Oral Administration of Lotus-Seed Resistant Starch Protects against Food Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
2.3. Induction of Food Allergy
2.4. Evaluation of Food Allergy
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Flow Cytometry
2.7. 16S rDNA Sequencing
2.8. Hematoxylin–Eosin Staining
2.9. Determination of SCFAs and BAs
2.10. Statistical Analysis
3. Results
3.1. Lotus-Seed Resistant Starch Exerted Anti-Allergic Effects in OVA-Induced Food Allergy Rodent Model
3.2. Lotus-Seed Resistant Starch Lowered Serum Concentrations of OVA-Specific Immunoglobulins and Allergy-Related Cytokine
3.3. Lotus-Seed Resistant Starch Intervention Regulated Th1/Th2 Balance and Lowered Serum Concentrations of Th2 Cytokines in OVA-Induced Allergic Mice
3.4. Lotus-Seed Resistant Starch Treatment Modulated the Gut Microbiota Composition, Fecal Short-Chain Fatty Acids, and Bile Acids of Mice with Food Allergies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Sun, X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits beta-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut. Pathog. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rosso, G.B.; Chung, C.K.K.; Bäriswyl, L.; Rodriguez, M.P.; Kim, B.S.; Engel, P.; Noti, M. High dietary fat intake induces a microbiota signature that promotes food allergy. J. Allergy Clin. Immunol. 2019, 144, 157–170.e8. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Li, Q.; Shi, Z.; Wu, H.; Zhang, H.; Tang, L.; Yi, R.; Su, H.; Sun, X. Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 2019, 55, 65–75. [Google Scholar] [CrossRef]
- Yamashita, H.; Shigemori, A.; Murata, M.; Tanaka, H.; Inagaki, N.; Tsutsui, M.; Kimura, M. Impact of orally-administered oligosaccharides in a murine model of food allergy. J. Funct. Foods 2021, 85, 104643. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Chen, Z.; Zou, C.; Liu, W.; Yang, L.; Fu, L.; Wang, Y.; Liu, G.-M.; Cao, M.-J. Depolymerized sulfated galactans from Eucheuma serra ameliorate allergic response and intestinal flora in food allergic mouse model. Int. J. Biol. Macromol. 2021, 166, 977–985. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. Int. Rev. J. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Lei, S.; Zhang, Y.; Zeng, H. Synergistic Effects of Lotus Seed Resistant Starch and Sodium Lactate on Hypolipidemic Function and Serum Nontargeted Metabolites in Hyperlipidemic Rats. J. Agric. Food Chem. 2021, 69, 14580–14592. [Google Scholar] [CrossRef] [PubMed]
- Enokida, M.; Minato, K.-I.; Yoshino, S.; Ohto, N.; Kuwahara, H.; Mizuno, M. Oral administration of Lactiplantibacillus plantarum 22A-3 exerts anti-allergic activity against intestinal food allergy mouse models sensitized and challenged with ovalbumin. Food Biosci. 2022, 47, 101785. [Google Scholar] [CrossRef]
- Hua, Y.L.; Jia, Y.Q.; Zhang, X.S.; Yuan, Z.W.; Ji, P.; Hu, J.J.; Wei, Y.M. Baitouweng Tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomed. Pharmacother. 2021, 137, 111320. [Google Scholar] [PubMed]
- Li, Q.; Tang, X.; Xu, J.; Ren, X.; Wang, R.; Jiang, S. Study on alleviation effect of stachyose on food allergy through TLR2/NF-kappaB signal pathway in a mouse model. Life Sci. 2021, 286, 120038. [Google Scholar]
- Ma, Y.; Liu, M.; Li, D.; Li, J.; Guo, Z.; Liu, Y.; Wan, S.; Liu, Y. Olive oil ameliorates allergic response in murine ovalbumin-induced food allergy by promoting intestinal mucosal immunity. Food Sci. Hum. Wellness 2023, 12, 801–808. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Piątek-Guziewicz, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Szczyrk, U.; et al. The Importance of Nutritional Aspects in the Assessment of Inflammation and Intestinal Barrier in Patients with Inflammatory Bowel Disease. Nutrients 2022, 14, 4622. [Google Scholar] [CrossRef]
- Ji, X.; Le Su, L.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Li, K.; Liu, X.; Zhang, S.; Zhao, L. Lentinan improves intestinal inflammation and gut dysbiosis in antibiotics-induced mice. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Lin, H.; Ma, X.; Yang, X.; Chen, Q.; Wen, Z.; Yang, M.; Fu, J.; Yin, T.; Lu, G.; Qi, J.; et al. Natural shikonin and acetyl-shikonin improve intestinal microbial and protein composition to alleviate colitis-associated colorectal cancer. Int. Immunopharmacol. 2022, 111, 109097. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Zheng, H.-Z.; Wang, R.; Chen, H.; Xiao, J.-X.; Zheng, B.; Liu, S.-L.; Ding, Y.-T. Ameliorative Effects of Peptides Derived from Oyster (Crassostrea gigas) on Immunomodulatory Function and Gut Microbiota Structure in Cyclophosphamide-Treated Mice. Mar. Drugs 2021, 19, 456. [Google Scholar] [CrossRef]
- Yao, L.; Yang, P.; Lin, Y.; Bi, D.; Yu, B.; Lin, Z.; Wu, Y.; Xu, H.; Hu, Z.; Xu, X. The regulatory effect of alginate on ovalbumin-induced gut microbiota disorders. J. Funct. Foods 2021, 86, 104727. [Google Scholar] [CrossRef]
- Sathe, S.K.; Liu, C.; Zaffran, V.D. Annual review of food science and technology. Food Allergy 2016, 7, 191–220. [Google Scholar]
- Helm, R.M.; Burks, A.W. Current Opinion in Allergy and Clinical Immunology. Anim. Model. Food Allergy 2002, 2, 541–546. [Google Scholar]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and Preventive Effects in Allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ho, H.-E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy, Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.; Prescott, S.L.; Palmer, D.J. Taking a prebiotic approach to early immunomodulation for allergy prevention. Expert Rev. Clin. Immunol. 2018, 14, 43–51. [Google Scholar] [CrossRef]
- Miyazato, S.; Tsuda, M.; Kishimoto, Y.; Hosono, A. Suppressive effect of dietary resistant maltodextrin on systemic immunity in a mouse model of food allergy. Biosci. Microbiota Food Heal. 2019, 38, 89–95. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, X.; Zheng, P.Y.; Luo, Y.; Lu, G.F.; Liu, Z.Q.; Huang, H.; Yang, P.C. Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J. Gastroenterol. Hepatol. 2010, 25, 928–934. [Google Scholar]
- Zhao, Y.; He, Z.; Hao, W.; Zhu, H.; Liu, J.; Ma, K.Y.; He, W.-S.; Chen, Z.-Y. Cholesterol-lowering activity of protocatechuic acid is mediated by increasing the excretion of bile acids and modulating gut microbiota and producing short-chain fatty acids. Food Funct. 2021, 12, 11557–11567. [Google Scholar] [CrossRef]
- Du, L.; Sun, Y.; Wang, Q.; Wang, L.; Zhang, Y.; Li, S.; Jin, H.; Yan, S.; Xiao, X. Integrated metabolomics and 16S rDNA sequencing to investigate the mechanism of immune-enhancing effect of health Tonic oral liquid. Food Res. Int. 2021, 144, 110323. [Google Scholar] [CrossRef]
- Liu, T.; Chen, P.; Munir, M.; Liu, L.; Li, C.; Li, A.; Fu, H. HMOs modulate immunoregulation and gut microbiota in a β-lactoglobulin-induced allergic mice model. J. Funct. Foods 2020, 70, 103993. [Google Scholar] [CrossRef]
- Schjødt, M.S.; Gürdeniz, G.; Chawes, B. The Metabolomics of Childhood Atopic Diseases: A Comprehensive Pathway-Specific Review. Metabolites 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, H.S.; Shin, E.; Do, S.-G.; Lee, C.-K.; Kim, Y.M.; Lee, M.B.; Min, K.Y.; Koo, J.; Kim, S.J.; et al. Polysaccharide isolated from Aloe vera gel suppresses ovalbumin-induced food allergy through inhibition of Th2 immunity in mice. Biomed. Pharmacother. 2018, 101, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-A.; Hwang, Y.-J.; Song, J. Anti-allergic effect of Aster yomena on ovalbumin-sensitized mouse and RHL-2H3 cells via Th1/Th2 cytokine balance. J. Funct. Foods 2018, 44, 1–8. [Google Scholar] [CrossRef]
- St John, A.L.; Ang, W.X.G.; Rathore, A.P.S.; Abraham, S.N. Reprograming immunity to food allergens. J. Allergy Clin. Immunol. 2018, 141, 1936–1939.e2. [Google Scholar]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
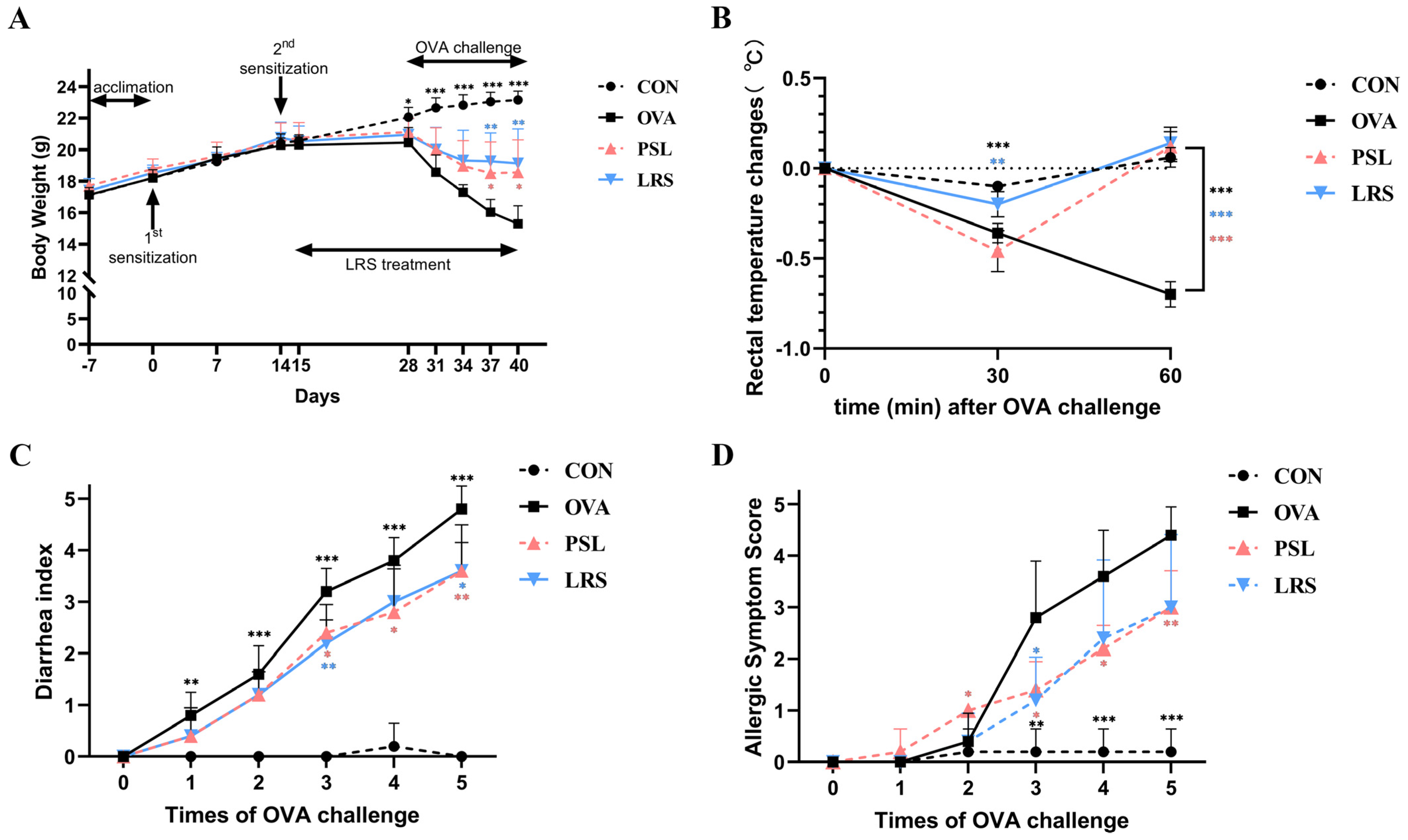
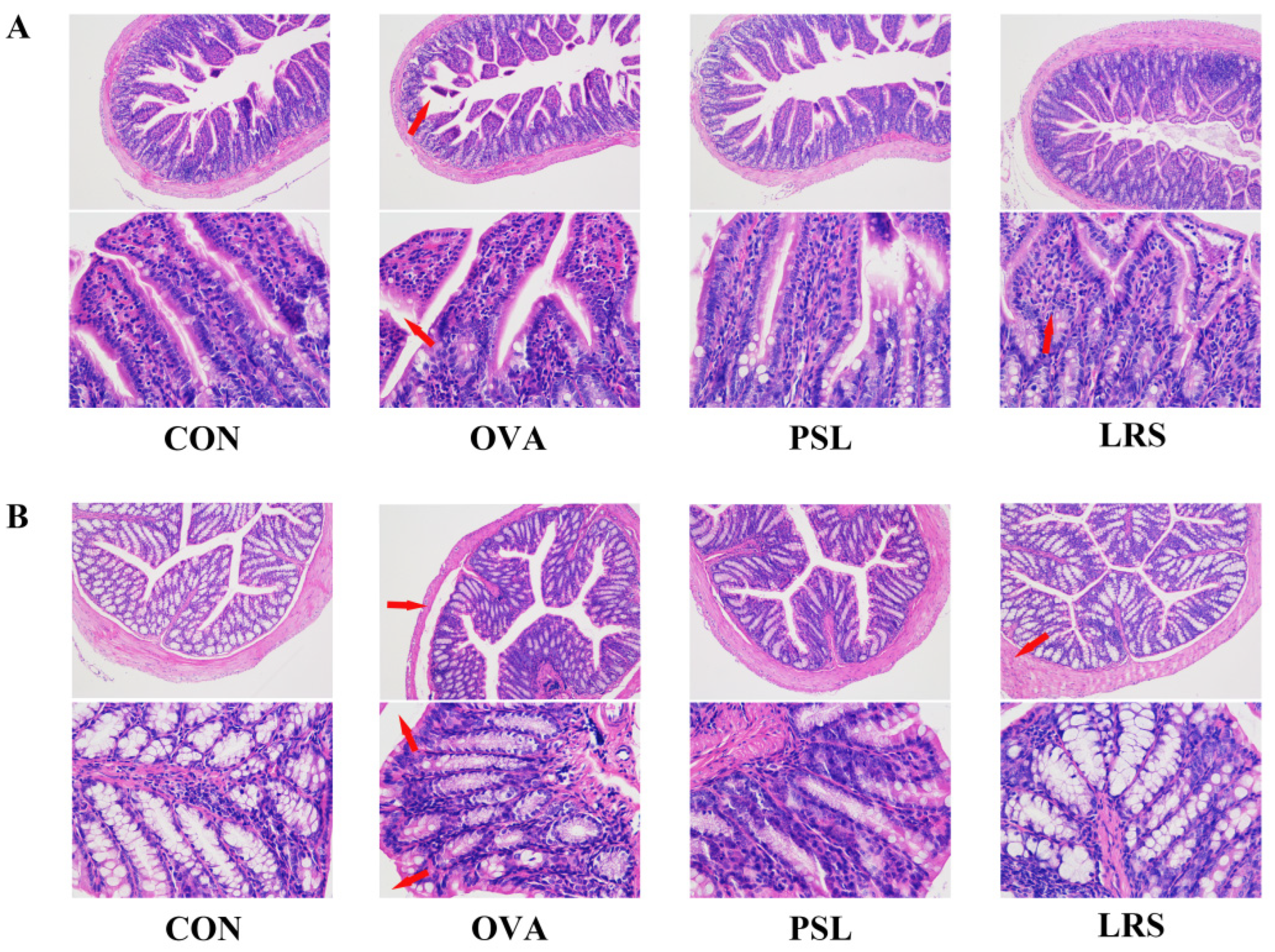

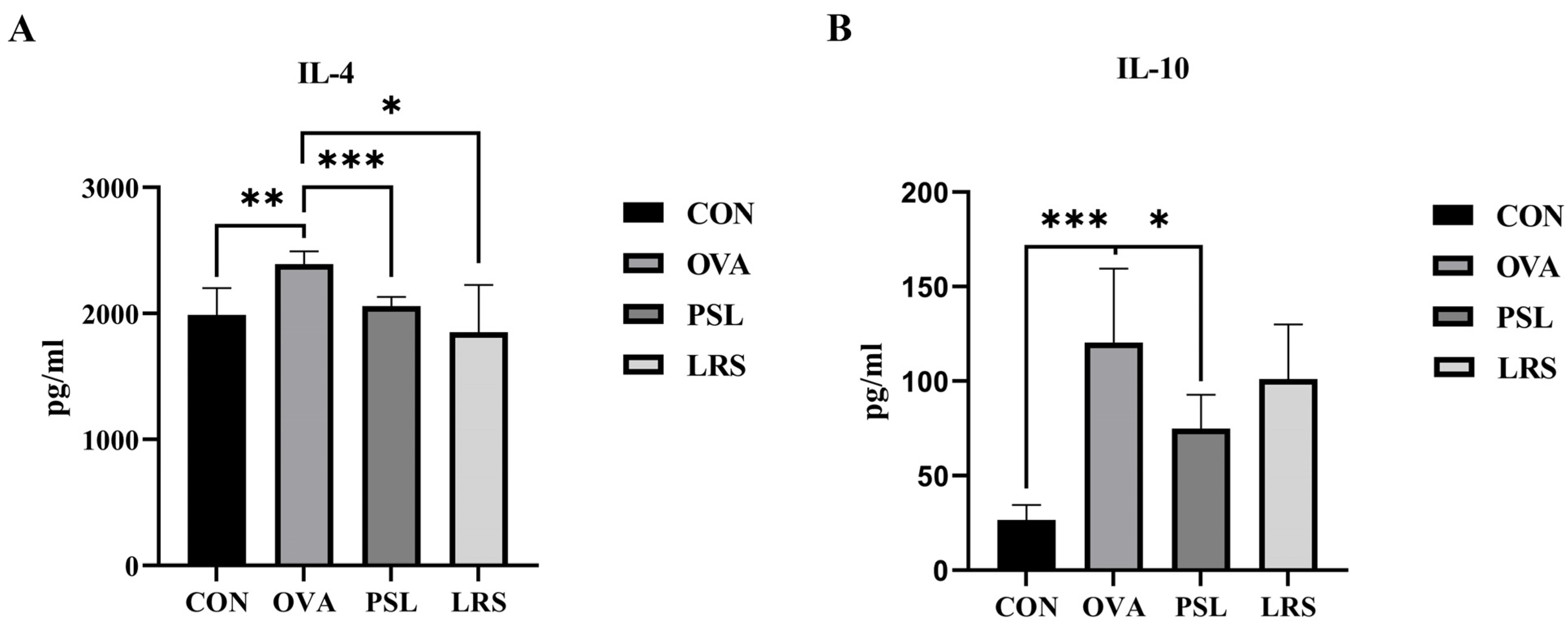

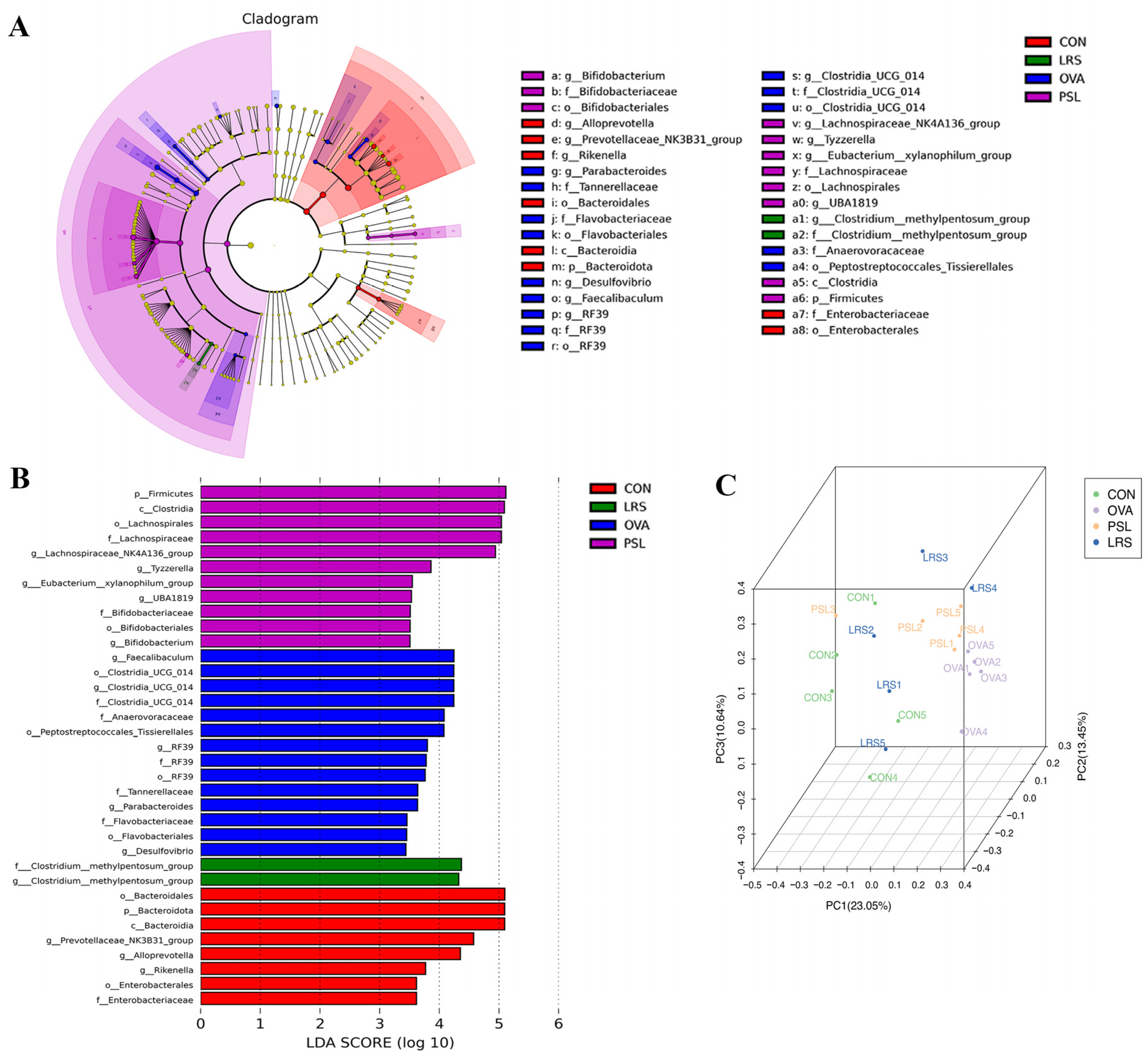


| CD4 + T cell content | CON | OVA | PSL | LRS |
|---|---|---|---|---|
| Th1 (%) | 1.36 ± 0.54* | 2.15 ± 1.34 | 2.07 ± 0.87 | 1.86 ± 0.88 |
| Th2 (%) | 1.17 ± 0.76* | 2.60 ± 0.78 | 2.08 ± 0.78 | 1.32 ± 0.80* |
| Th1/Th2 | 1.39 ± 0.49 | 0.81 ± 0.36 | 1.10 ± 0.67 | 1.49 ± 0.50* |
| SCFAs (μg/g) | Control | OVA | PSL | LRS |
|---|---|---|---|---|
| Acetic acid | 1194.48 ± 124.82 a | 1037.60 ± 195.36 b | 1154.31 ± 250.92 b | 1205.22 ± 302.80 b |
| Butyric acid | 394.66 ± 97.40 a | 306.99 ± 95.27 b | 373.35 ± 161.86 b | 436.92 ± 139.79 b |
| Isobutyric acid | 22.80 ± 2.24 a | 13.64 ± 3.75 b | 19.68 ± 8.33 b | 18.97 ± 7.18 b |
| Isovaleric acid | 14.47 ± 0.94 a | 8.10 ± 1.31 b | 12.80 ± 5.59 a | 11.36 ± 4.13 a |
| Pentanoic acid | 17.65 ± 3.64 | 20.46 ± 4.42 | 21.00 ± 8.86 | 19.06 ± 8.19 |
| Propionic acid | 364.59 ± 53.03 a | 274.69 ± 65.62 b | 280.89 ± 79.36 b | 304.34 ± 116.38 b |
| Bile Acids (ng/g) | CON | OVA | PSL | LRS |
|---|---|---|---|---|
| CDCA | 149.63 ± 82.16 | 77.87 ± 86.60 | 117.74 ± 95.83 | 89.88 ± 67.16 |
| CA | 732.98 ± 344.10 | 1041.95 ± 390.68 | 831.78 ± 167.68 * | 939.84 ± 465.74 |
| GCA | 12.92 ± 6.83 | 10.20 ± 6.33 | 10.15 ± 9.07 | 3.93 ± 3.02 * |
| TCDCA | 74.95 ± 89.24 | 42.22 ± 25.71 | 40.56 ± 21.48 | 31.97 ± 22.89 |
| TCA | 2194.48 ± 1419.93 | 1867.41 ± 927.63 | 1836.84 ± 674.11 | 1687.60 ± 379.88 |
| LCA | 1020.97 ± 581.76 | 733.73 ± 939.00 | 1325.27 ± 1042.11 | 884.69 ± 594.49 |
| DCA | 2312.20 ± 1845.32 | 1940.72 ± 663.47 | 3702.57 ± 1486.00 | 2704.53 ± 1942.32 |
| UDCA | 168.62 ± 104.83 | 121.00 ± 98.37 | 233.62 ± 89.91 * | 170.46 ± 106.78 |
| GLCA | 1.34 ± 1.72 * | 7.12 ± 5.53 | 6.07 ± 4.00 | 2.81 ± 1.99 * |
| TLCA | 8.98 ± 11.71 | 6.46 ± 2.51 | 7.48 ± 3.06 | 4.34 ± 3.48 |
| TUDCA | 139.41 ± 105.88 | 23.54 ± 17.04 | 92.73 ± 70.15 | 60.68 ± 50.33 |
| TDCA | 98.91 ± 100.31 | 31.99 ± 21.70 | 81.34 ± 40.87 | 56.67 ± 72.48 |
| EALCA | 6187.93 ± 2638.41 | 3736.37 ± 4052.82 | 5291.85 ± 4361.71 | 4948.35 ± 1971.00 |
| HDCA | 1133.45 ± 702.83 | 1390.67 ± 674.78 | 1404.02 ± 689.62 | 1281.71 ± 810.33 |
| α-MCA | 16,125.42 ± 5693.38 | 12,831.89 ± 3895.25 | 19,127.22 ± 7009.88 | 14,603.84 ± 5598.79 |
| β-MCA | 4397.20 ± 3288.10 | 3293.72 ± 919.33 | 5699.42 ± 1437.25 * | 4071.21 ± 1424.47 |
| α-TMCA | 324.92 ± 192.09 | 387.32 ± 51.84 | 530.31 ± 165.58 | 378.89 ± 122.55 |
| THDCA | 74.95 ± 89.24 | 42.22 ± 25.71 | 40.56 ± 21.48 | 31.97 ± 22.89 |
| Iso-LCA | 6958.30 ± 3205.64 | 3851.44 ± 4773.45 | 5905.51 ± 5359.43 | 5452.63 ± 2410.86 |
| M-DCA | 137.60 ± 129.33 | 158.93 ± 86.15 | 213.68 ± 87.20 | 152.94 ± 110.68 |
| β-TMCA-Na | 1146.03 ± 734.61 | 1351.69 ± 258.84 | 1882.41 ± 683.18 | 1296.47 ± 452.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Lin, Z.; Li, L.; Zheng, B.; Zeng, H.; Wang, Y.; Zhang, Y. Oral Administration of Lotus-Seed Resistant Starch Protects against Food Allergy. Foods 2023, 12, 737. https://doi.org/10.3390/foods12040737
Hu J, Lin Z, Li L, Zheng B, Zeng H, Wang Y, Zhang Y. Oral Administration of Lotus-Seed Resistant Starch Protects against Food Allergy. Foods. 2023; 12(4):737. https://doi.org/10.3390/foods12040737
Chicago/Turabian StyleHu, Jiamiao, Zhongjing Lin, Lanxin Li, Baodong Zheng, Hongliang Zeng, Yanbo Wang, and Yi Zhang. 2023. "Oral Administration of Lotus-Seed Resistant Starch Protects against Food Allergy" Foods 12, no. 4: 737. https://doi.org/10.3390/foods12040737
APA StyleHu, J., Lin, Z., Li, L., Zheng, B., Zeng, H., Wang, Y., & Zhang, Y. (2023). Oral Administration of Lotus-Seed Resistant Starch Protects against Food Allergy. Foods, 12(4), 737. https://doi.org/10.3390/foods12040737









