Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials of the Study
4.2. Plasma Levels of Cytokines
4.3. The Plasma Selenium
4.4. Determination of Glutathione Peroxidase (GPx) 3 and (EC) 1.11.1.9 Activity
4.5. Selenoprotein P (SeP)
4.6. Stress Parameters
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomer, Y.; Davies, T. Searching for the Autoimmune Thyroid Disease Susceptibility Genes: From Gene Mapping to Gene Function. Endocr. Rev. 2003, 24, 694–717. [Google Scholar] [CrossRef]
- Hansen, P.S.; Brix, T.H.; Iachine, I.; Kyvik, K.O.; Hegedüs, L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: A study of healthy Danish twins. Eur. J. Endocrinol. 2006, 154, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Docimo, G.; Cangiano, A.; Romano, R.M.; Pignatelli, M.F.; Offi, C.; Paglionico, V.A.; Galdiero, M.; Donnarumma, G.; Nigro, V.; Esposito, D.; et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020, 11, 586529. [Google Scholar] [CrossRef]
- Matos-Santos, A.; Nobre, E.L.; Costa, J.G.; Nogueira, P.J.; Macedo, A.; Galvão-Teles, A.; de Castro, J.J. Relationship between the number and impact of stressful life events and the onset of Graves’ disease and toxic nodular goitre. Clin. Endocrinol. 2001, 55, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Johnson, E.; Kalogera, C.; Seferiadis, K.; Tsolas, O. The effect of thyrotoxicosis on adrenocortical reserve. Eur. J. Endocrinol. 2000, 142, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Fukao, A.; Takamatsu, J.; Murakami, Y.; Sakane, S.; Miyauchi, A.; Kuma, K.; Hayashi, S.; Hanafusa, T. The relationship of psychological factors to the prognosis of hyperthyroidism in antithyroid drug-treated patients with Graves’ disease. Clin. Endocrinol. 2003, 58, 550–555. [Google Scholar] [CrossRef]
- Effraimidis, G.; Tijssen, J.G.; Brosschot, J.F.; Wiersinga, W.M. Involvement of stress in the pathogenesis of autoimmune thyroid disease: A prospective study. Psychoneuroendocrinology 2012, 37, 1191–1198. [Google Scholar] [CrossRef]
- Damian, L.; Ghiciuc, C.M.; Dima-Cozma, L.C.; Ungureanu, M.C.; Cozma, S.; Patacchioli, F.R.; Lupusoru, C.E. No definitive evidence for a connection between autoimmune thyroid diseases and stress in women. Neuro Endocrinol. Lett. 2016, 37, 155–162. [Google Scholar]
- Blotta, M.H.; DeKruyff, R.H.; Umetsu, D.T. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J. Immunol. 1997, 158, 5589–5595. [Google Scholar] [CrossRef]
- DeKruyff, R.H.; Fang, Y.; Umetsu, D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998, 160, 2231–2237. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Markomanolaki, Z.S.; Tigani, X.; Siamatras, T.; Bacopoulou, F.; Tsartsalis, A.; Artemiadis, A.; Megalooikonomou, V.; Vlachakis, D.; Chrousos, G.P.; Darviri, C. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J. Mol. Biochem. 2019, 8, 3–12. [Google Scholar] [PubMed]
- Moncayo, R.; Moncayo, H. The WOMED model of benign thyroid disease: Acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2014, 3, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshiuchi, K.; Kumano, H.; Nomura, S.; Yoshimura, H.; Ito, K.; Kanaji, Y.; Kuboki, T.; Suematsu, H. Psychosocial factors influencing the short-term outcome of antithyroid drug therapy in Graves’ disease. Psychosom. Med. 1998, 60, 592–596. [Google Scholar] [CrossRef]
- Porcelli, B.; Pozza, A.; Bizzaro, N.; Fagiolini, A.; Costantini, M.C.; Terzuoli, L.; Ferretti, F. Association between stressful life events and autoimmune diseases: A systematic review and meta-analysis of retrospective case-control studies. Autoimmun. Rev. 2016, 15, 325–334. [Google Scholar] [CrossRef]
- Radosavljević, V.R.; Janković, S.M.; Marinković, J.M. Stressful life events in the pathogenesis of Graves’ disease. Eur. J. Endocrinol. 1996, 134, 699–701. [Google Scholar] [CrossRef]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, 241–252. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Pathogenesis of thyroid autoimmune disease: The role of cellular mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- Figueroa-Vega, N.; Alfonso-Pérez, M.; Benedicto, I.; Sánchez-Madrid, F.; González-Amaro, R.; Marazuela, M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 2010, 95, 953–962. [Google Scholar] [CrossRef]
- Peng, D.; Xu, B.; Wang, Y.; Guo, H.; Jiang, Y. A high frequency of circulating Th22 and Th17 cells in patients with new onset graves’ disease. PLoS ONE 2013, 8, e68446. [Google Scholar] [CrossRef]
- Esfahanian, F.; Ghelich, R.; Rashidian, H.; Jadali, Z. Increased Levels of Serum Interleukin-17 in Patients with Hashimoto’s Thyroiditis. Indian J. Endocrinol. Metab. 2017, 21, 551–554. [Google Scholar]
- Chrousos, G.P.; Elenkov, I.J. Interactions of the endocrine and immune systems. In Endocrinology, 5th ed.; De Groot, L.J., Jameson, J.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; Volume 1, pp. 799–818. [Google Scholar]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Vita, R.; Lapa, D.; Trimarchi, F.; Benvenga, S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves’ disease. Endocrine 2015, 48, 254–263. [Google Scholar] [CrossRef]
- Matsubayashi, S.; Tamaí, H.; Matsumoto, Y.; Tamagawa, K.; Mukuta, T.; Morita, T.; Kubo, C. Graves’disease after the onset of panic disorder. Psychother. Psychosom. 1996, 65, 277–280. [Google Scholar] [CrossRef]
- Effraimidis, G.; Strieder, T.G.; Tijssen, J.G.; Wiersinga, W.M. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: A prospective study. Eur. J. Endocrinol. 2011, 164, 107–113. [Google Scholar] [CrossRef]
- Strieder, T.G.; Prummel, M.F.; Tijssen, J.G.; Brosschot, J.F.; Wiersinga, W.M. Stress is not associated with thyroid peroxidase autoantibodies in euthyroid women. Brain Behav. Immun. 2005, 19, 203–206. [Google Scholar] [CrossRef]
- Terzidis, K.; Panoutsopoulos, A.; Mantzou, A.; Tourli, P.; Papageorgiou, G.; Saltiki, K.; Mara, C.; Alevizaki, M. Lower early morning plasma cortisol levels are associated with thyroid autoimmunity in the elderly. Eur. J. Endocrinol. 2010, 162, 307–313. [Google Scholar] [CrossRef]
- Müssig, K.; Künle, A.; Säuberlich, A.L.; Weinert, C.; Ethofer, T.; Saur, R.; Klein, R.; Häring, H.U.; Klingberg, S.; Gallwitz, B.; et al. Thyroid peroxidase antibody positivity is associated with symptomatic distress in patients with Hashimoto’s thyroiditis. Brain Behav. Immun. 2012, 26, 559–563. [Google Scholar] [CrossRef]
- Plaza, A.; Garcia-Esteve, L.; Torres, A. Childhood physical abuse as a common risk factor for depression and thyroid dysfunction in the earlier postpartum. Psychiatry Res. 2012, 200, 329–335. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The role of stress in the clinical expression of thyroid autoimmunity. Ann. N. Y. Acad. Sci. 2006, 1088, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Common modifications of selenocysteine in selenoproteins. Essays Biochem. 2020, 64, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite. Nutrients 2020, 12, 1067. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Coplan, L.; Lichtbroun, B.; Krosser, A.; Lichtbroun, M.; Bragazzi, N.L.; Amital, H.; Afek, A.; Shoenfeld, Y. The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmun. Rev. 2018, 17, 967–983. [Google Scholar] [CrossRef]
- Goodyer, I.M. Recent undesirable life events: Their influence on subsequent psychopathology. Eur. Child. Adolesc. Psychiatry 1996, 5 (Suppl. 1), 33–37. [Google Scholar] [CrossRef]
- Vinokur, A.; Caplan, R.D. Cognitive and affective components of life events: Their relations and effects on well-being. Am. J. Community Psychol. 1986, 14, 351–370. [Google Scholar] [CrossRef]
- Zake, T.; Skuja, S.; Kalere, I.; Konrade, I.; Groma, V. Upregulated tissue expression of T helper (Th) 17 pathogenic interleukin (IL)-23 and IL-1β in Hashimoto’s thyroiditis but not in Graves’ disease. Endocr. J. 2019, 66, 423–430. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Minciullo, P.; Saitta, S.; Giovinazzo, S.; Certo, R.; Campennì, A.; Trimarchi, F.; Gangemi, S.; Benvenga, S. Serum interleukin-22 (IL-22) is increased in the early stage of Hashimoto’s thyroiditis compared to non-autoimmune thyroid disease and healthy controls. Hormones 2014, 13, 338–344. [Google Scholar] [CrossRef]
- Seshadri, S.; Pope, R.L.; Zenewicz, L.A. Glucocorticoids Inhibit Group 3 Innate Lymphocyte IL-22 Production. J. Immunol. 2018, 201, 1267–1274. [Google Scholar] [CrossRef]
- Mori, S.; Maher, P.; Conti, B. Neuroimmunology of the Interleukins 13 and 4. Brain Sci. 2016, 6, 18. [Google Scholar] [CrossRef]
- Rütti, S.; Howald, C.; Arous, C.; Dermitzakis, E.; Halban, P.A.; Bouzakri, K. IL-13 improves beta-cell survival and protects against IL-1beta-induced beta-cell death. Mol. Metab. 2015, 5, 122–131. [Google Scholar] [CrossRef]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef]
- Fraternale, A.; Brundu, S.; Magnani, M. Glutathione and glutathione derivatives in immunotherapy. Biol. Chem. 2017, 398, 261–275. [Google Scholar] [CrossRef]
- Rebuffat, S.A.; Nguyen, B.; Robert, B.; Castex, F.; Peraldi-Roux, S. Antithyroperoxidase antibody-dependent cytotoxicity in autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2008, 93, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative Stress and Advanced Glycation End Products in Hashimoto’s Thyroiditis. Thyroid 2016, 26, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Mantzou, E.; Koutras, D.A. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur. J. Endocrinol. 2003, 148, 389–393. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated with Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Thomson, C.D.; Rea, H.M.; Doesburg, V.M.; Robinson, M.F. Selenium concentrations and glutathione peroxidase activities in whole blood of New Zealand residents. Br. J. Nutr. 1977, 37, 457–460. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Gudkov, S.V.; Turovsky, E.A. Modulation of the Functional State of Mouse Neutrophils by Selenium Nanoparticles In Vivo. Int. J. Mol. Sci. 2022, 23, 13651. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Sarason, I.G.; Johnson, J.H.; Siegel, J.M. Assessing the impact of life changes: Development of the Life Experiences Survey. J. Consult. Clin. Psychol. 1978, 46, 932–946. [Google Scholar] [CrossRef]
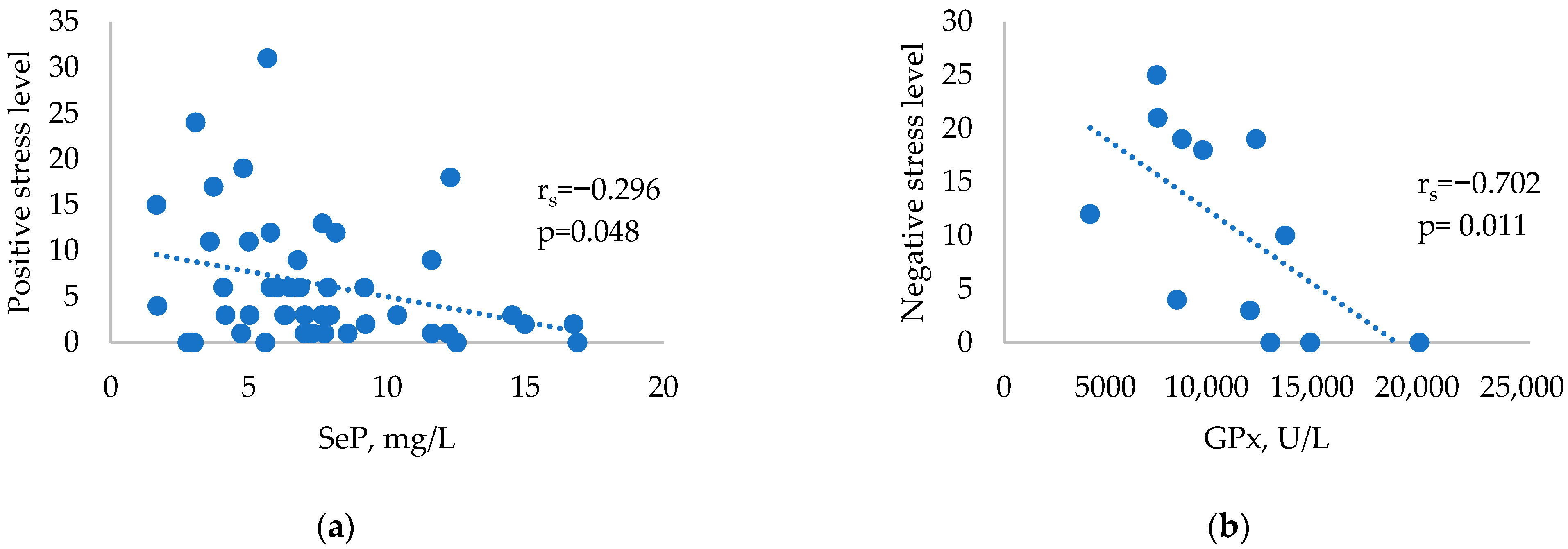
| HT Patients (n = 47) | GD Patients (n = 13) | Controls (n = 49) | p Value | |
|---|---|---|---|---|
| Sex (female/male) | 44/3 | 11/2 | 38/11 | 0.072 |
| Age (years) | 41 (27–50) | 41 (29–57) | 30 (26.5–46.5) | 0.247 |
| TSH (μIU/mL) | 2.19 (1.47–3.89) | 0.0001 (0.0000–0.0004) | 1.12 (0.88–1.75) | <0.001 |
| FT4 (ng/dL) | 0.91 (0.86–0.99) | 1.97 (1.75–2.55) | 0.98 (0.92–1.07) | <0.001 |
| FT3 (ng/dL) | 0.32 (0.29–0.34) | 1.21 (0.98–2.20) | 0.32 (0.30–0.34) | <0.001 |
| TPOAb (IU/mL) | 293.04 (125.77–530.59) | 215.12 (22.63–1093.52) | 0.46 (0.21–0.80) | <0.001 |
| TgAb (U/mL) | <20 # (<20 #–45.28) | <20 # (<20 #–146.80) | <20 # | - |
| TR-Ab (IU/L) | <1.58 | 12.13 (4.30–24.54) | <1.58 | - |
| Se (µg/L) | 93.19 (71.64–118.65) | 71.33 (58.92–104.38) | 90.93 (71.64–118.65 | 0.287 |
| GPx (U/L) | 12827.5 (10191.5–15006) | 10571.5 (7526.75–13194.5) | 12962 (9350–14792) | 0.246 |
| SeP (mg/L) | 6.92 (4.94–9.50) | 5.79 (4.52–7.71) | 6.41 (4.37–7.85) | 0.315 |
| Total number of major life events | 7.5 (5.75–11) | 7 (4.5–16) | 7 (3–9) | 0.355 |
| Number of major life events in the last 0–6 months | 4 (2–7) | 4 (1.4–6) | 3 (1–6) | 0.477 |
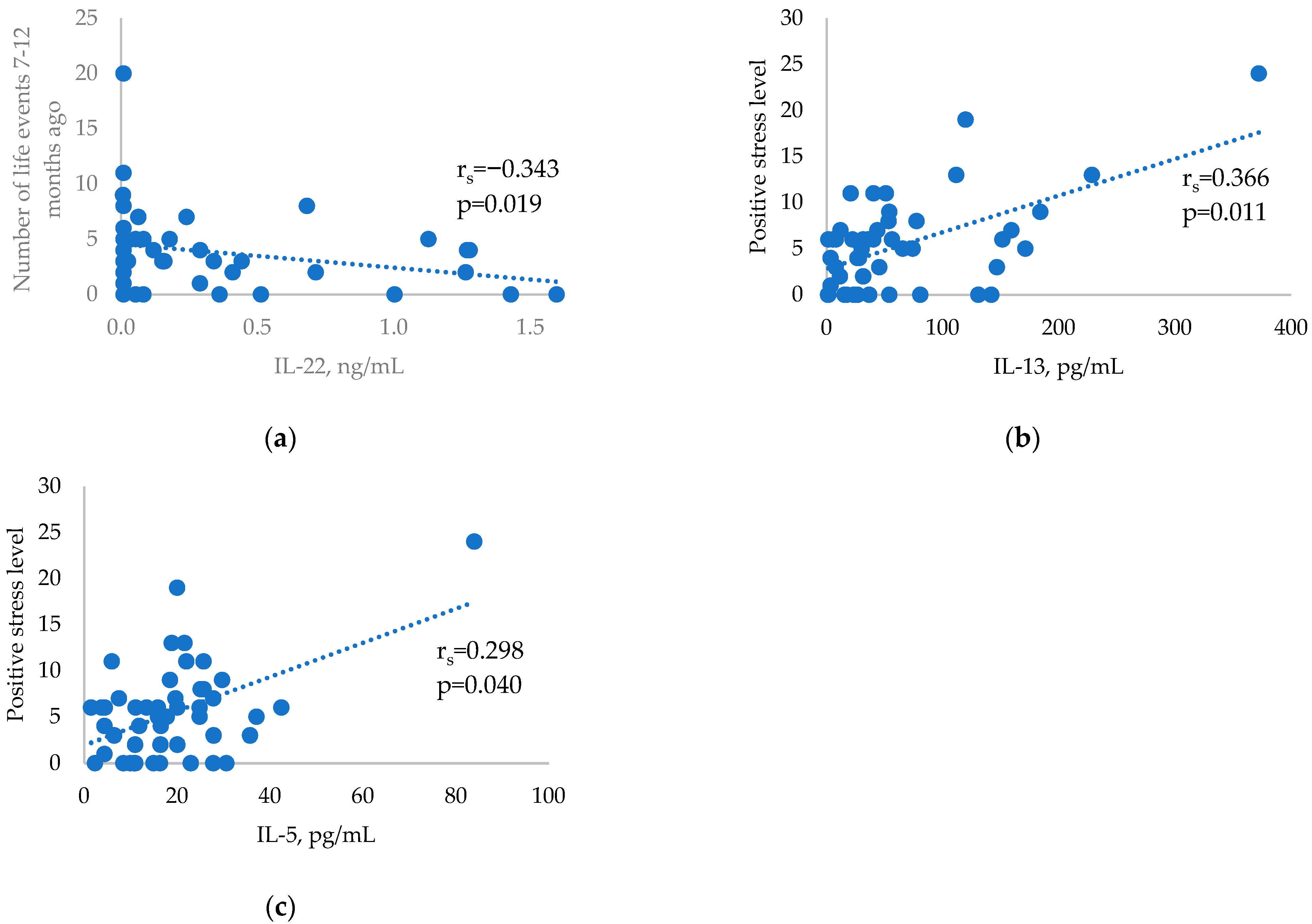
| Number of major life events in the last 7–12 months | 3 (1–5) | 3 (0–13) | 2 (0.25–4.75) | 0.348 |
| Number of major life events with no impact | 0 (0–1.25) | 0 (0–2) | 0 (0–1) | 0.209 |
| Negative stress level | 7.5 (4–12.25) | 10 (1–19) | 6 (3–8) | 0.103 |
| Positive stress level | 3 (1–9.5) | 5 (2–12.5) | 5 (1.25–7) | 0.664 |
| HT Patients (n = 47) | GD Patients (n = 13) | Controls (n = 49) | p | |
|---|---|---|---|---|
| IFN-γ (pg/mL) | 24.47 (18.74–37.49) | 22.75 (14.99–32.55) | 20.55 (9.71–37.63) | 0.343 |
| IL-10 (pg/mL) | 9.25 (5.94–15.21) | 8.70 (5.84–10.51) | 8.80 (5.37–15.39) | 0.761 |
| IL-13 (pg/mL) | 33.26 (19.42–60.92) | 23.49 (16.97–59.69) | 36.90 (16.58–79.21) | 0.577 |
| IL-17a (pg/mL) | 12.95 (9.16–19.61) | 12.37 (9.82–16.43) | 11.79 (5.96–18.55) | 0.530 |
| IL-22 (ng/mL) | 0.12 (0.00–0.51) | 0.14 (0.00–0.32) | 0.37 (0.15–0.57) | 0.063 |
| IL-2 (pg/mL) | 0.22 (0.09–0.38) | 0.11 (0.07–0.27) | 0.26 (0.09–0.42) | 0.164 |
| IL-4 (ng/mL) | 0.31 (0.17–0.64) | 0.27 (0.18–0.63) | 0.37 (0.10–0.78) | 0.821 |
| IL-23 (ng/mL) | 2.83 (1.43–4.94) | 2.31 (1.81–3.27) | 3.42 (2.08–4.83) | 0.357 |
| IL-5 (pg/mL) | 15.89 (11.00–25.98) | 14.53 (11.28–22.84) | 16.51 (9.50–24.99) | 0.783 |
| IL-6 (pg/mL) | 6.58 (0.00–31.50) | 3.66 (0.00–20.36) | 11.28 (1.29–49.09) | 0.345 |
| TPO-Ab Level | Tg-Ab Level | |||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient | p Value | Spearman’s Correlation Coefficient | p Value | |
| Total number of major life events | 0.220 | 0.023 | 0.216 | 0.039 |
| Number of major life events in the last 0–6 months | 0.117 | 0.231 | 0.134 | 0.204 |
| Number of major life events in the last 7–12 months | 0.211 | 0.029 | 0.124 | 0.241 |
| Number of major life events with no impact | 0.228 | 0.018 | 0.187 | 0.074 |
| Negative stress levels | 0.218 | 0.024 | 0.215 | 0.039 |
| Positive stress levels | 0.100 | 0.304 | 0.050 | 0.636 |
| HT vs. Controls | GD vs. Controls | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| IL-13 (pg/mL) | 0.99 (0.98–1.00) | 0.103 | 0.99 (0.96–1.01) | 0.288 |
| IL-22 (ng/mL) | 1.66 (0.93–2.99) | 0.088 | 0.05 (0.01–1.12) | 0.059 |
| IL-5 (pg/mL) | 1.05 (0.99–1.11) | 0.115 | 1.11 (0.97–1.27) | 0.130 |
| No. of major life events with negative impact | 1.10 (1.01–1.19) | 0.028 | 1.10 (0.99–1.23) | 0.088 |
| Se (µg/L) | 1.00 (0.98–1.01) | 0.551 | 0.98 (0.95–1.00) | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaivode, I.; Zake, T.; Strele, I.; Upmale-Engela, S.; Gogins, D.; Gersone, G.; Skesters, A.; Dambrova, M.; Konrade, I. Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients. Int. J. Mol. Sci. 2023, 24, 2440. https://doi.org/10.3390/ijms24032440
Vaivode I, Zake T, Strele I, Upmale-Engela S, Gogins D, Gersone G, Skesters A, Dambrova M, Konrade I. Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients. International Journal of Molecular Sciences. 2023; 24(3):2440. https://doi.org/10.3390/ijms24032440
Chicago/Turabian StyleVaivode, Ieva, Tatjana Zake, Ieva Strele, Sabine Upmale-Engela, Deniss Gogins, Gita Gersone, Andrejs Skesters, Maija Dambrova, and Ilze Konrade. 2023. "Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients" International Journal of Molecular Sciences 24, no. 3: 2440. https://doi.org/10.3390/ijms24032440
APA StyleVaivode, I., Zake, T., Strele, I., Upmale-Engela, S., Gogins, D., Gersone, G., Skesters, A., Dambrova, M., & Konrade, I. (2023). Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients. International Journal of Molecular Sciences, 24(3), 2440. https://doi.org/10.3390/ijms24032440







