Evaluation of the Possible Protective Role of Nobiletin against Arsenic-Induced Liver Damage in Male Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Assessment of As level
2.5. Biochemical Analysis
2.6. Assessment of Liver Function Markers
2.7. Inflammatory Markers
2.8. Apoptosis
2.9. Histomorphometry
2.10. Statistical Analysis
3. Results
3.1. Defensive Role of NOB on Endogenous Antioxidant Profile
3.2. Defensive Role of NOB on Liver Function Indices
3.3. Effect of NOB on Inflammatory Markers
3.4. Effect of NOB on Pro-Apoptotic and Anti-Apoptotic Markers
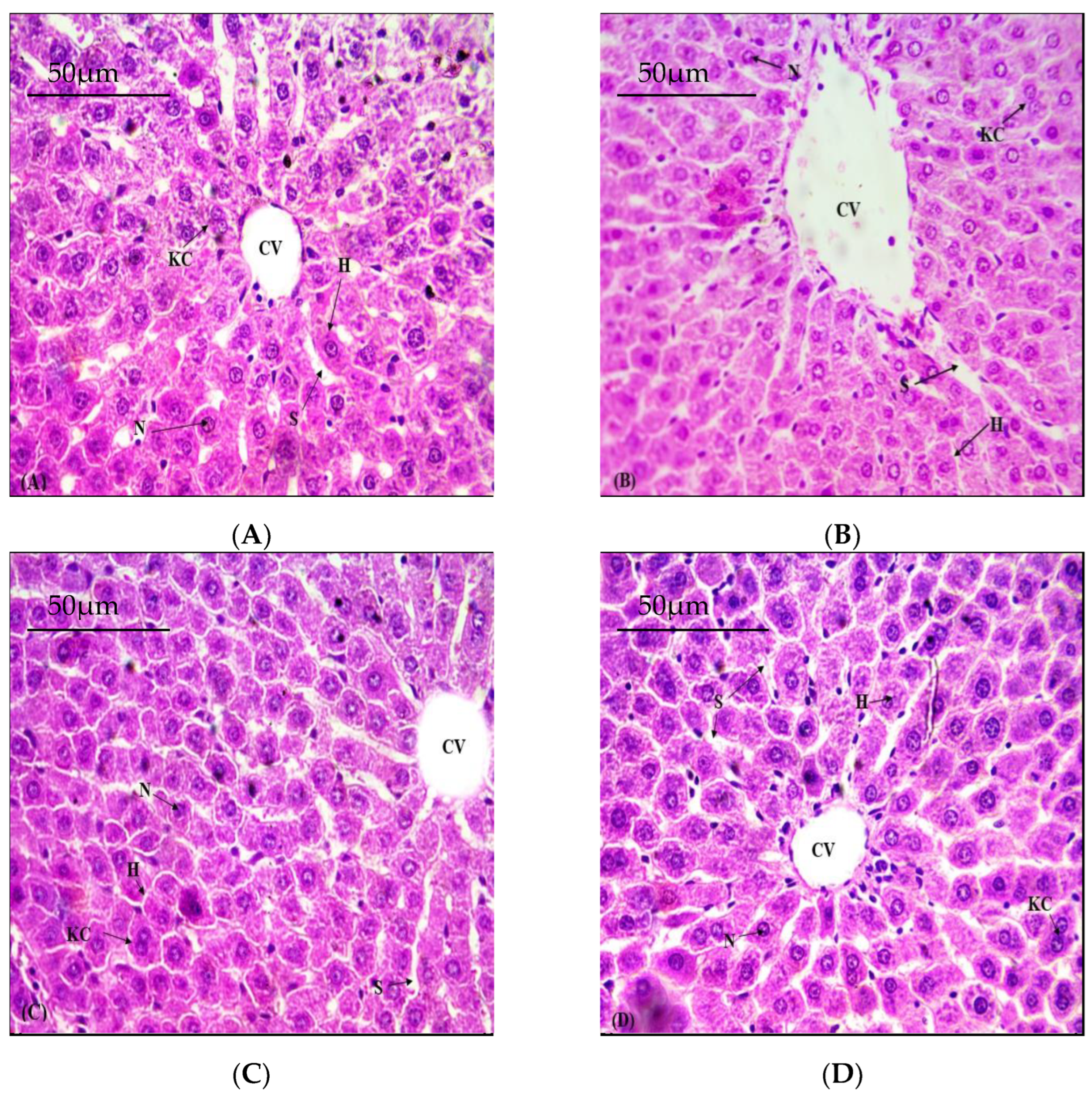
3.5. Defensive Role of NOB on Hepatic Structural Viability
3.6. Effects of NOB on As Accumulation in Hepatic Tissues
| Group | As Level (µg/gm Tissue) | CAT (U/mg Protein) | SOD (U/mg Protein) | POD (Nanomole) | GSH (μM/g Tissue) | GSR (nm NADPH Oxidized/min/mg Tissue) |
|---|---|---|---|---|---|---|
| Control | 1.43 ± 0.10 a | 7.36 ± 0.33 a | 5.36 ± 0.12 a | 7.16 ± 0.21 a | 17.54 ± 0.37 a | 3.80 ± 0.04 a |
| As (50 mg/kg) | 30.8 ± 2.29 b | 4.25 ± 0.36 b | 3.03 ± 0.04 b | 3.07 ± 0.13 b | 6.766 ± 0.40 b | 2.02 ± 0.09 b |
| As (50 mg/kg) + NOB (25 mg/kg each) | 6.32 ± 0.35 c | 7.21 ± 0.23 a | 4.56 ± 0.07 c | 6.52 ± 0.15 c | 14.23 ± 0.28 c | 3.06 ± 0.06 c |
| NOB (25 mg/kg) | 0.85 ± 0.04 a | 7.33 ± 0.26 a | 4.77 ± 0.06 c | 7.05 ± 0.17 a | 17.43 ± 0.51 a | 3.48 ± 0.07 ac |
| Group | TAS (mmol/L) | TOS (µmol/L) | TBARS (nm TBARS/min/mg Tissue) | H2O2 (nM/min/mg Protein) |
|---|---|---|---|---|
| Control | 2.99 ± 0.06 a | 8.99 ± 0.23 a | 14.29 ± 0.68 a | 1.45 ± 0.05 a |
| As (50 mg/kg) | 1.47 ± 0.14 b | 14.3 ± 0.38 b | 25.14 ± 1.09 b | 6.04 ± 0.09 b |
| As (50 mg/kg) + NOB (25 mg/kg each) | 2.53 ± 0.12 a | 10.3 ± 0.25 c | 17.54 ± 0.19 c | 2.38 ± 0.08 c |
| NOB (25 mg/kg) | 2.96 ± 0.11 a | 9.21 ± 0.39 a | 14.84 ± 0.36 a | 1.78 ± 0.07 a |
| Group | ALT (U/l) | AST (U/l) | ALP (U/l) |
|---|---|---|---|
| Control | 37.93 ± 6.37 a | 50.55 ± 2.87 a | 77.81 ± 2.94 a |
| As (50 mg/kg) | 131.4 ± 5.47 b | 146.2 ± 37.6 b | 144.1 ± 3.21 b |
| As (50 mg/kg) + NOB (25 mg/kg each) | 65.03 ± 3.21 c | 66.51 ± 3.54 c | 94.59 ± 4.06 c |
| NOB (25 mg/kg) | 38.25 ± 7.03 a | 46.63 ± 3.66 d | 79.21 ± 3.09 a |
| Group | NF-κB (ng/g Tissue) | TNF-α (ng/g Tissue) | IL-1β (ng/g Tissue) | IL-6 (ng/g Tissue) | COX-2 (ng/g Tissue) |
|---|---|---|---|---|---|
| Control | 12.25 ± 0.36 a | 7.12 ± 0.13 a | 21.39 ± 0.87 a | 7.01 ± 0.22 a | 17.56 ± 0.69 a |
| As (50 mg/kg) | 65.99 ± 1.90 b | 17.28 ± 0.36 b | 87.40 ± 1.30 b | 33.16 ± 0.80 b | 71.84 ± 1.42 b |
| As (50 mg/kg) + NOB (25 mg/kg each) | 23.62 ±1.34 c | 8.98 ± 0.35 c | 33.44 ± 1.28 c | 18.79 ± 0.64 c | 27.35 ± 1.47 c |
| NOB (25 mg/kg) | 12.08 ± 0.43 a | 7.09 ± 0.13 a | 21.15 ± 0.84 a | 6.97 ± 0.23 c | 17.51 ± 0.69 a |
| Group | Bcl-2 | Bax | Caspase-3 | Caspase-9 |
|---|---|---|---|---|
| Control | 15.85 ± 0.57 a | 2.96 ± 0.11 a | 1.64 ± 0.04 a | 3.31 ± 0.11 a |
| As (50 mg/kg) | 3.5 ± 0.41 b | 9.43 ± 0.56 b | 8.41 ± 0.50 b | 12.74 ± 0.60 b |
| As (50 mg/kg) + NOB (25 mg/kg each) | 12.39 ± 0.56 c | 5.08 ± 0.19 c | 3.28 ± 0.20 c | 5.97 ± 0.37 c |
| NOB (25 mg/kg) | 15.94 ± 0.51 a | 2.94 ± 0.12 a | 1.60 ± 0.05 a | 3.28 ± 0.11 c |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.; Sharaf, R.; Khan, M.Z.; Saleemi, M.K.; Mahmood, F. Arsenic toxicity in broiler chicks and its alleviation with ascorbic acid: A toxico-patho-biochemical study. Int. J. Agric. Biol. 2013, 15, 1105–1111. [Google Scholar]
- Hall, A.H. Chronic arsenic poisoning. Toxicol. Lett. 2002, 128, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, B. Assessment of arsenic and iron contamination of groundwater in four development blocks of Lakhimpur District, Assam, India. Der Chem. Sin. 2011, 2, 316–323. [Google Scholar]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef]
- Rani, V.U.; Sudhakar, M.; Ramesh, A. Protective effect of Pueraria tuberosa Linn. in arsenic induced nephrotoxicity in rats. Asian J. Pharma. Res. 2017, 7, 15. [Google Scholar] [CrossRef]
- Namratha, M.L.; Lakshman, M.; Jeevanalatha, M.; Kumar, B.A. Assessment of vitamin C protective activity in glyphosate-induced hepatotoxicity in rats. Pak. Vet. J. 2021, 41, 439–445. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Aziz, S.; Faheem, M.; Abbas, K.; Nasir, S.; Naz, H.; Ali, A.; Imran, M. Orientin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. Pak. Vet. J. 2021, 41, 574–578. [Google Scholar] [CrossRef]
- Liu, J.; Waalkes, M.P. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008, 105, 24–32. [Google Scholar] [CrossRef]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef]
- Lu, Y.H.; Su, M.Y.; Huang, H.Y.; Yuan, C.G. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci. Lett. 2010, 484, 6–11. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Wang, R.; Zhao, X. Preventive effect of flavonoid extract from the peel of Gonggan (Citrus reticulata Blanco Var. Gonggan) on CCl4-induced acute liver injury in mice. J. Inflamm. Res. 2021, 14, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid. Based Complement. Altern. Med. 2016, 1. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, W.Y.; Yang, Z.; Che, Y.; Jin, Y.G.; Liao, H.H.; Wang, S.S.; Deng, W.; Tang, Q.Z. Nobiletin, a polymethoxy flavonoid, protects against cardiac hypertrophy induced by pressure-overload via inhibition of NAPDH oxidases and endoplasmic reticulum stress. Cell Physiol. Biochem. 2017, 42, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Tahir, A.; Samad, A.; Anwar, H. ;Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol. 2021, 40, 403–416. [Google Scholar] [CrossRef]
- Brooks, R.R.; Ryan, D.E.; Zhang, H. Atomic absorption spectrometry and other instrumental methods for quantitative measurements of arsenic. Anal. Chim. Acta 1981, 131, 1–16. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130. [Google Scholar]
- Carlberg, I.N.C.E.R.; Mannervik, B.E.N.G.T. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Sharma, S.D.; Rezazadeh, H.; Hasan, N.; Abdulla, M.; Athar, M.J.R.R. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep. 1996, 2, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Keisari, Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. Cell Immunol. 1981, 59, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PloS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bansal, M.; Soni, G.; Bhatnagar, D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005, 156, 101–111. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Y.; Zhang, L.; Guo, Q. Oxidative stress and liver disease. Hepatol. Res. 2012, 42, 741–749. [Google Scholar] [CrossRef]
- Usoh, I.F.; Akpan, E.J.; Etim, E.O.; Farombi, E.O. Antioxidant actions of dried flower extracts of Hibiscus sabdariffa L. on sodium arsenite-induced oxidative stress in rats. Pak. J. Nutr. 2005, 4, 135–141. [Google Scholar]
- He, J.; Huang, B.; Ban, X.; Tian, J.; Zhu, L.; Wang, Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 2012, 141, 104–110. [Google Scholar] [CrossRef]
- Garcia-Chavez, E.; Santamaría, A.; Dıiaz-Barriga, F.; Mandeville, P.; Juarez, B.I.; Jimenez-Capdeville, M.E. Arsenite-induced formation of hydroxyl radical in the striatum of awake rats. Brain Res. 2003, 976, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yucel, N.; Wang, Y.X.; Mai, T.; Porpiglia, E.; Lund, P.J.; Markov, G.; Garcia, B.A.; Bendall, S.C.; Angelo, M.; Blau, H.M. Glucose metabolism drives histone acetylation landscape transitions that dictate muscle stem cell function. Cell Rep. 2019, 27, 3939–3955. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Ansari, U.A.; Siddiqui, A.J.; Iqbal, D.; Khan, J.; Banawas, S.; Alshehri, B.; Alshahrani, M.M.; Alsagaby, S.A.; Redhu, N.S.; et al. Nobiletin ameliorates cellular damage and stress response and restores neuronal identity altered by sodium arsenate exposure in human iPSCs-derived hNPCs. Pharmaceuticals 2022, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Al-Mulhim, A.S.; Jresat, I. Telmisartan treatment attenuates arsenic-induced hepatotoxicity in mice. Toxicology 2012, 300, 149–157. [Google Scholar] [CrossRef]
- Muthumani, M.; Miltonprabu, S. Ameliorative efficacy of tetrahydrocurcumin against arsenic induced oxidative damage, dyslipidemia and hepatic mitochondrial toxicity in rats. Chem. Biol. Interact. 2015, 235, 95–105. [Google Scholar] [CrossRef]
- Hall, M.N.; Niedzwiecki, M.; Liu, X.; Harper, K.N.; Alam, S.; Slavkovich, V.; Ilievski, V.; Levy, D.; Siddique, A.B.; Parvez, F.; et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ. Health Perspect. 2013, 121, 1068–1074. [Google Scholar] [CrossRef]
- Blann, A. What is the purpose of liver function tests? Nurs. Times 2014, 110, 17–19. [Google Scholar]
- Carobene, A.; Braga, F.; Roraas, T.; Sandberg, S.; Bartlett, W.A. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin. Chem. Lab. Med. 2013, 51, 1997–2007. [Google Scholar] [CrossRef]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Zhong, Q.; Sun, Y.; Xu, Y.; Khan, A.; Guo, J.; Wang, Z.; Sun, N.; Li, H. The therapeutic effect and mechanism of physalin on LPS-induced acute lung injury in rats. Pak. Vet. J. 2021, 41, 372–378. [Google Scholar]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, S.; Wang, M.; Chen, J.; Huang, S.; Wei, Z.; Cheng, Z.; Wang, H.; Long, M.; Li, P. Curcumin inhibits zearalenone-induced apoptosis and oxidative stress in Leydig cells via modulation of the PTEN/Nrf2/Bip signaling pathway. Food Chem. Toxicol. 2020, 141, 111385. [Google Scholar] [CrossRef]
- Venkatadri, R.; Muni, T.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, 2104. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Sarabi, P.Z.; Athari, S.S.; Esmaeilzadeh, A. Therapeutics strategies against cancer stem cell in breast cancer. Int. J. Biochem. Cell Biol. 2019, 109, 76–81. [Google Scholar] [CrossRef]
- Gu, P.; Zhu, L.; Liu, Y.; Zhang, L.; Liu, J.; Shen, H. Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. Int. Immunopharmacol. 2017, 50, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Green, D.R. PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle 2009, 8, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, B.; Shadjou, N.; Charoudeh, H.N.; Rashidi, M.R. Recent advances in electrochemical and electrochemiluminescence based determination of the activity of caspase-3. Microchim. Acta 2017, 184, 3651–3662. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Wu, J.; Wang, L.; Wang, J.; Lv, K.; Liu, S.; Wang, M.; Guan, W.; Liu, J.; et al. Nobiletin Protects against acute liver injury via targeting c-Jun N-terminal kinase (JNK)-induced apoptosis of hepatocytes. J. Agric. Food Chem. 2020, 68, 7112–7120. [Google Scholar] [CrossRef]
- Noman, A.S.M.; Dilruba, S.; Mohanto, N.C.; Rahman, L.; Khatun, Z.; Riad, W.; Al Mamun, A.; Alam, S.; Aktar, S.; Chowdhury, S.; et al. Arsenic-induced histological alterations in various organs of mice. J. Cytol. Histol. 2015, 6, 323. [Google Scholar] [CrossRef]
- Gora, R.H.; Baxla, S.L.; Kerketta, P.; Patnaik, S.; Roy, B.K. Hepatoprotective activity of Tephrosia purpurea against arsenic induced toxicity in rats. Indian J. Pharmacol. 2014, 46, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sumedha, N.C.; Miltonprabu, S. Diallyl trisulfide ameliorates arsenic-induced hepatotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. Hum. Exp. Toxicol. 2015, 34, 506–525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijaz, M.U.; Ahmed, A.; Al-Ghanim, K.A.; Al-Misned, F.; Riaz, M.N.; Kaimkhani, Z.A.; Mahboob, S. Evaluation of the Possible Protective Role of Nobiletin against Arsenic-Induced Liver Damage in Male Albino Rats. Toxics 2023, 11, 110. https://doi.org/10.3390/toxics11020110
Ijaz MU, Ahmed A, Al-Ghanim KA, Al-Misned F, Riaz MN, Kaimkhani ZA, Mahboob S. Evaluation of the Possible Protective Role of Nobiletin against Arsenic-Induced Liver Damage in Male Albino Rats. Toxics. 2023; 11(2):110. https://doi.org/10.3390/toxics11020110
Chicago/Turabian StyleIjaz, Muhammad Umar, Aqsa Ahmed, Khalid Abdullah Al-Ghanim, Fahad Al-Misned, Mian Nadeem Riaz, Zahid Ali Kaimkhani, and Shahid Mahboob. 2023. "Evaluation of the Possible Protective Role of Nobiletin against Arsenic-Induced Liver Damage in Male Albino Rats" Toxics 11, no. 2: 110. https://doi.org/10.3390/toxics11020110
APA StyleIjaz, M. U., Ahmed, A., Al-Ghanim, K. A., Al-Misned, F., Riaz, M. N., Kaimkhani, Z. A., & Mahboob, S. (2023). Evaluation of the Possible Protective Role of Nobiletin against Arsenic-Induced Liver Damage in Male Albino Rats. Toxics, 11(2), 110. https://doi.org/10.3390/toxics11020110





