Estuar. Coast. Shelf Sci. 2006, 66, 97–110. [Google Scholar] [CrossRef]
- Phang, V.X.H.; Chou, L.M.; Friess, D.A. Ecosystem Carbon Stocks across a Tropical Intertidal Habitat Mosaic of Mangrove Forest, Seagrass Meadow, Mudflat and Sandbar. Earth Surf. Process. Landforms 2015, 40, 1387–1400. [Google Scholar] [CrossRef]
- Kruitwagen, G.; Nagelkerken, I.; Lugendo, B.R.; Mgaya, Y.D.; Bonga, S.E.W. Importance of Different Carbon Sources for Macroinvertebrates and Fishes of an Interlinked Mangrove-Mudflat Ecosystem (Tanzania). Estuar. Coast. Shelf Sci. 2010, 88, 464–472. [Google Scholar] [CrossRef]
- Corte, G.N.; Checon, H.H.; Shah Esmaeili, Y.; Lefcheck, J.S.; Amaral, A.C.Z. Mangrove Fragments as Key Coastal Reservoirs of Taxonomic and Functional Biodiversity. Biodivers. Conserv. 2021, 30, 1573–1593. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the Effects of Marine Biodiversity on Communities and Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Coccia, C.; Almeida, B.A.; Green, A.J.; Gutiérrez, A.B.; Carbonell, J.A. Functional Diversity of Macroinvertebrates as a Tool to Evaluate Wetland Restoration. J. Appl. Ecol. 2021, 58, 2999–3011. [Google Scholar] [CrossRef]
- Barnes, R.S.K.; Hendy, I.W. Seagrass-Associated Macrobenthic Functional Diversity and Functional Structure along an Estuarine Gradient. Estuar. Coast. Shelf Sci. 2015, 164, 233–243. [Google Scholar] [CrossRef]
- Barnes, R.S.K. What Does Measuring Species Diversity in Estuarine Seagrass Systems Actually Assess? Mar. Environ. Res. 2021, 172, 105500. [Google Scholar] [CrossRef]
- Barbanera, A.; Markesteijn, L.; Kairo, J.; Juma, G.A.; Karythis, S.; Skov, M.W. Functional Responses of Mangrove Fauna to Forest Degradation. Mar. Freshw. Res. 2022, 73, 762–763. [Google Scholar] [CrossRef]
- Henseler, C.; Nordström, M.C.; Törnroos, A.; Snickars, M.; Pecuchet, L.; Lindegren, M.; Bonsdorff, E. Coastal Habitats and Their Importance for the Diversity of Benthic Communities: A Species- and Trait-Based Approach. Estuar. Coast. Shelf Sci. 2019, 226, 106272. [Google Scholar] [CrossRef]
- Wong, M.C.; Dowd, M. Patterns in Taxonomic and Functional Diversity of Macrobenthic Invertebrates Across Seagrass Habitats: A Case Study in Atlantic Canada. Estuaries Coasts 2015, 38, 2323–2336. [Google Scholar] [CrossRef]
- Paganelli, D.; Marchini, A.; Occhipinti-Ambrogi, A. Functional Structure of Marine Benthic Assemblages Using Bio-logical Traits Analysis (BTA): A Study along the Emilia-Romagna Coastline (Italy, North-West Adriatic Sea). Estuar. Coast. Shelf Sci. 2012, 96, 245–256. [Google Scholar] [CrossRef]
- Paula, J.; Bandeira, S. An Introduction to the Maputo Bay. In The Maputo Bay Ecosystem; Paula, J., Bandeira, S., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 3–10. ISBN 978-9987-9559-3-0. [Google Scholar]
- Machava-António, V.; Fernando, A.; Cravo, M.; Massingue, M.; Lima, H.; Macamo, C.; Bandeira, S.; Paula, J. A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa). Forests 2022, 13, 1466. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Bandeira, S. Maputo Bay’s Coastal Habitats. In The Maputo Bay Ecosystem; Paula, J., Bandeira, S., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 21–30. [Google Scholar]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial Bias in the GBIF Database and Its Effect on Modeling Species’ Geographic Distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Smith, V.S.; Blagoderov, V. Bringing Collections out of the Dark. Zookeys 2012, 209, 1–6. [Google Scholar] [CrossRef]
- Cannicci, S.; Lee, S.Y.; Bravo, H.; Cantera-Kintz, J.R.; Dahdouh-Guebas, F.; Fratini, S.; Fusi, M.; Jimenez, P.J.; Nordhaus, I.; Porri, F.; et al. A Functional Analysis Reveals Extremely Low Redundancy in Global Mangrove Invertebrate Fauna. Proc. Natl. Acad. Sci. USA 2021, 118, e2016913118. [Google Scholar] [CrossRef]
- Bender, I.M.A.; Kissling, W.D.; Böhning-Gaese, K.; Hensen, I.; Kühn, I.; Nowak, L.; Töpfer, T.; Wiegand, T.; Dehling, D.M.; Schleuning, M. Projected Impacts of Climate Change on Functional Diversity of Frugivorous Birds along a Tropical Elevational Gradient. Sci. Rep. 2019, 9, 17708. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Brum, F.T.; Cássia-Silva, C.; Maracahipes, L.; Carlucci, M.B.; Collevatti, R.G.; Bacon, C.D. Incongruent Spatial Distribution of Taxonomic, Phylogenetic, and Functional Diversity in Neotropical Cocosoid Palms. Front. For. Glob. Chang. 2021, 4, 739468. [Google Scholar] [CrossRef]
- Granado-Lorencio, C.; Guisande, C.; Pelayo-Villamil, P.; Manjarrés-Hernández, A.; García-Roselló, E.; Heine, J.; Pérez-Costas, E.; González-Vilas, L.; González-Dacosta, J.; Lobo, J.M. Diversity Dimensions of Freshwater Fish Species around the World. J. Geogr. Inf. Syst. 2021, 13, 106371. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Fourcade, Y. Temporal Analysis of GBIF Data Reveals the Restructuring of Communities Following Climate Change. J. Anim. Ecol. 2022, 1–12. [Google Scholar] [CrossRef]
- Toussaint, A.; Brosse, S.; Bueno, C.G.; Pärtel, M.; Tamme, R.; Carmona, C.P. Extinction of Threatened Vertebrates Will Lead to Idiosyncratic Changes in Functional Diversity across the World. Nat. Commun. 2021, 12, 5162. [Google Scholar] [CrossRef]
- da Silva, A.; Rafael, J. Geographical and Socio-Economic Setting of Maputo Bay. In The Maputo Bay Ecosystem; Paula, J., Bandeira, S., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 11–20. [Google Scholar]
- Bento, M.; Niza, H.; Cartaxana, A.; Bandeira, S.; Paula, J.; Marçal Correia, A. A Dataset of Marine Macroinvertebrate Diversity from Mozambique and São Tomé and Príncipe; Version 1.6; Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa: Lisboa, Portugal, Occurrence Dataset. [CrossRef]
- Bento, M. A Dataset of Marine Macroinvertebrate Diversity from Maputo Bay, Mozambique. Available online: https://www.gbif.org/derivedDataset/10.15468/dd.2qg6gj (accessed on 22 January 2023).
- Bento, M.; Niza, H.; Cartaxana, A.; Bandeira, S.; Paula, J.; Correia, A.M. Mind the Gaps: Taxonomic, Geographic and Temporal Data of Marine Invertebrate Databases from Mozambique and São Tomé and Príncipe. Diversity 2023, 15, 70. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional Diversity (FD), Species Richness and Community Composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Lepš, J.; de Bello, F.; Lavorel, S.; Berman, S. Quantifying and Interpreting Functional Diversity of Natural Communities: Practical Considerations Matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional Diversity: Back to Basics and Looking Forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Moore, J.C. Diversity, Taxonomic versus Functional. Encycl. Biodivers. Second Ed. 2013, 2, 648–656. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Rao, R. Diversity and Dissimilarity. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Pélissier, R.; Couteron, P.; Dray, S.; Sabatier, D. Consistency between Ordination Techniques and Diversity Measurements: Two Strategies for Species Occurrence Data. Ecology 2003, 84, 242–251. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Theory of Mathematical Communication, 10th ed.; The University of Illinois Press: Chicago, IL, USA, 1964; pp. 1–131. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A Further Biodiversity Index Applicable to Species Lists: Variation in Taxonomic Distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A Taxonomic Distinctness Index and Its Statistical Properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Paula, J.; Bandeira, S.; Msangameno, D.; Queiroga, H. Measures of Biological Diversity. In Exercises in Marine Biodiversity and Ecology—A Training Manual Using the Intertidal Habitats of the WIO Region; WIOMSA: Zanzibar Town, Tanzania, 2021; pp. 59–78. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual PRIMER-E; Primer-E Ldt: Plymouth, UK, 2006; pp. 1–91. [Google Scholar]
- Chitará-Nhandimo, S.; Chissico, A.; Mubai, M.E.; de Cabral, A.S.; Guissamulo, A.; Bandeira, S. Seagrass Invertebrate Fisheries, Their Value Chains and the Role of LMMAs in Sustainability of the Coastal Communities—Case of Southern Mozambique. Diversity 2022, 14, 170. [Google Scholar] [CrossRef]
- Tavares, A.I.; Assis, J.; Patrício, A.R.; Ferreira, R.; Cheikh, M.A.S.; Bandeira, S.; Regalla, A.; Santos, I.; Potouroglou, M.; Nicolau, S.; et al. Seagrass Connectivity on the West Coast of Africa Supports the Hypothesis of Grazer-Mediated Seed Dispersal. Front. Mar. Sci. 2022, 9, 852. [Google Scholar] [CrossRef]
- Cheikh, M.A.S.; Bandeira, S.O.; Traganos, D.; Poursanidis, D.; Vegh, T. Seagrasses of West Africa: New Discoveries, Distribution Limits, Ecosystem Services and Prospects for Management. Diversity 2022, 15, 5. [Google Scholar] [CrossRef]
- Amone-Mabuto, M.; Hollander, J.; Lugendo, B.; Adams, J.B.; Bandeira, S. A Field Experiment Exploring Disturbance-and-Recovery, and Restoration Methodology of Zostera Capensis to Support Its Role as a Coastal Protector. Nord. J. Bot. 2023, 2023, e03632. [Google Scholar] [CrossRef]
- de Oliveira, S.E.; Bozelli, R.L. How Planktonic Microcrustaceans Respond to Environment and Affect Ecosystem: A Functional Trait Perspective. Int. Aquat. Res. 2019, 11, 207–223. [Google Scholar] [CrossRef]
- Faith, D.; Collen, B.; Ariño, A.; Patricia Koleff, P.K.; Guinotte, J.; Kerr, J.; Chavan, V. Bridging the Biodiversity Data Gaps: Recommendations to Meet Users’ Data Needs. Biodivers. Inform. 2013, 8, 41–58. [Google Scholar] [CrossRef]
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating Species Diversity and Distribution in the Era of Big Data: To What Extent Can We Trust Public Databases? Glob. Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Neves, I.Q.; da Mathias, M.L.; Bastos-Silveira, C. Mapping Knowledge Gaps of Mozambique’s Terrestrial Mammals. Sci. Rep. 2019, 9, 18184. [Google Scholar] [CrossRef] [Green Version]
- Indo-Pacific Seagrass Network (IPSN). Available online: https://indopacificseagrassnetwork.wordpress.com/ (accessed on 26 December 2022).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, M.; Paula, J.; Bandeira, S.; Correia, A.M. Catching the Drift of Marine Invertebrate Diversity through Digital Repositories—A Case Study of the Mangroves and Seagrasses of Maputo Bay, Mozambique. Diversity 2023, 15, 242. https://doi.org/10.3390/d15020242
Bento M, Paula J, Bandeira S, Correia AM. Catching the Drift of Marine Invertebrate Diversity through Digital Repositories—A Case Study of the Mangroves and Seagrasses of Maputo Bay, Mozambique. Diversity. 2023; 15(2):242. https://doi.org/10.3390/d15020242
Chicago/Turabian StyleBento, Marta, José Paula, Salomão Bandeira, and Alexandra Marçal Correia. 2023. "Catching the Drift of Marine Invertebrate Diversity through Digital Repositories—A Case Study of the Mangroves and Seagrasses of Maputo Bay, Mozambique" Diversity 15, no. 2: 242. https://doi.org/10.3390/d15020242
APA StyleBento, M., Paula, J., Bandeira, S., & Correia, A. M. (2023). Catching the Drift of Marine Invertebrate Diversity through Digital Repositories—A Case Study of the Mangroves and Seagrasses of Maputo Bay, Mozambique. Diversity, 15(2), 242. https://doi.org/10.3390/d15020242