Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells via Phagocytosis
Abstract
1. Introduction
2. Results
2.1. Alteration of RAW 264.7 Cell Properties following Long-Term Culture in Medium Containing THGP
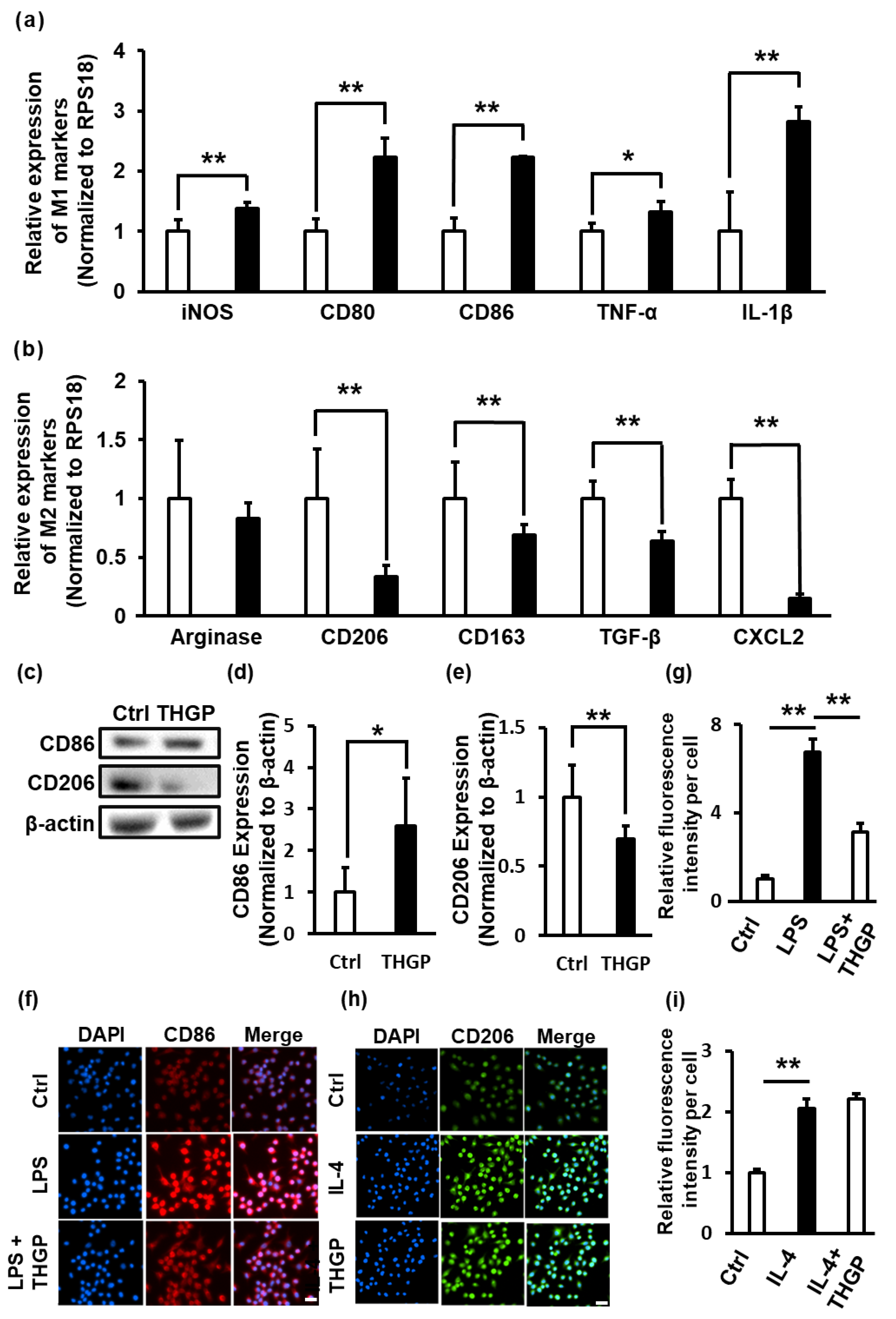
2.2. THGP Shifted Macrophages toward M1 Polarization
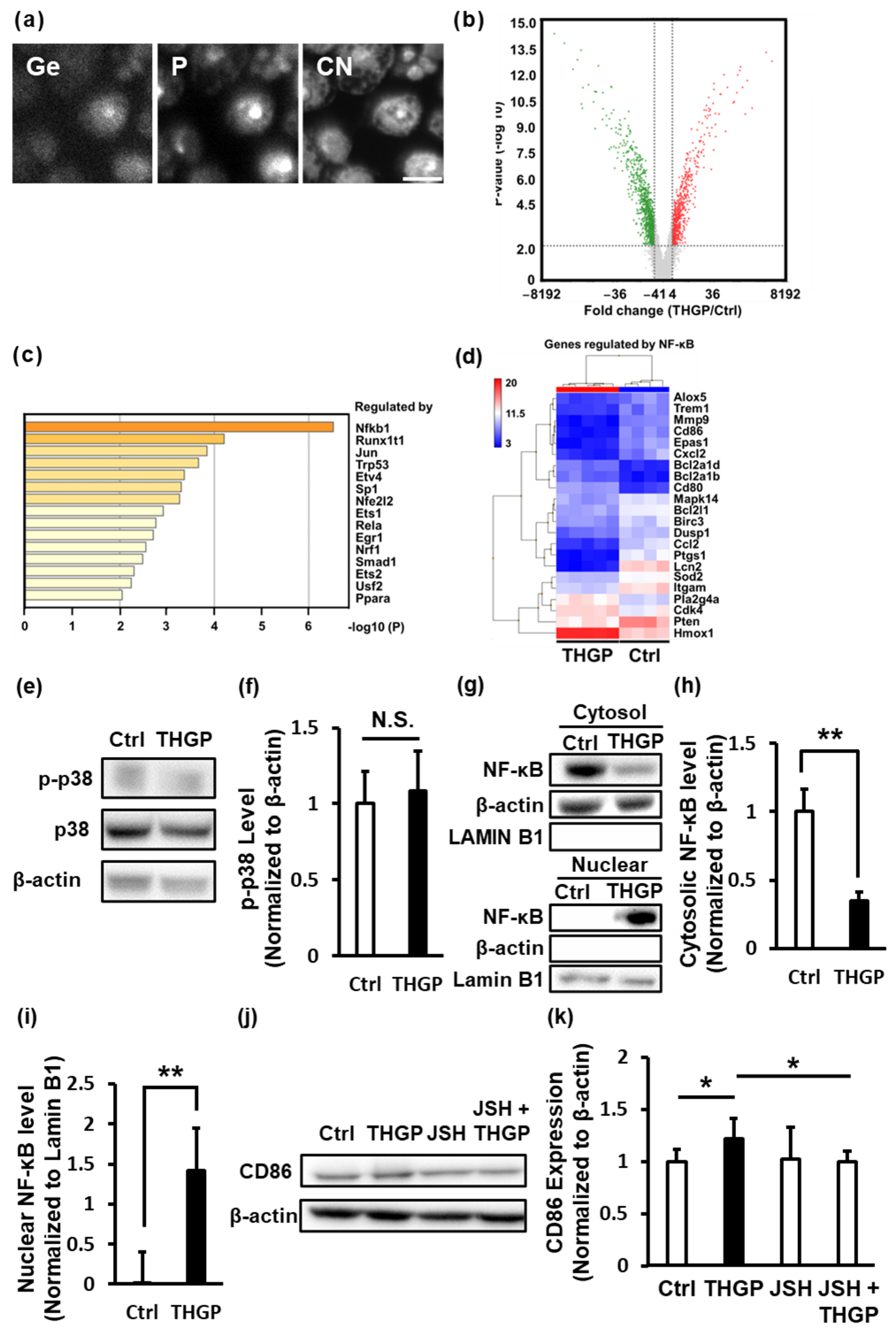
2.3. THGP Induces the Differentiation of Macrophages toward M1 Polarization by Activating NF-κB Signaling
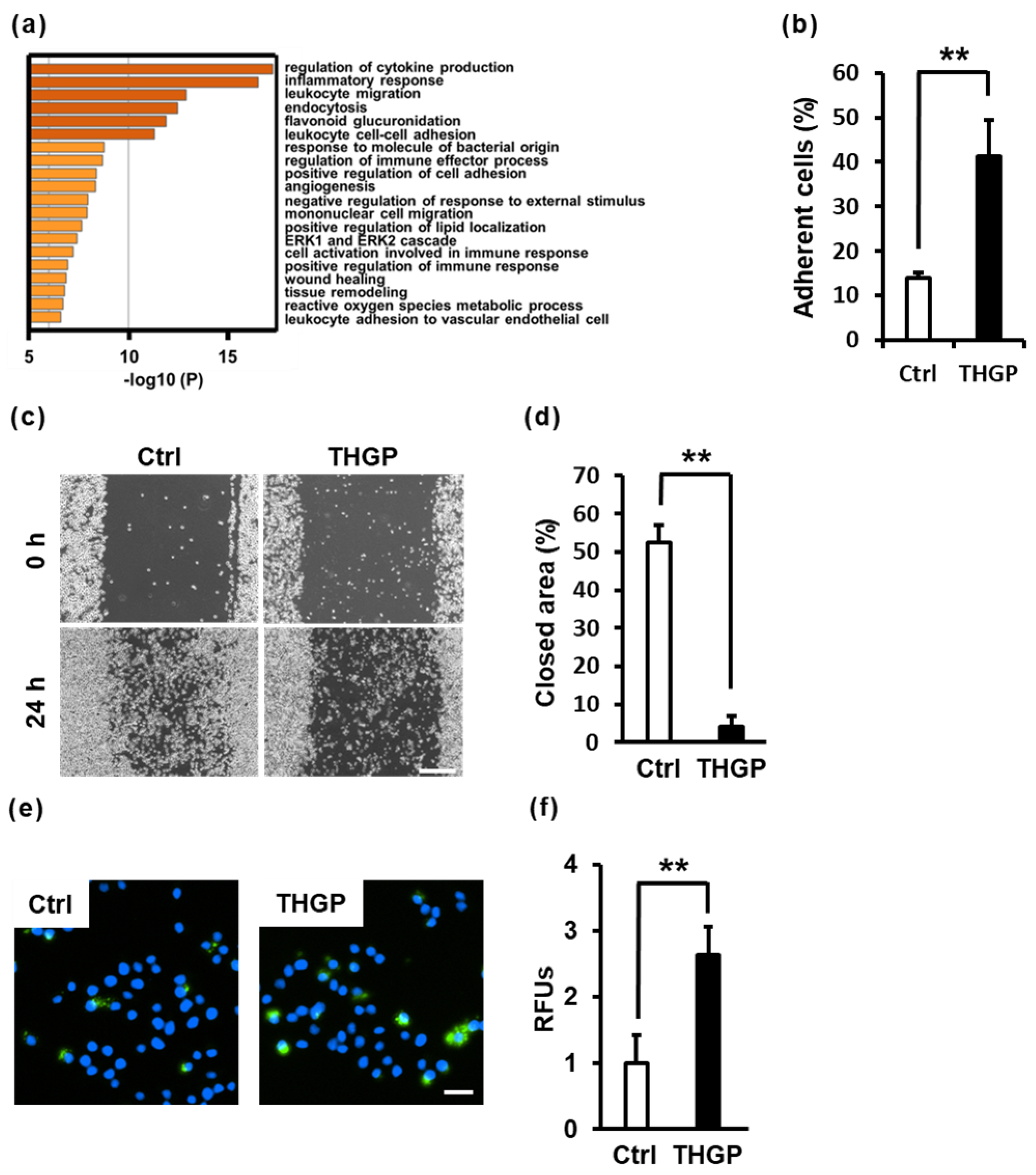
2.4. THGP Alters Macrophage Functions Such as Adhesion, Migration and Phagocytosis
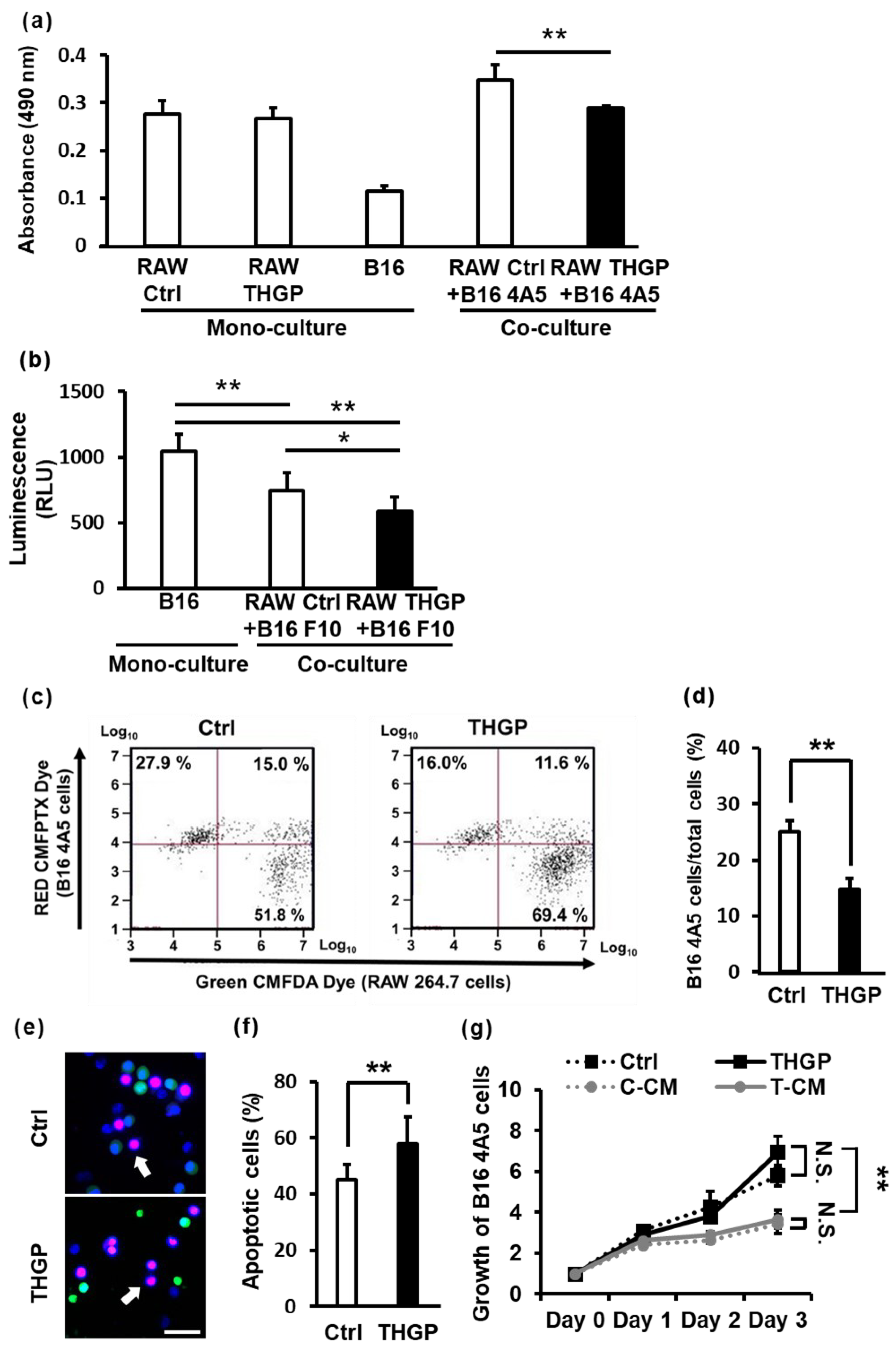
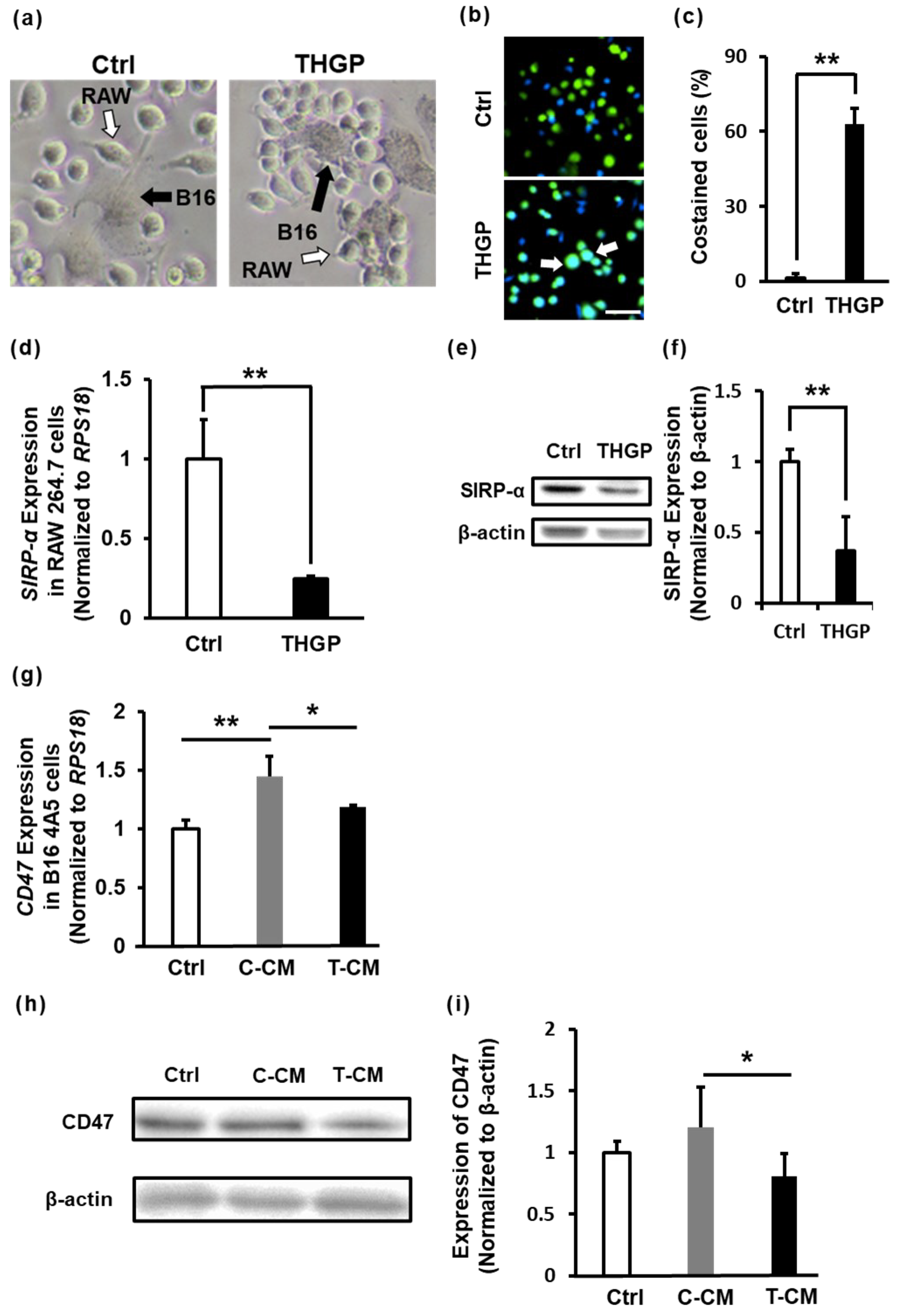
2.5. THGP Increases the Cytotoxicity of RAW 264.7 Cells toward B16 Mouse Melanoma Cells
2.6. THGP Increases the SIRP-α–CD47 Axis Related to Aggregation and Phagocytosis
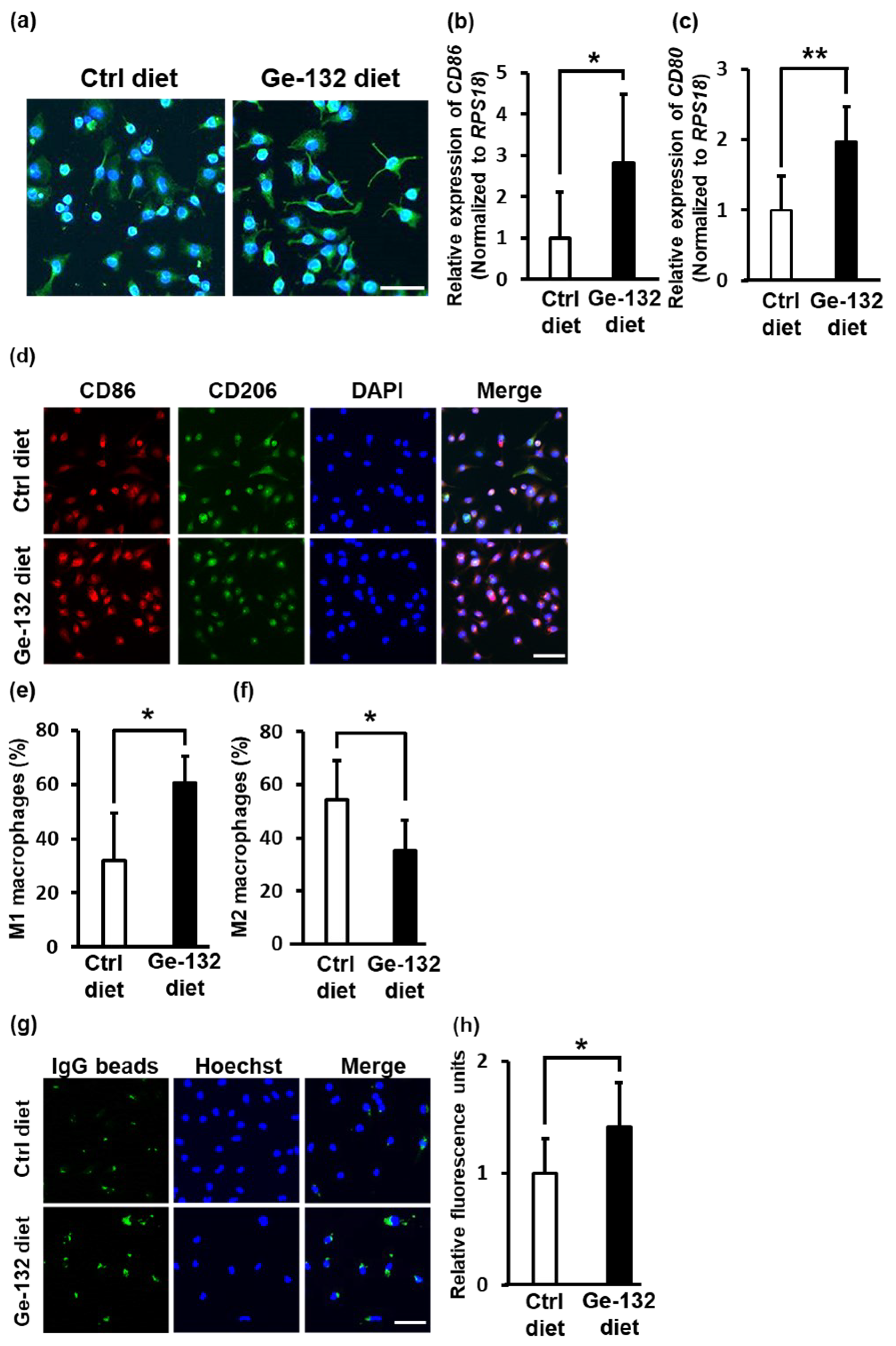
2.7. Ge-132 Induced M1 Macrophage Polarization in Mouse-Derived Primary Intraperitoneal Macrophages In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Growth Assay (MTS Assay)
4.3. Analysis of Cell Size and Internal Complexity
4.4. Immunofluorescence Staining
4.5. Quantitative Polymerase Chain Reaction (PCR)
4.6. Western Blotting
4.7. Induction of M1 or M2 Polarization
4.8. Isotope Microscopy
4.9. Microarray Analysis
4.10. NF-κB Inhibitor Test
4.11. Cell Adhesion Assay
4.12. Cell Migration Assay
4.13. Phagocytosis Assay
4.14. Coculture Model with MTS Assays
4.15. Coculture Model with Luciferase Assays
4.16. Coculture Model with Fluorescence Staining
4.17. Coculture Model with PI and Hoechst Staining
4.18. Collection of Mouse Peritoneal Macrophages
4.19. Conditioned Medium
4.20. Video Recording of Cocultured RAW 26 4.7 Cells and B16 4A5 Cells
4.21. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. Mech. Dis. 2019, 15, 123. [Google Scholar] [CrossRef]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2018, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Xiang, Y.-R.; Liu, L. “Eating” Cancer Cells by Blocking CD47 Signaling: Cancer Therapy by Targeting the Innate Immune Checkpoint. Cancer Transl. Med. 2017, 3, 200–208. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage Polarity in Cancer: A Review. J. Cell. Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting Macrophages: Therapeutic Approaches in Cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Oronsky, B.; Carter, C.; Reid, T.; Brinkhaus, F.; Knox, S.J. Just Eat It: A Review of CD47 and SIRP-α Antagonism. Semin. Oncol. 2020, 47, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S.; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPα Antibodies as a Potential New Tool for Cancer Immunotherapy. JCI Insight 2017, 2, e89140. [Google Scholar] [CrossRef] [PubMed]
- Gül, N.; Egmond, M. van Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015, 75, 5008–5013. [Google Scholar] [CrossRef]
- Gutiérrez-Seijo, A.; García-Martínez, E.; Barrio-Alonso, C.; Parra-Blanco, V.; Avilés-Izquierdo, J.A.; Sánchez-Mateos, P.; Samaniego, R. Activin A Sustains the Metastatic Phenotype of Tumor-Associated Macrophages and Is a Prognostic Marker in Human Cutaneous Melanoma. J. Investig. Dermatol. 2022, 142, 653–661.e2. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-Associated Macrophages: From Basic Research to Clinical Application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene 2020, 30, 291–297. [Google Scholar] [CrossRef]
- Lombardo, N.; Corte, M.D.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Duca, E.D.; Bennardo, L.; Nisticò, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef]
- Pavri, S.N.; Clune, J.; Ariyan, S.; Narayan, D. Malignant Melanoma: Beyond the Basics. Plast. Reconstr. Surg. 2016, 138, 330e–340e. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Saito, Y.; Kotani, T.; Matozaki, T. CD47-signal Regulatory Protein α Signaling System and Its Application to Cancer Immunotherapy. Cancer Sci. 2018, 109, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in Effect of Germanium or Germanium Compounds on Animals—A Review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Zhang, C.L.; Li, T.H.; Niu, S.H.; Wang, R.F.; Fu, Z.L.; Guo, F.Q.; Yang, M. Synthesis and Evaluation of Novel Organogermanium Sesquioxides As Antitumor Agents. Bioinorg. Chem. Appl. 2009, 2009, 908625. [Google Scholar] [CrossRef]
- Kumano, N.; Ishikawa, T.; Koinumaru, S.; Kikumoto, T.; Suzuki, S.; Nakai, Y.; Konno, K. Antitumor Effect of the Organogermanium Compound Ge-132 on the Lewis Lung Carcinoma (3LL) in C57BL/6 (B6) Mice. Tohoku J. Exp. Med. 1985, 146, 97–104. [Google Scholar] [CrossRef]
- Aso, H.; Suzuki, F.; Yamagughi, T.; Hayashi, Y.; Ebina, T.; Ishida, N. Induction of Interferon and Activation of NK Cells and Macrophages in Mice by Oral Administration of Ge-132, an Organic Germanium Compound. Microbiol. Immunol. 1985, 29, 65–74. [Google Scholar] [CrossRef]
- Ning, K.; Liu, W.-W.; Huang, J.-L.; Lu, H.-T.; Sun, X.-J. Effects of Hydrogen on Polarization of Macrophages and Microglia in a Stroke Model. Med. Gas Res. 2018, 8, 154. [Google Scholar] [CrossRef]
- Liu, C.-P.; Zhang, X.; Tan, Q.-L.; Xu, W.-X.; Zhou, C.-Y.; Luo, M.; Li, X.; Huang, R.-Y.; Zeng, X. NF-ΚB Pathways Are Involved in M1 Polarization of RAW 264.7 Macrophage by Polyporus Polysaccharide in the Tumor Microenvironment. PLoS ONE 2017, 12, e0188317. [Google Scholar] [CrossRef]
- Heinrich, F.; Lehmbecker, A.; Raddatz, B.B.; Kegler, K.; Tipold, A.; Stein, V.M.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W.; Ulrich, R.; et al. Morphologic, Phenotypic, and Transcriptomic Characterization of Classically and Alternatively Activated Canine Blood-Derived Macrophages in Vitro. PLoS ONE 2017, 12, e0183572. [Google Scholar] [CrossRef]
- Hu, F.; Lesney, P. FThe isolation and cytology of two pigment cell strains from B16 mouse melanomas. Cancer Res. 1964, 24, 1634–1643. [Google Scholar]
- Fidler, I.J. Biological Behavior of Malignant Melanoma Cells Correlated to Their Survival in Vivo. Cancer Res. 1975, 35, 218–224. [Google Scholar] [PubMed]
- Huang, Y.; Ma, Y.; Gao, P.; Yao, Z. Targeting CD47: The Achievements and Concerns of Current Studies on Cancer Immunotherapy. J. Thorac. Dis. 2017, 9, E168–E174. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, Y.K.; Krupa, M.; Nguyen, A.N.; Do, B.H.; Chung, B.; Vu, T.T.T.; Kim, S.C.; Choe, H. Crotamine Stimulates Phagocytic Activity by Inducing Nitric Oxide and TNF-α via P38 and NFκ-B Signaling in RAW 264.7 Macrophages. BMB Rep. 2016, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Shigeru, A.; Miyoko, Y.; Kaori, I. Improved Rheumatoid Arthritis Case with Ge-132 Administration Evaluated by Clinically and Immunologically Using Two-Color Flow Cytometry. Jpn. J. Clin. Immunol. 1990, 13, 80–86. [Google Scholar] [CrossRef]
- Laria, A.; Lurati, A.M.; Marrazza, M.; Mazzocchi, D.; Re, K.; Scarpellini, M. The Macrophages in Rheumatic Diseases. J. Inflamm. Res. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Teng, G.; Zhou, H.; Dong, C. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol. Trace Elem. Res. 2020, 198, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Shimada, Y.; Takeda, T.; Aso, H.; Nakamura, T. The Organogermanium Compound 3-(Trihydroxygermyl) Propanoic Acid (THGP) Suppresses Inflammasome Activation Via Complexation with ATP. Int. J. Mol. Sci. 2022, 23, 13364. [Google Scholar] [CrossRef]
- Nakamura, T.; Nagura, T.; Akiba, M.; Sato, K.; Tokuji, Y.; Ohnishi, M.; Osada, K. Promotive Effects of the Dietary Organic Germanium Poly-Trans-[(2-Carboxyethyl) Germasesquioxane] (Ge-132) on the Secretion and Antioxidative Activity of Bile in Rodents. J. Health Sci. 2010, 56, 72–80. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Johann, D.J. Cancer and the Tumor Microenvironment: A Review of an Essential Relationship. Cancer Chemother. Pharmacol. 2009, 63, 571–582. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. MMP9 Induction by Vascular Endothelial Growth Factor Receptor-1 Is Involved in Lung-Specific Metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.; Shao, G.; Wang, C.; Lin, C.; Fang, H.; Balajee, A.S.; Bhagat, G.; Hei, T.K.; Zhao, Y. TGFBI Deficiency Predisposes Mice to Spontaneous Tumor Development. Cancer Res. 2009, 69, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.P.; Minogue, A.M.; Falvey, A.; Lynch, M.A. Involvement of IGF-1 and Akt in M1/M2 Activation State in Bone Marrow-Derived Macrophages. Exp. Cell Res. 2015, 335, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Czimmerer, Z.; Daniel, B.; Horvath, A.; Rückerl, D.; Nagy, G.; Kiss, M.; Peloquin, M.; Budai, M.M.; Cuaranta-Monroy, I.; Simandi, Z.; et al. The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages. Immunity 2018, 48, 75–90.e6. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Zeigler, A.C.; Nelson, A.R.; Lindsey, M.L.; Saucerman, J.J. Network Analysis Reveals a Distinct Axis of Macrophage Activation in Response to Conflicting Inflammatory Cues. J. Immunol. 2021, 206, 883–891. [Google Scholar] [CrossRef]
- Takeda, T.; Doiyama, S.; Azumi, J.; Shimada, Y.; Tokuji, Y.; Yamaguchi, H.; Nagata, K.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium Suppresses Cell Death Due to Oxidative Stress in Normal Human Dermal Fibroblasts. Sci. Rep. 2019, 9, 13637. [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Serrano, C.; Villegas-Sepúlveda, N.; Maravillas-Montero, J.L.; Romero-Ramírez, S.; Shibayama, M.; Medina-Contreras, O.; Nava, P.; Santos-Argumedo, L. Myosin 1F Regulates M1-Polarization by Stimulating Intercellular Adhesion in Macrophages. Front. Immunol. 2019, 9, 3118. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, D.-X.; Yu, X.-J.; Sun, H.-W.; Xu, Y.-T.; Zhang, Y.-J.; Xu, J. Macrophages Induce CD47 Upregulation via IL-6 and Correlate with Poor Survival in Hepatocellular Carcinoma Patients. Oncoimmunology 2019, 8, e1652540. [Google Scholar] [CrossRef]
- Munakata, T.; Arai, S.; Kuwano, K.; Furukawa, M.; Tomita, Y. Induction of Interferon Production by Natural Killer Cells by Organogermanium Compound, Gel32. J. Interferon Res. 1987, 7, 69–76. [Google Scholar] [CrossRef]
- Suzuki, F. Antitumor Activity of Ge-132, a New Organogermanium Compound, in Mice Is Expressed through the Functions of Macrophages and T Lymphocytes. Cancer Chemother. 1985, 12, 1445–1452. [Google Scholar]
- Suzuki, F.; Brutkiewicz, R.R.; Pollard, R.B. Ability of Sera from Mice Treated with Ge-132, an Organic Germanium Compound, to Inhibit Experimental Murine Ascites Tumours. Br. J. Cancer 1985, 52, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F. Suppression of Tumor Growth by Peritoneal Macrophages Isolated from Mice Treated with Carboxyethylgermanium Sesquioxide (Ge-132). Cancer Chemother. 1985, 12, 2122–2128. [Google Scholar]
- Mizuno, N.; Nishibori, E.; Oka, M.; Jomori, T.; Takata, M.; Kumasaka, T. Structural Basis for Polymer Packing and Solvation Properties of the Organogermanium Crystalline Polymer Propagermanium and Its Derivatives. J. Pharm. Sci. 2015, 104, 2482–2488. [Google Scholar] [CrossRef]
- Hirayama, C.; Suzuki, H.; Ito, M.; Okumura, M.; Oda, T. Propagermanium: A Nonspecific Immune Modulator for Chronic Hepatitis B. J. Gastroenterol. 2003, 38, 525–532. [Google Scholar] [CrossRef]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 Signaling Axis in Cancer Metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef]
- Kobatake, K.; Ikeda, K.; Nakata, Y.; Yamasaki, N.; Ueda, T.; Kanai, A.; Sentani, K.; Sera, Y.; Hayashi, T.; Koizumi, M.; et al. Kdm6a Deficiency Activates Inflammatory Pathways, Promotes M2 Macrophage Polarization, and Causes Bladder Cancer in Cooperation with P53 Dysfunction. Clin. Cancer Res. 2020, 26, 2065–2079. [Google Scholar] [CrossRef]
- Kikuchi, S.; Noguchi, K.; Wakai, K.; Hamazaki, Y.; Tozawa, K.; Jomori, T.; Sasako, M.; Miwa, H. Propagermanium Induces NK Cell Maturation and Tends to Prolong Overall Survival of Patients with Refractory Cancer. Anticancer Res. 2019, 39, 4687–4698. [Google Scholar] [CrossRef] [PubMed]
- Molgora, M.; Supino, D.; Mavilio, D.; Santoni, A.; Moretta, L.; Mantovani, A.; Garlanda, C. The Yin-yang of the Interaction between Myelomonocytic Cells and NK Cells. Scand. J. Immunol. 2018, 88, e12705. [Google Scholar] [CrossRef]
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No Carcinogenicity of Poly-Trans-[(2-Carboxyethyl) Germasesquioxane] (Ge-132): 26-Week Feeding Study Using RasH2 Mice. Fundam. Toxicol. Sci. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Sanai, T.; Okuda, S.; Onoyama, K.; Oochi, N.; Takaichi, S.; Mizuhira, V.; Fujishima, M. Chronic Tubulointerstitial Changes Induced by Germanium Dioxide in Comparison with Carboxyethylgermanium Sesquioxide. Kidney Int. 1991, 40, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity Study of Poly-Trans-[(2-Carboxyethyl)Germasesquioxane] (Ge-132) in F344 Rats. Fundam. Toxicol. Sci. 2018, 5, 127–140. [Google Scholar] [CrossRef]
- Nakayama, S.; Tsuji, T.; Usami, K. Acute toxicity study of organic germanium (Ge-132)in mice and rats. Syowa Igakkai Zasshi 1986, 46, 227–235. [Google Scholar] [CrossRef]
- Miyao, K.; Onishi, T.; Asai, K.; Tomizawa, S.; Suzuki, F. Toxicology and Phase I Studies on a Novel Organogermanium Compound, Ge-132. Curr. Chemother. Infect. Dis. 1979, 2, 1527–1529. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azumi, J.; Takeda, T.; Shimada, Y.; Zhuang, T.; Tokuji, Y.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells via Phagocytosis. Int. J. Mol. Sci. 2023, 24, 1885. https://doi.org/10.3390/ijms24031885
Azumi J, Takeda T, Shimada Y, Zhuang T, Tokuji Y, Sakamoto N, Aso H, Nakamura T. Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells via Phagocytosis. International Journal of Molecular Sciences. 2023; 24(3):1885. https://doi.org/10.3390/ijms24031885
Chicago/Turabian StyleAzumi, Junya, Tomoya Takeda, Yasuhiro Shimada, Tao Zhuang, Yoshihiko Tokuji, Naoya Sakamoto, Hisashi Aso, and Takashi Nakamura. 2023. "Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells via Phagocytosis" International Journal of Molecular Sciences 24, no. 3: 1885. https://doi.org/10.3390/ijms24031885
APA StyleAzumi, J., Takeda, T., Shimada, Y., Zhuang, T., Tokuji, Y., Sakamoto, N., Aso, H., & Nakamura, T. (2023). Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells via Phagocytosis. International Journal of Molecular Sciences, 24(3), 1885. https://doi.org/10.3390/ijms24031885





