The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals and Husbandry Practices
2.2. Treatment Groups
2.2.1. Blood Sampling and Analysis
2.2.2. Colostrum Collection
2.2.3. Characterization of Nutritional Profiles of Colostrum, Colostrum Replacer and Milk
2.3. Wistar Rat Model
2.4. Tissue Collection and Histological Analysis
2.5. Statistical Analysis
3. Results
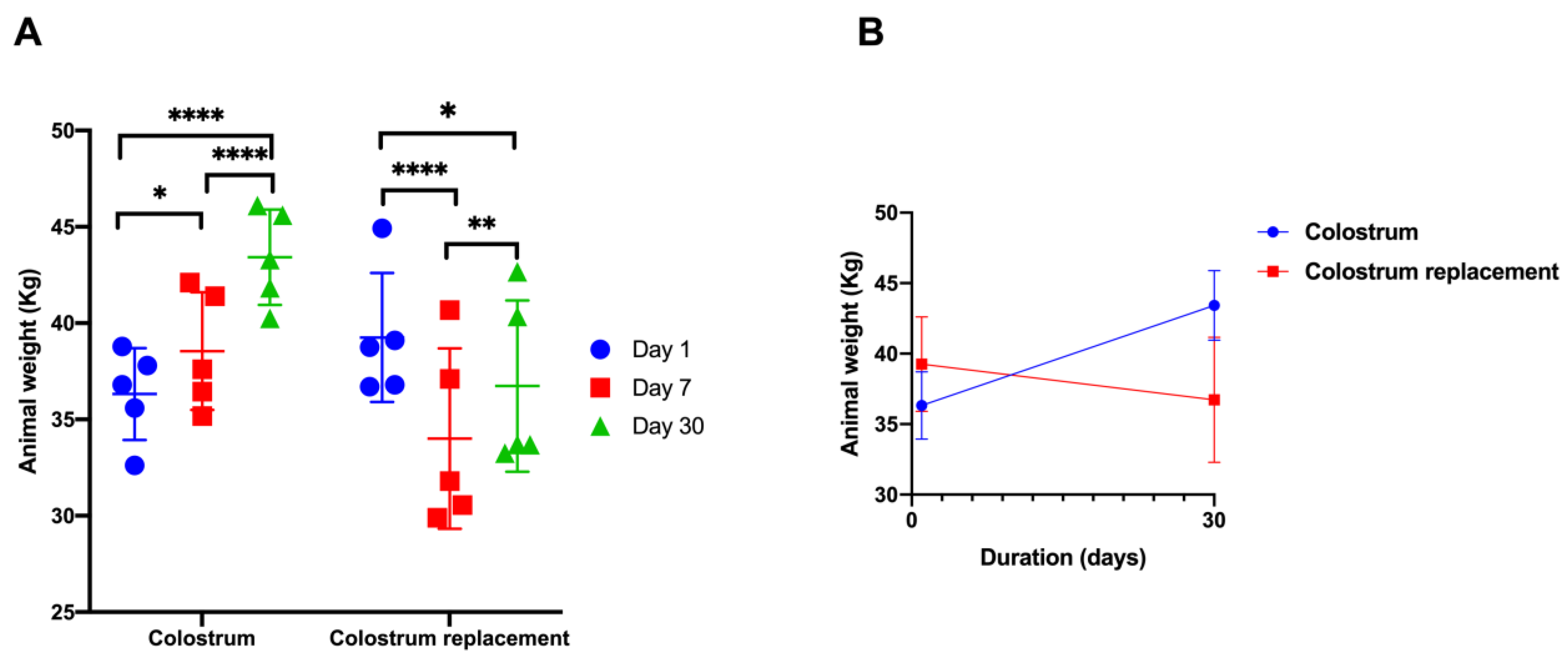
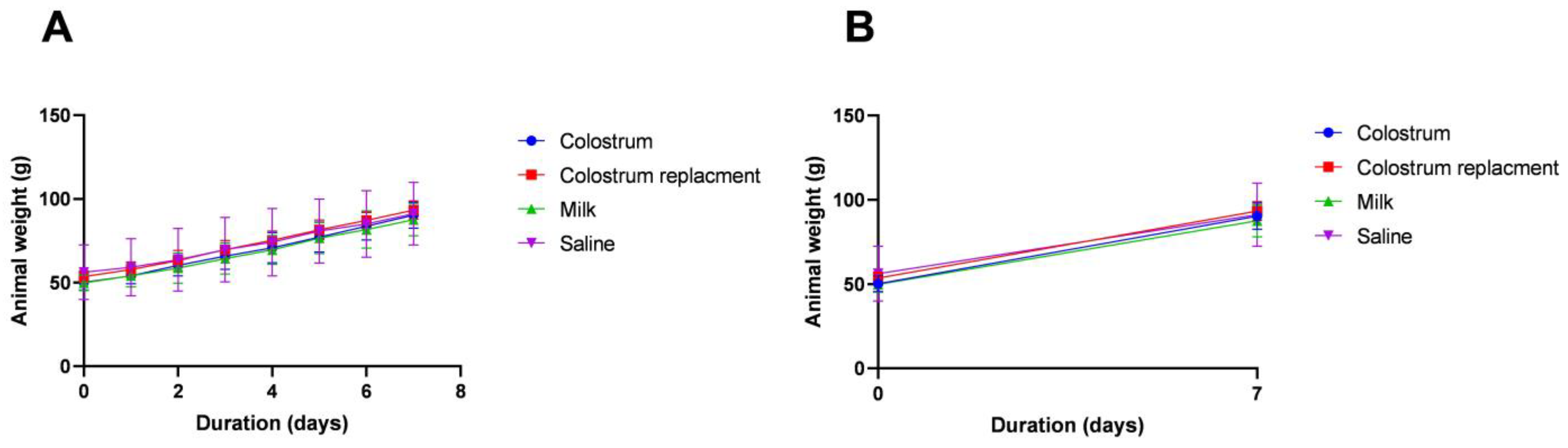
3.1. Body Mass Changes in Calves
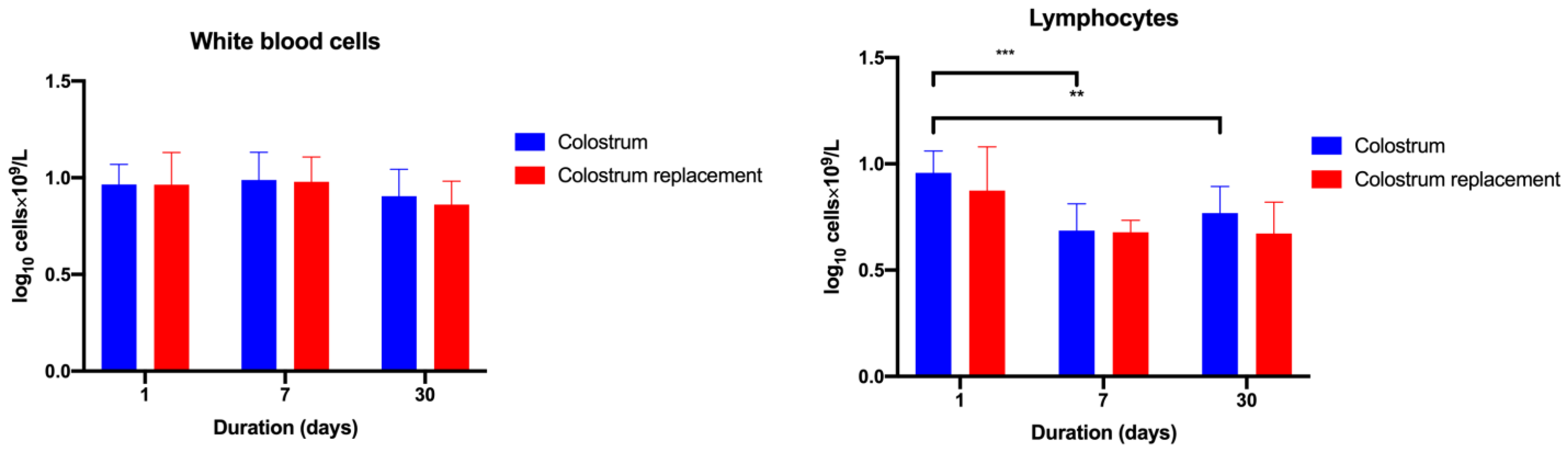
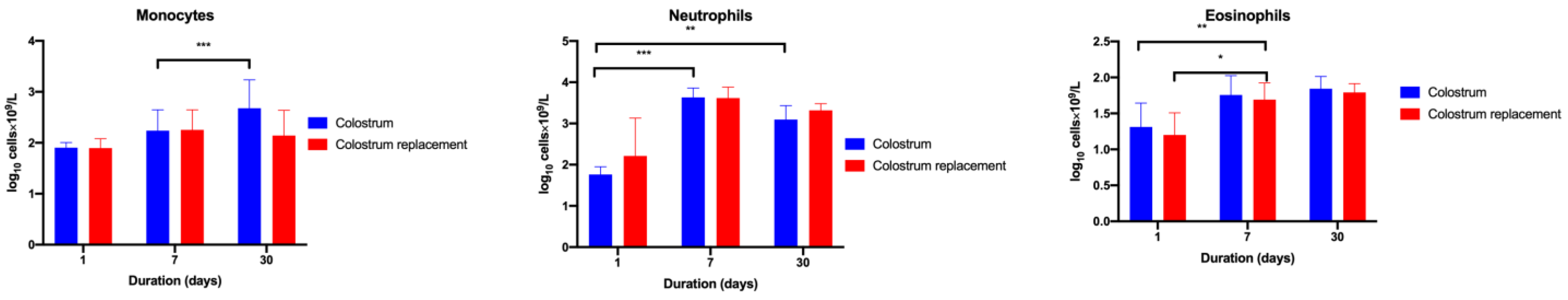
3.2. Administration of Bovine Colostrum Modulates Lymphoid and Myeloid Blood Cell Populations in Calves
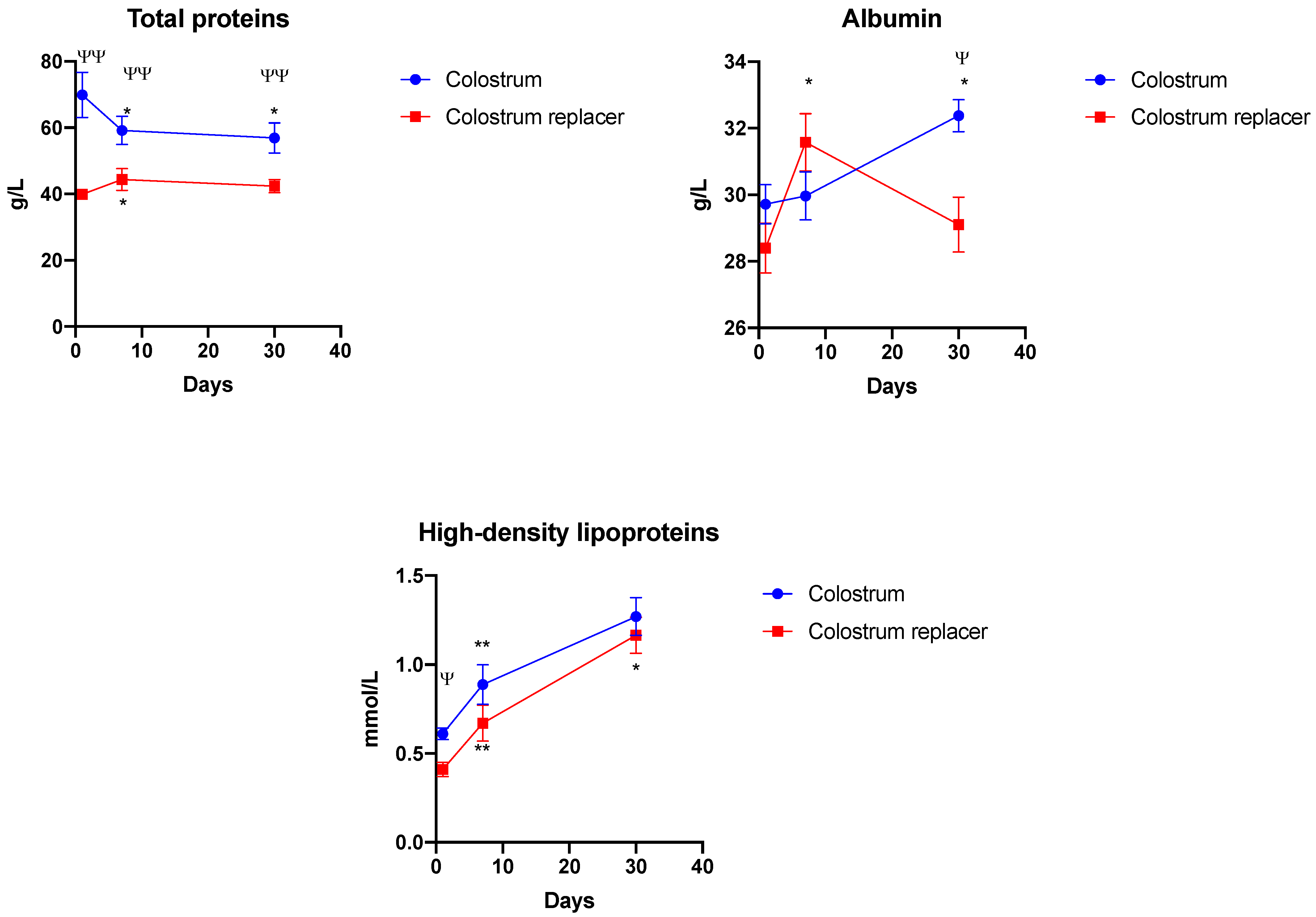
3.3. The Effect of Early Colostrum Administration on Biochemical Renal Markers in Blood
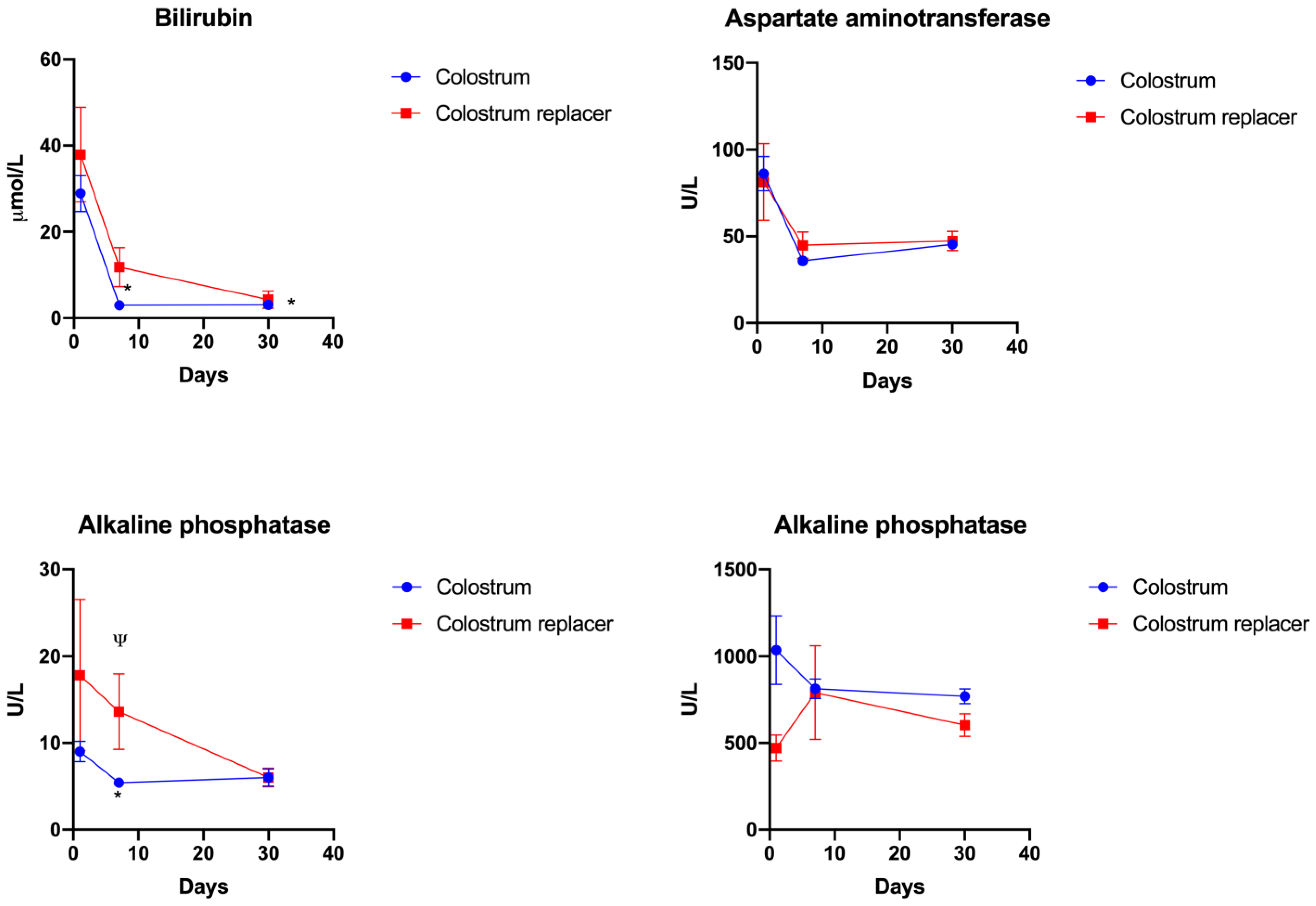
3.4. The Effect of Early Colostrum Administration on Biochemical Liver Markers in Blood
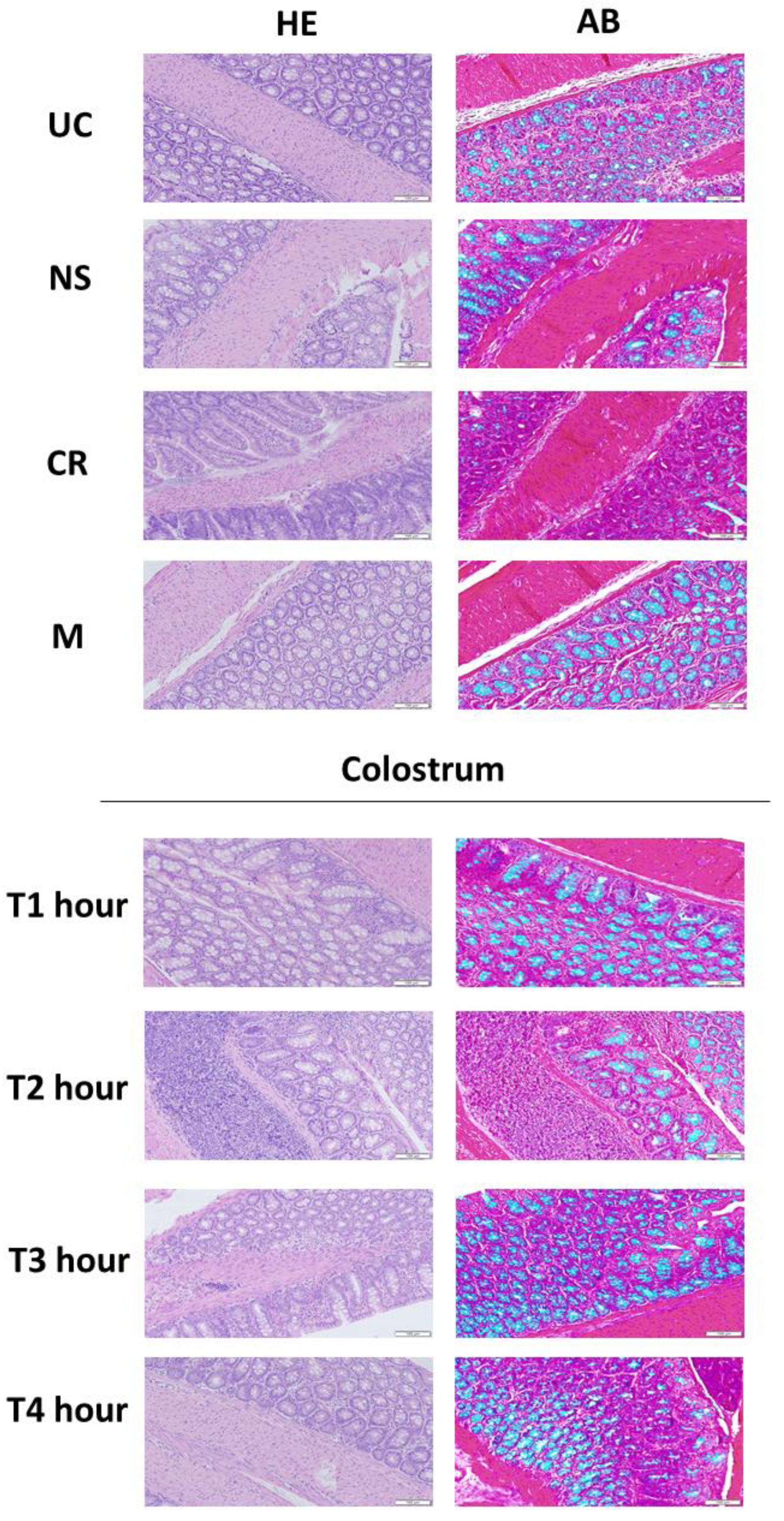
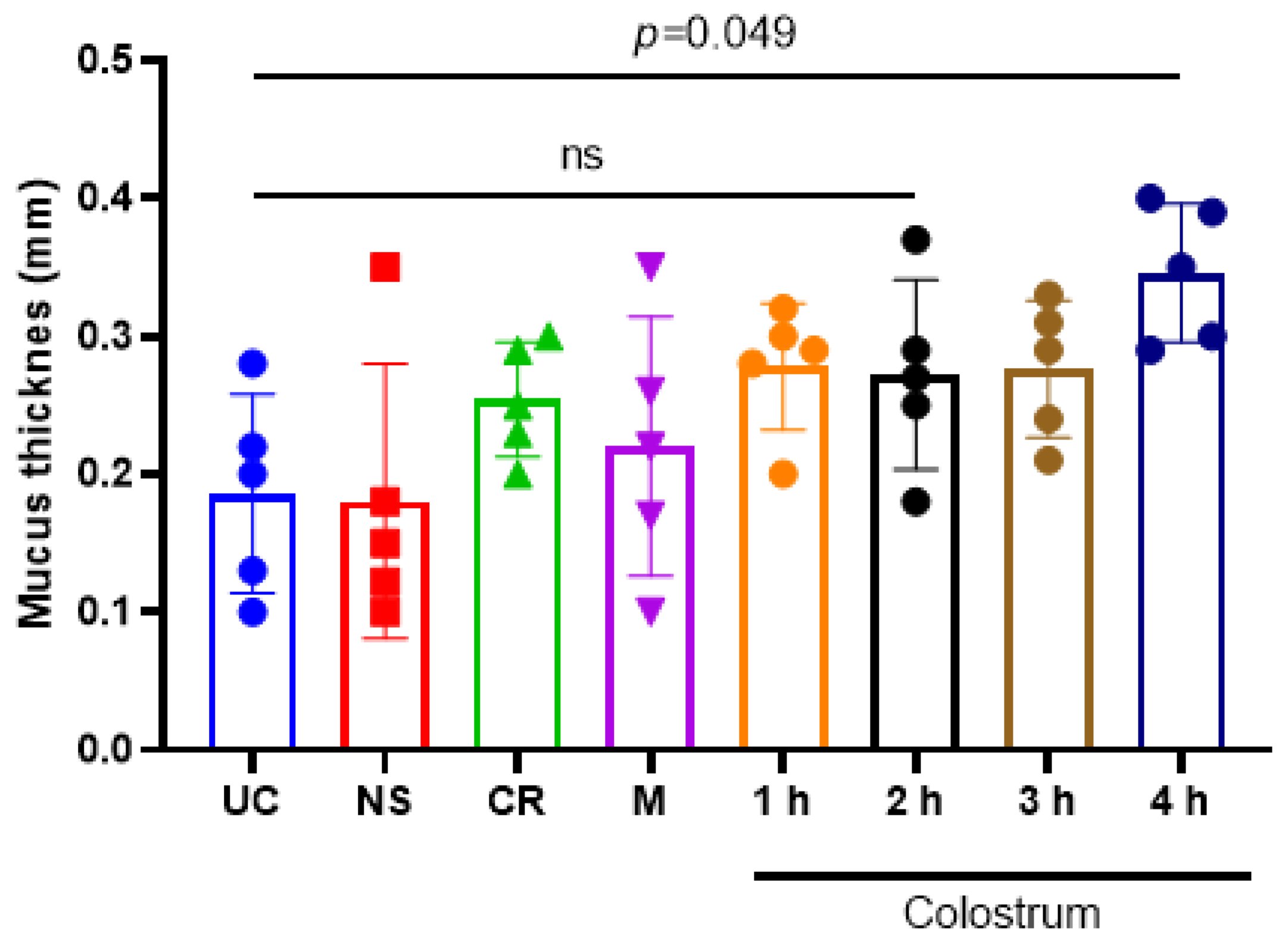
3.5. Bovine Colostrum Enhances the Mucus Production in the Intestine of Wistar Rats by Colostrum-Collection in a Time-Dependent Manner
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godden, S.M.; Smolenski, D.J.; Donahue, M.; Oakes, J.M.; Bey, R.; Wells, S.; Sreevatsan, S.; Stabel, J.; Fetrow, J. Heat-Treated Colostrum and Reduced Morbidity in Preweaned Dairy Calves: Results of a Randomized Trial and Examination of Mechanisms of Effectiveness. J. Dairy Sci. 2012, 95, 4029–4040. [Google Scholar] [CrossRef]
- Allore, H.G.; Oltenacu, P.A.; Erb, H.N. Effects of Season, Herd Size, and Geographic Region on the Composition and Quality of Milk in the Northeast. J. Dairy Sci. 1997, 80, 3040–3049. [Google Scholar] [CrossRef]
- Morrill, K.M.; Conrad, E.; Lago, A.; Campbell, J.; Quigley, J.; Tyler, H. Nationwide Evaluation of Quality and Composition of Colostrum on Dairy Farms in the United States. J. Dairy Sci. 2012, 95, 3997–4005. [Google Scholar] [CrossRef]
- Zarei, S.; Reza Ghorbani, G.; Khorvash, M.; Martin, O.; Hossein Mahdavi, A.; Riasi, A. The Impact of Season, Parity, and Volume of Colostrum on Holstein Dairy Cows Colostrum Composition. Agric. Sci. 2017, 8, 572–581. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Dunn, A.; Ashfield, A.; Earley, B.; Welsh, M.; Gordon, A.; McGee, M.; Morrison, S.J. Effect of Concentrate Supplementation during the Dry Period on Colostrum Quality and Effect of Colostrum Feeding Regimen on Passive Transfer of Immunity, Calf Health, and Performance. J. Dairy Sci. 2017, 100, 357–370. [Google Scholar] [CrossRef]
- Godhia, L.M.; Patel, N. Colostrum—Its Composition, Benefits as A Nutraceutical: A Review. Curr. Res. Nutr. Food Sci. 2013, 1, 37–47. [Google Scholar] [CrossRef]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef]
- le Cozler, Y.; Guatteo, R.; le Dréan, E.; Turban, H.; Leboeuf, F.; Pecceu, K.; Guinard-Flament, J. IgG1 Variations in the Colostrum of Holstein Dairy Cows. Animal 2015, 10, 230–237. [Google Scholar] [CrossRef]
- Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; Reinhardt, T.A.; Sonstegard, T.S. MicroRNA Expression Profiles of Bovine Milk Exosomes in Response to Staphylococcus aureus Infection. BMC Genom. 2015, 16, 806. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, W.K.; Kim, E.J. Combined Effects of Bovine Colostrum and Glutamine in Diclofenac-Induced Bacterial Translocation in Rat. Clin. Nutr. 2005, 24, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.F.; Greenhill, N.S.; Rowan, A.M.; Schollum, L.M. The Safety of New Zealand Bovine Colostrum: Nutritional and Physiological Evaluation in Rats. Food Chem. Toxicol. 2007, 45, 229–236. [Google Scholar] [CrossRef]
- Mobley, C.B.; Toedebusch, R.G.; Lockwood, C.M.; Heese, A.J.; Zhu, C.; Krieger, A.E.; Cruthirds, C.L.; Hofheins, J.C.; Company, J.M.; Wiedmeyer, C.E.; et al. Herbal Adaptogens Combined with Protein Fractions from Bovine Colostrum and Hen Egg Yolk Reduce Liver TNF-α Expression and Protein Carbonylation in Western Diet Feeding in Rats. Nutr. Metab. 2014, 11, 19. [Google Scholar] [CrossRef]
- Jungi, T.W.; Peterhans, E.; Pfister, H.; Fey, H. The Interaction of Ruminant IgG with Receptor Type II for IgG on Human Phagocytes. Immunology 1989, 66, 143. [Google Scholar]
- Fischer-Tlustos, A.J.; Lopez, A.; Hare, K.S.; Wood, K.M.; Steele, M.A. Effects of Colostrum Management on Transfer of Passive Immunity and the Potential Role of Colostral Bioactive Components on Neonatal Calf Development and Metabolism. Can. J. Anim. Sci. 2021, 101, 405–426. [Google Scholar] [CrossRef]
- Fischer, A.J.; Song, Y.; He, Z.; Haines, D.M.; Guan, L.L.; Steele, M.A. Effect of Delaying Colostrum Feeding on Passive Transfer and Intestinal Bacterial Colonization in Neonatal Male Holstein Calves. J. Dairy Sci. 2018, 101, 3099–3109. [Google Scholar] [CrossRef]
- Patel, S.; Gibbons, J. Ensuring Optimal Colostrum Transfer to Newborn Dairy Calves. Cattle Pract. 2014, 22, 95–104. [Google Scholar]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut Microbiome Colonization and Development in Neonatal Ruminants: Strategies, Prospects, and Opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Beaver, A.; Petersen, C.; Weary, D.M.; Finlay, B.B.; von Keyserlingk, M.A. Differences in the Fecal Microbiota of Dairy Calves Reared with Differing Sources of Milk and Levels of Maternal Contact. JDS Commun. 2021, 2, 200–206. [Google Scholar] [CrossRef]
- Rosa, F.; Michelotti, T.C.; St-Pierre, B.; Trevisi, E.; Osorio, J.S. Early Life Fecal Microbiota Transplantation in Neonatal Dairy Calves Promotes Growth Performance and Alleviates Inflammation and Oxidative Stress during Weaning. Animals 2021, 11, 2704. [Google Scholar] [CrossRef]
- Hue, D.T.; Skirving, R.; Chen, T.; Williams, J.L.; Bottema, C.D.K.; Petrovski, K. Colostrum Source and Passive Immunity Transfer in Dairy Bull Calves. J. Dairy Sci. 2021, 104, 8164–8176. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; Bruce German, J.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef] [PubMed]
- le Naour, J.; Montégut, L.; Joseph, A.; Garbin, K.; Vacchelli, E.; Kroemer, G.; Pol, J.G.; Maiuri, M.C. Improved Swiss-Rolling Method for Histological Analyses of Colon Tissue. MethodsX 2022, 9, 101630. [Google Scholar] [CrossRef] [PubMed]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef]
- Levieux, D.; Ollier, A. Bovine Immunoglobulin G, β-Lactoglobulin, α-Lactalbumin and Serum Albumin in Colostrum and Milk during the Early Post Partum Period. J. Dairy Res. 1999, 66, 421–430. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Nocek, J.E.; Braund, D.G.; Warner, R.G. Influence of Neonatal Colostrum Administration, Immunoglobulin, and Continued Feeding of Colostrum on Calf Gain, Health, and Serum Protein. J. Dairy Sci. 1984, 67, 319–333. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kataoka, S.; Yamanaka, H.; Kirisawa, R.; Iwai, H. Detection of Cytokines in Bovine Colostrum. Vet. Immunol. Immunopathol. 2000, 76, 183–190. [Google Scholar] [CrossRef]
- Wilm, J.; Costa, J.H.C.; Neave, H.W.; Weary, D.M.; von Keyserlingk, M.A.G. Technical Note: Serum Total Protein and Immunoglobulin G Concentrations in Neonatal Dairy Calves over the First 10 Days of Age. J. Dairy Sci. 2018, 101, 6430–6436. [Google Scholar] [CrossRef]
- Fidler, A.P.; Alley, M.L.; Smith, G.W. Short Communication: Serum Immunoglobulin G and Total Protein Concentrations in Dairy Calves Fed a Colostrum-Replacement Product. J. Dairy Sci. 2011, 94, 3609–3612. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The Gastrointestinal Mucus System in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Park, S.J.; Cheon, J.H. Effect of Colostrum on the Symptoms and Mucosal Permeability in Patients with Irritable Bowel Syndrome: A Randomized Placebo-Controlled Study. Intest. Res. 2014, 12, 80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noel, G.; In, J.G.; Lemme-Dumit, J.M.; DeVine, L.R.; Cole, R.N.; Guerrerio, A.L.; Campbell, J.D.; Kovbasnjuk, O.; Pasetti, M.F. Human Breast Milk Enhances Intestinal Mucosal Barrier Function and Innate Immunity in a Healthy Pediatric Human Enteroid Model. Front. Cell Dev. Biol. 2021, 9, 1833. [Google Scholar] [CrossRef]
- van Tassell, M.L.; Miller, M.J. Lactobacillus Adhesion to Mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigaleviciute, R.; Planciuniene, R.; Prikockyte, I.; Radzeviciute-Valciuke, E.; Baleviciute, A.; Zelvys, A.; Zinkeviciene, A.; Zigmantaite, V.; Kucinskas, A.; Matusevicius, P.; et al. The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves. Vet. Sci. 2023, 10, 128. https://doi.org/10.3390/vetsci10020128
Grigaleviciute R, Planciuniene R, Prikockyte I, Radzeviciute-Valciuke E, Baleviciute A, Zelvys A, Zinkeviciene A, Zigmantaite V, Kucinskas A, Matusevicius P, et al. The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves. Veterinary Sciences. 2023; 10(2):128. https://doi.org/10.3390/vetsci10020128
Chicago/Turabian StyleGrigaleviciute, Ramune, Rita Planciuniene, Ieva Prikockyte, Eivina Radzeviciute-Valciuke, Austeja Baleviciute, Augustinas Zelvys, Aukse Zinkeviciene, Vilma Zigmantaite, Audrius Kucinskas, Paulius Matusevicius, and et al. 2023. "The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves" Veterinary Sciences 10, no. 2: 128. https://doi.org/10.3390/vetsci10020128
APA StyleGrigaleviciute, R., Planciuniene, R., Prikockyte, I., Radzeviciute-Valciuke, E., Baleviciute, A., Zelvys, A., Zinkeviciene, A., Zigmantaite, V., Kucinskas, A., Matusevicius, P., & Kavaliauskas, P. (2023). The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves. Veterinary Sciences, 10(2), 128. https://doi.org/10.3390/vetsci10020128





