Abstract
Abiotic stresses, such as a drought and heat, are potential constraints limiting wheat production across the globe. This current perspective study intended to characterize the performance of exotic synthetic hexaploid (SH) wheat genotypes on a physiological, biochemical, and agronomic basis under field-based drought and heat conditions. The tri-replicate experiments were conducted in two seasons using two-factorial arrangements in a randomized complete block design (RCBD) with stresses as one factor and genotypes as another factor. The recorded data were statistically analyzed using computer-based software statistix8.1 and R-studio. In this study, all the physiological parameters (total chlorophyll, stomatal conductance, photosynthesis rate, transpiration rate, and cell membrane stability percentage), biochemical stress markers (antioxidant enzymes, glycine betaine, and proline), and agronomic traits (flag leaf area, plant height, tillers per plant, spike length, grains per spike, and thousand grain weight) varied significantly under separate and combined regimes of drought and heat stresses. All traits varied in same direction, excluding glycine betaine and proline, which varied in the opposite direction because of stress, as explicated by correlation analysis. Furthermore, PCA and heatmap analysis confirmed that the expression of the traits varied more significantly because of combined regimes of drought and heat stresses as compared to controlled and isolated applications. Interestingly, synthetic hexaploid (SH) genotypes depicted similar responses to individual and integrated regimes of drought and heat stresses. The current study proved that deciphering the physiological, biochemical, and agronomic performance of wheat genotypes under stress can provide effective criteria for the future selection of wheat germplasm for breeding against drought and heat stresses.
1. Introduction
Wheat (Triticum aestivum L.) is an important cereal crop used as a main staple food in many countries in the world. Various abiotic stresses individually or unanimously impair the annual wheat yield [1]. The current decade has included drought and heat stresses which became the most eminent factors in determining crop productivity and food security [2]. The severe prevalence of drought and heat stresses is causing a substantial loss in the crop yield through adverse effects on plant growth, biochemistry, physiology, and reproduction [3,4]. Drought and heat stresses caused wheat productivity to suffer a 40% reduction, as reviewed by Fahad et al. [1]. In arid regions, wheat production is potentially reduced by terminal heat stress, and the effect is further intensified when combined with drought stress [5]. The optimum temperature for wheat during its reproductive stage is 15–20 °C, where a per degree Celsius rise can decrease the yield by up to 21% [6]. Heat stress has various implications during the reproductive stages of wheat: it shortens the life cycle, impairs pollen viability, and disrupts grain settings, which collectively leads toward a decline in crop productivity [7]. In general, arid regions of the world are rain fed, and drought combines with heat stress particularly at the terminal stages of wheat [5]. Heat and drought stresses both dramatically impair various physiological activities such as stomatal conductance, the transpiration rate, chlorophyll content, and membrane integrity [8]. Severe drought stress disrupts plant photosynthesis by destroying the enzymes involved in chlorophyll synthesis, and these effects are further intensified when drought and heat stress are combined [9]. Abiotic stresses such as drought and heat also create oxidative stress within the plant cellular system; this is caused by the generation of reactive oxygen species (ROS) that disrupt the membrane fluidity and integrity by enhancing lipid peroxidation [10]. A plant is not a passive system; instead, it reacts to any sort of stress through the activation of various homeostatic mechanisms [11]. From this perspective, a plant triggers the activities of various antioxidant enzymes such as superoxide dismutase, catalase, and peroxidase, which regulate various pathways involved in the scavenging of ROS [12]. Moreover, abiotic stresses impose osmotic stress that perturbs the plant water balance, causing the plant to release various osmoprotectants, such as proline and glycine betaine [13]. Proline is an important amino acid that tends to alleviate the impacts of drought and heat stress through an antioxidant defense system that involves scavenging ROS [14]. In the same way, glycine betaine is an important osmolyte that sustains growth and ensures plant survival by counteracting the metabolic dysfunctions imposed by drought and heat stresses [15,16]. In addition to physiological and biochemical changes, wheat exhibits various agronomic changes such as a reduction in the flag leaf area, plant height, number of tillers, spike length, grain per spike, and thousand grain weight due to abiotic stresses [16]. The creation of physiological and biochemical disequilibrium within the plant under the conditions of stress severely compromises the crop yield [17]. The tendency of plants to respond to stress varies based upon the diversity of their genetic architecture. Drought and heat tolerance is a multigenic trait regulated by many genes that have been eroded from bread wheat by its self-pollinated nature and continuous domestication process [18]. In this regard, synthetic hexaploid wheat is considered a valuable source of novel genes and diversity that can be incorporated in elite bread wheat cultivars without facing any reproductive barrier [19]. Synthetic hexaploid wheat (2n = 6x = 42, AABBDD) is produced artificially by an interspecific cross of durum wheat (2n = 4x = 28, AABB, T. turgidum L.) and goat grass (2n = 2x = 14, DD, Aegilops tauschii Coss.) [20]. Furthermore, lines derived from synthetic hexaploid wheat help breeders to restore the novel genes that were lost during the evolutionary process [19]. Synthetic hexaploid wheat is also more tolerant of drought and heat stresses as it contains many genes inherited from its wild progenitors [20]. This perspective current study aimed to evaluate the performance of synthetic hexaploid wheat genotypes under individual and combined applications of drought and heat stresses on the physiological, biochemical, and agronomic factors under field conditions. Furthermore, this study aimed to test the suitability of these genotypes as a valuable breeding material to avoid damage from drought and heat stress.
2. Material and Methods
In the current study, different synthetic hexaploid (SH) wheat genotypes (Table 1) collected from the NARC (National Agricultural Research Center) Islamabad, Pakistan were evaluated under field conditions at Hada-Alsham (21.802° North, 39.729° East, 254 m a.s.l.), the research area of King Abdulaziz University, Jeddah, Saudi Arabia. During the growing season (October to March), the experimental site was characterized by an annual rainfall of 53 mm, 50–65% humidity, and a temperature of 19–35 °C (Table 2). The textural class of soil was loamy sand with a moderately alkaline pH of 8.3. The tri-replicate experiments were conducted in two runs (2020–21 and 2021–22) using a two-factorial arrangement in a randomized complete block design (RCBD) with stresses as one factor and genotypes as another factor.

Table 1.
List of synthetic hexaploid genotypes characterized for physiological, biochemical, and morphological traits under separate and combined regimes of in field drought and heat stress.

Table 2.
Average meteorological data of research station during cropping seasons 2020–21 and 2021–22.
2.1. Crop Husbandry, Treatments, and Data Collection
Soil followed by a power tiller was harrowed and well laddered. The remains and leftovers of a previous crop were removed. A set of ten SH wheat genotypes were checked under controlled (irrigated), individual, and combined levels of drought (rain fed) and heat (site temperature) stresses during two cropping seasons (2020–21 and 2021–22). The size of each plot was approximately 7.2 m2, with 6 rows of plants each at 20 cm apart. Furthermore, the soil was augmented with nitrogen and P2O5 each at the rate of 50 kg ha−1. For the individual level of the control and drought treatments, the wheat genotypes followed early sowing (mid-October) for preventing them from terminal heat stress. On the other hand, wheat genotypes subjected to heat and drought + heat treatments followed late sowing (mid-November). The data for all physiological and biochemical parameters were recorded after the crop attained physiological maturity at the heading stage and continued until the grain setting. The data were collected on a weekly basis and averaged. On the other hand, the data for the yield parameters were recorded after harvesting. For the data estimation, five to ten plants were randomly selected for each treatment.
2.2. Estimation of Physiological Traits
The physiological traits of the stomatal conductance (Gs), photosynthesis rate (Pn), and transpiration rate (Tr) were determined from an attached flag leaf between 8 a.m. and 10 p.m. using Syrus-3 (Decagon Devices, Pullman, WA, USA). The total chlorophyll content was measured using SPAD-502 (Spectrum Technologies, Bridgend, United Kingdom) apparatus. The cell membrane stability percentage (CMSP) was estimated with the help of formula CMSP = [(1 − (X1/X2))/(1 − (Y1/Y2))} × 100, whereas X1 = the conductance of the stress-treated sample before autoclaving, X2 = the conductance of the stress-treated sample after autoclaving, Y1 = the conductance of the control sample before autoclaving, and Y2 = the conductance of the control sample after autoclaving.
2.3. Estimation of Biochemical Traits
Among the biochemical traits, the activities of the antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalases (CAT) were estimated by using respective enzyme assay kits. For this purpose, 1 g of frozen leaf samples were homogenously crushed in 1 mL of 0.1 M ice cold Tri-HCL buffer following the procedure used by Djanaguiraman et al. [21]. Subsequently, the mixture was centrifuged at 4 °C for 15 min at 20,000× g and the supernatant was isolated for the assay of the enzymatic activities. The activity of the SOD was recorded using an SOD-assay kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s guidelines. The catalase activity was recorded using a CAT-assay kit (Sigma-Aldrich, St. Louis, MO, USA) following the given instructions. In addition, a peroxidase-assay kit (Cell Biolabs Inc, San Diego, CA, USA) was used for estimating the plants’ activity according to the given standard protocol. The proline content was quantified using a UV-Vis spectrophotometer (DeNovix, Wilmington, NC, USA) based on its reactivity with ninhydrin and the absorbance was recorded at 508 nm for the proline–ninhydrin condensation product. On the other hand, the glycine betaine (GB) content from the selected samples was recorded using high-performance liquid chromatography (HPLC) (Shimadzu Corp, Kyoto, Japan) following the protocol demonstrated by Ma et al. [22]. For this purpose, a plant extract of 20–100 μL was used and the eluted GB (retention time 4–5 min) was estimated by recording the absorbance at 200 nm with the help of a diode-array spectrophotometer (Bioevopeak, Jinan, Shandong, China). Subsequently, the quantification was completed by comparing the surface areas of the peaks with those obtained with pure standard GB solutions in the range of 0.05–4 mM.
2.4. Estimation of Agronomic Traits
The agronomic traits flag leaf area (FLA) was recorded with the help of the formula used by Shah et al. [17]. The plant height (PH) from randomly selected plants was calculated from the shoot base to the apex by using a meter rod and was averaged for analysis. The spike length (SL) from randomly selected plants was measured with the help of a scale and averaged for analysis. In the same way, the number of tillers per plant (TPP) and grains per spike (GPS) were simply counted from randomly selected plants and averaged for statistical analysis. The thousand grain weight (TGW) was recorded with the help of the electric weighing balance (Bioevopeak, Jinan, Shandong, China).
2.5. Statistical Analysis
The recorded data were subjected to statistical analysis using computer-based program Statistix8.1 and R-program [17]. The ANOVA was performed and LSD was calculated at (p ≤ 0.05). Furthermore, for the delineation of the traits’ association and expression correlation, PCA and heatmap analysis were performed.
3. Results
3.1. Physiological Traits
Among the individual factors, the stress treatments showed a significant effect (p ≤ 0.01), while the genotypes showed no significant effect on the mean values of the physiological traits such as the total chlorophyll, stomatal conductance (Gs), photosynthesis (Pn), transpiration rate (Tr), and cell membrane stability percentage (CMSP), as indicated in Table 3. All the physiological traits illustrated a significant reduction for all the level of stresses compared to the control; however, this reduction was more dramatic when drought and heat stresses acted together (Table 3). In the same way, a two-way interaction of the genotypes and stress (genotypes × stress) showed a significant (p ≤ 0.05) effect on the mean values of all the physiological traits. All the genotypes showed a significant decline in the mean values of the physiological parameters in a parallel way under the individual and combined treatment of the stresses; however, this decline was more dramatic due to the combined application of the stresses as compared to their individual treatments (Table 3, Table 4, Table 5 and Table 6).

Table 3.
Variation in physiological parameters of different synthetic hexaploid wheat genotypes due to individual and combined application of drought and heat stresses during 2020–21 and 2021–22.

Table 4.
Variation in biochemical parameters of different synthetic hexaploid wheat genotypes due to individual and combined application of drought and heat stresses during 2020–21 and 2021–22.

Table 5.
Variation in agronomical parameters of different synthetic hexaploid wheat genotypes due to individual and combined application of drought and heat stresses during 2020–21 and 2021–22.

Table 6.
Variation in physiological, biochemical, and agronomic traits (averaged 2020–21 and 2021–22) due to interaction effect of synthetic hexaploid wheat genotypes with individual and combined applications of drought and heat stresses.
3.2. Biochemical Traits
All biochemical traits such as antioxidant enzymes (SOD, POD, and CAT), glycine betaine, and proline depicted a significant (p ≤ 0.01) change due to the individual factor of stress, while they also showed a non-significant change due to the individual factor of the genotypes (Table 4). Both separate and combined regimes of drought and heat stresses recorded a significant decrease in the activities of the antioxidant enzymes and a significant (p ≤ 0.01) increase in the concentrations of glycine betaine and proline as compared to the control; however, this decrease and increase were at the maximum due to the combined application as compared to the individual application of drought and heat stresses (Table 4). In the same way, the biochemical traits showed a statistically significant (p ≤ 0.05) variation due to the interaction effect of the genotypes and stress (genotype × stress). All the synthetic hexaploid (SH) wheat genotypes depicted the maximum increase in the activities of the SOD, POD, CAT, glycine betaine, and proline due to the combined treatments as compared to the individual treatments of drought and heat stresses (Table 6).
3.3. Agronomic Traits
The agronomic traits, such as the flag leaf area (FLA), plant height (PH), tillers per plant (TPP), spike length (SL), grains per spike (GPS), and thousand grain weight (TGW), recorded a significant (p ≤ 0.01) alteration due to the individual effect of stress while they also showed a non-significant alteration due to the individual effect of the genotypes (Table 5). Both the separate and combined regimes of drought and heat stresses manifested a statistically distinct reduction in all the agronomic traits as compared to the control; however, this reduction was the highest for the combined regime as compared to the individual regimes of stress. On the other hand, a two-way interaction of the genotypes and stress (genotypes × stress) significantly (p ≤ 0.05) affected the mean values of all the agronomic traits. All synthetic hexaploid (SH) wheat genotypes revealed a more dramatic reduction in the agronomic traits due to the combined regimes as compared to the separate regimes of drought and heat stresses (Table 6).
3.4. Correlation, PCA and Heatmap Analysis
The correlation analysis revealed a significant paired association among all the physiological, biochemical, and agronomic traits (Figure 1). Among the physiological traits, the Chl, Gs, Pn, and CMSP recorded a significant positive paired association in the same direction with the activities of the antioxidant enzymes (SOD, POD, and CAT) and agronomic traits, such as the FLA, PH, SL, GPS, and TGW. Contrarily, the glycine betaine (GB) and proline content revealed a significant negative paired association in the opposite direction with the physiological traits (Chl, Gs, Pn, and CMSP), antioxidant enzymes (SOD, POD, and CAT), and agronomic traits (FLA, PH, SL, GPS, and TGW). The principal component analysis revealed (PCA) the differential extent of the association of the traits due to the separate and combined treatments of drought and heat stresses as illustrated by the differential dispersion of the traits’ clusters with respect to the origin in the PCA scattered plot (Figure 2). This explicated that the nature of the association of the traits is strongly associated with the type of stress treatment. However, no remarkable effect on the orientation of the traits cluster with respect to the origin was noticed in the PCA scattered plot due to the synthetic hexaploid (SH) wheat genotypes, which explicated that the trend of association of the traits is independent of the nature of genotypes (Figure 3). On the other hand, the PCA scattered graph showed a considerable deviation of the traits’ clusters from the origin due to the interaction effect of the genotypes and stress (Figure 4). Moreover, the PCA plot depicted that the performance of all the genotypes varied to a great extent with respect to the traits’ associations due to the combined treatments of drought and heat stresses; however, individual treatments of drought and heat stresses did not make a remarkable difference in the performance of the genotypes in terms of the traits’ associations. In addition, the heatmap cluster analysis also authenticated that the extent of association of the traits varied significantly with the type of stress treatment (Figure 5).
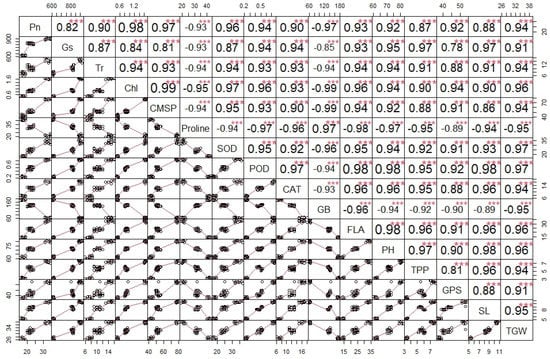
Figure 1.
Correlation chart showing the significance of association of physiological, biochemical, and agronomic traits in synthetic hexaploid wheat genotypes. Pn; photosynthesis, Gs; stomatal conductance, Tr; transpiration rate, CMSP; cell membrane stability percentage, GB; glycine betaine, SOD; superoxide dismutase, POD; peroxidase, CAT; catalase, FLA; flag leaf area, PH; plant height, TPP; tiller per plant, SL; spike length, GPS; grains per spike, TGW; thousand grain weight. *** = significant at p ≤ 0.001.
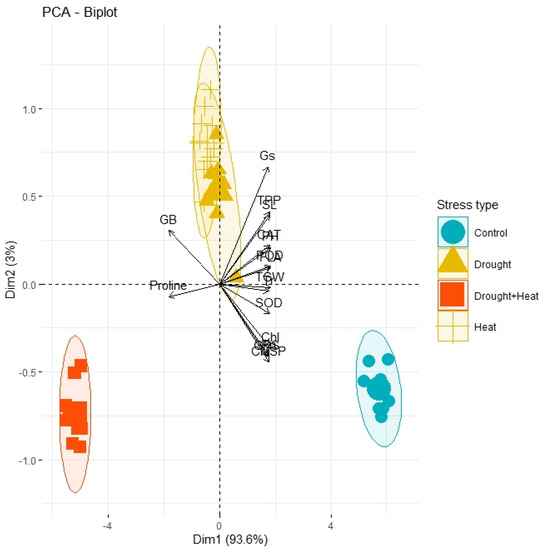
Figure 2.
PCA plot showing the differential divergence of treatments circles with respect to proximity association of physiological, biochemical, and agronomic traits while extent of divergence of circles is directly associated with variation in the effect of treatments. Pn; photosynthesis, Gs; stomatal conductance, Tr; transpiration rate, CMSP; cell membrane stability percentage, GB; glycine betaine, SOD; superoxide dismutase, POD; peroxidase, CAT; catalase, FLA; flag leaf area, PH; plant height, TPP; tiller per plant, SL; spike length, GPS; grains per spike, TGW; thousand grain weight.
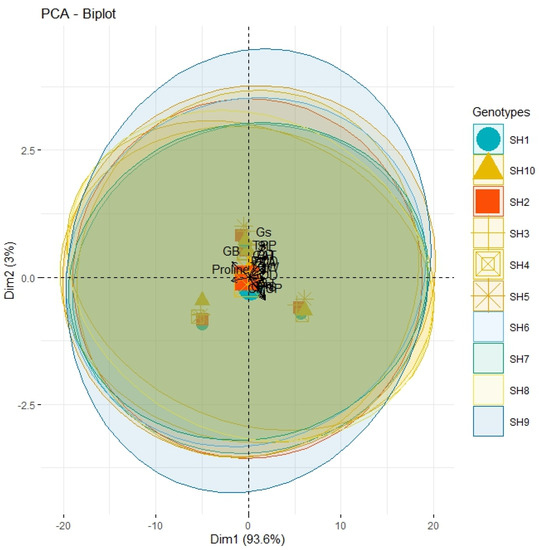
Figure 3.
PCA with no scatter circle indicating analogous impact of synthetic SH wheat genotypes on proximity association of physiological, biochemical, and agronomic traits. Pn; photosynthesis, Gs; stomatal conductance, Tr; transpiration rate, CMSP; cell membrane stability percentage, GB; glycine betaine, SOD; superoxide dismutase, POD; peroxidase, CAT; catalase, FLA; flag leaf area, PH; plant height, TPP; tiller per plant, SL; spike length, GPS; grains per spike, TGW; thousand grain weight.
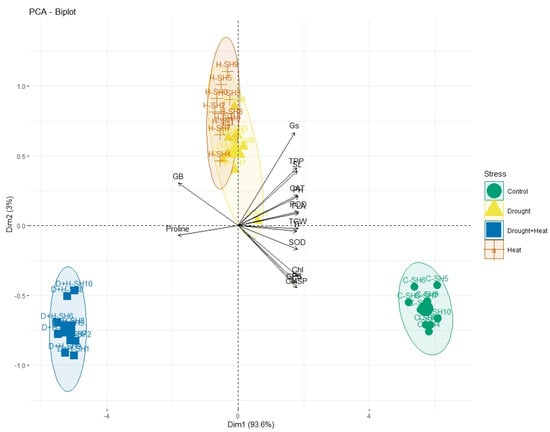
Figure 4.
PCA plot showing the differential divergence of treatment interaction (genotypes × stress) circles with respect to proximity association of physiological, biochemical, and agronomic traits while extent of divergence of circles is directly associated with variation in the effect of interacting treatments. Pn; photosynthesis, Gs; stomatal conductance, Tr; transpiration rate, CMSP; cell membrane stability percentage, GB; glycine betaine, SOD; superoxide dismutase, POD; peroxidase, CAT; catalase, FLA; flag leaf area, PH; plant height, TPP; tiller per plant, SL; spike length, GPS; grains per spike, TGW; thousand grain weight.
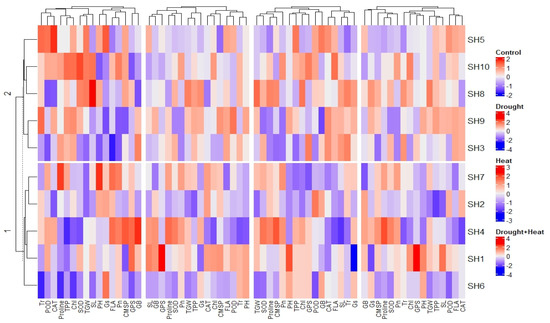
Figure 5.
Heatmap dendrogram revealing varying extent of traits association in SH wheat genotypes due to control, separte, and combined regimes of drought and heat stresses. Pn; photosynthesis, Gs; stomatal conductance, Tr; transpiration rate, CMSP; cell membrane stability percentage, GB; glycine betaine, SOD; superoxide dismutase, POD; peroxidase, CAT; catalase, FLA; flag leaf area, PH; plant height, TPP; tiller per plant, SL; spike length, GPS; grains per spike, TGW; thousand grain weight.
4. Discussion
The current study intended to characterize the different genotypes of SH wheat under separate and combined regimes of drought and heat stresses under a field condition at physiological, biochemical, and agronomic levels. All the genotypes depicted comparatively similar responses to the separate and combined regimes of drought and heat stresses (Table 3, Table 4, Table 5 and Table 6). Abiotic stresses directly affected the plants’ metabolic activities via an interruption of the numerous physiological and metabolic activities [10]. For instance, drought and heat stresses are capable of damaging the plants’ photosystem, thylakoid membrane, and the enzymes involved in chlorophyll synthesis, which leads to a dramatic reduction in the chlorophyll (chl) content and photosynthesis rate (Pn), as reviewed by Fahad et al. [2]. Moreover, both a high temperature and drought can trigger the production of ROS, which destroys the membrane integrity, due to lipid peroxidation and protein degradation, which leads towards cell membrane damage and more electrolyte leakage [23]. Furthermore, abiotic stresses disrupt the plant water relations, including the water potential and osmotic potential, which interrupts various exchange mechanisms such as the stomatal conductance (Gs) and transpiration (Tr) [11]. In consistence with these findings, the current study recorded a dramatic reduction in the chlorophyll, Pn, Gs, Tr, and CMSP under both individual and integrated spans of drought and heat stresses (Table 3 and Table 6). Correspondingly, Algahabari et al. [8], Shah et al. [17], and Qaseem et al. [24] also reported a significant reduction in the chlorophyll, Pn, Gs, Tr, and CMSP under isolated and combined versions of drought and heat stresses. Furthermore, the simultaneous occurrence of drought and heat stress aggravates the symptoms of stress [9]. A decline in the activities of antioxidant enzymes during the regimes of stress is a potential cause of the generation of ROS that interfere with the plant’s natural defense mechanism, as reviewed by Qayyum et al. [12] and Caverzan et al. [25]. In fact, drought and heat stresses elicit the production of ROS, owing to the suppression of the activities of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) [26]. Correspondingly, Alghabari et al. [8] and Nasirzadeh et al. [27] recorded a decline in the activities of these enzymes in wheat under heat and drought, respectively. In parallel with these findings, the current study reported a significant decrease in the activities of these enzymes due to drought and heat stresses (Table 4 and Table 6). Plants are not passive objects; instead, they respond to counter the effect of any external stimulus causing an imbalance in the plants’ homeostatic equilibrium through the activation of various mechanisms involved in the synthesis of different metabolites [10]. In this context, plants conduct an osmotic readjustment by raising the levels of various osmoprotectants, such as glycine betaine and proline [13,24], as confirmed by the present study (Table 4 and Table 6). Plants have several morphological adaptations to nullify the effect of stress; the reduction in the flag leaf area (FLA) is one of them [17]. This is the most probable reason for the reduction in Pn, Tr, and Gs that leads to less accumulation of photosynthates, causing a reduction in the overall agronomic productivity, demonstrated by fewer tillers per plant (TPP), the plant height (PH), the spike length (SL), the grains per spike (GPS), and the thousand grain weight (TGW), as confirmed by numerous researchers [8,14,17]. In consistence with these findings, the current study reported a significant decline in the agronomic traits due to drought and heat stresses (Table 4 and Table 6). Furthermore, high-temperature stress severely impacted the pollen viability, which hinders the pollination and grain setting process, while the concurrence of drought stress intensified the hazards of heat stress [7]. This is another potential reason for a declined agronomic productivity. Abiotic stress perturbs the chlorophyll content and physiological processes, such as Pn, Gs, and Tr, which results in the availability of less photosynthates for enhancing the agronomic productivity in the form of TPP, SL, GPS, and TGW [8,17]. In the same way, the physiological activities are strongly correlated with the activities of the antioxidant enzymatic and osmolyte concentration [12]. Therefore, equilibrium of the physiological and biochemical processes is strongly correlated with the ultimate agronomic yield, as proven by the correlation analysis in the current study (Figure 1). However, drought and heat stress change the extent of association and expression of physiological, biochemical, and agronomic traits, as proven by the principal component (PCA) and heatmap analysis (Figure 2 and Figure 5). These findings were in complete agreement with Alghabari et al. [8] and Shah et al. [17], who proved that during stress, all traits varied in the paired pattern. Interestingly, in the current study, all SH wheat genotypes exhibited analogous behavior to individual and combined regimes of drought and heat stress (Figure 3 and Figure 4). This can be attributed to their very close association in terms of the genetic architecture [19]. Although their behavior varied with the type of stress, they survived. Hence, this synthetic hexaploid wheat germplasm can serve as potential stock for breeding against drought and heat stress. Moreover, the current characterization of these genotypes on a physiological, biochemical, and agronomic basis can provide substantial help for genetic elucidations in the future. Moreover, crop productivity is an outcome of physio-chemical equilibrium that a tolerant plant is sure to retain under conditions of abiotic stresses. In this context, the current study concluded that synthetic wheat is better able to retain its physio-chemical equilibrium under drought and heat stresses; hence, it can serve as a bridge to introgress such novel traits into elite bread wheat varieties without facing any barrier of sexuality.
Author Contributions
Z.H.S. and H.S. came up with the idea, while F.A. executed the experiment. Z.H.S. and F.A. wrote the manuscript and H.S. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Siddique, K.H.M.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Geno-types/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Basu, S.; Kumar, S.; Kumar, G.; Prakash, V.; Kumar, S.; Mishra, J.S.; Bhatt, B.; Malviya, N.; Singh, G.; et al. Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crops Res. 2017, 206, 106–114. [Google Scholar] [CrossRef]
- Alghabari, F.; Shah, Z.H.; Elfeel, A.A.; Alyami, J.H. Biochemical and Physiological Responses of Thermostable Wheat Genotypes for Agronomic Yield under Heat Stress during Reproductive Stages. Agronomy 2021, 20, 80. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Daur, I.; Nawaz, M.A.; Ahmad, M.Q.; Rana, I.A.; Atif, R.M.; Yang, S.H.; Chung, G. Redox and Ionic Homeostasis Regulations against Oxidative, Salinity and Drought Stress in Wheat (A Systems Biology Approach). Front Genet. 2017, 8, 141. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Qayyum, A.; Al Ayoubi, S.; Sher, A.; Bibi, Y.; Ahmad, S.; Shen, Z.; Jenks, M.A. Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 2021, 28, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Zhang, X.Y.; Li, F.; Luo, Y.; Wang, W. Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P.; Bayat, H. Effect of Water Deficit Stress on Seedling Biomass and Physio-Chemical Characteristics in Different Species of Wheat Possessing the D Genome. Agronomy 2019, 9, 522. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of Wheat (Triticum aestivum L.) Genotypes for Drought Tolerance through Agronomic and Physiological Response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Shah, S.M.D.M.; Shabbir, G.; Malik, S.I.; Raja, N.I.; Shah, Z.H.; Rauf, M.; Zahrani, Y.A.; Alghabari, F.; Alsamadany, H.; Shahzad, K.; et al. Delineation of Physiological, Agronomic and Genetic Responses of Different Wheat Genotypes under Drought Condition. Agronomy 2022, 12, 1056. [Google Scholar] [CrossRef]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.; Jamshed, M.; Samuel, M.A. Abiotic stress signaling in wheat–an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Yang, W.; Kishii, M.; Mao, L. Synthetic hexaploid wheat: Yesterday, today, and tomorrow. Engineering 2018, 4, 552–558. [Google Scholar] [CrossRef]
- Pradhan, G.P.; Prasad, P.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Effects of drought and high temperature stress on synthetic hexaploid wheat. Funct. Plant Biol. 2012, 39, 190–198. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Shayeganpour, A.; Brocks, D.R.; Lavasanifar, A.; Samuel, J. High-performance liquid chromatography analysis of curcumin in rat plasma: Application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed. Chrom. 2007, 21, 546–552. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, Tolerance, Adaptation, and Mitigation of Heat Stress on Wheat under Changing Climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Majidi Hervan, E.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Commun. 2021, 49, 83–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).