Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Blood Samples for Total PSA and Circulating Cell Free DNA Concentration and Integrity
2.1.1. Blood Sample Collection
2.1.2. Measurement of Total PSA
2.1.3. Preparation of the Plasma Sample for DNA Extraction
2.1.4. Circulating Cell Free DNA Extraction from Plasma
2.1.5. Circulating Cell Free DNA Quantification
2.1.6. Data Collection
2.1.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sikaris, K. Prostate specific antigen. Clin. Biochem. Rev. 1996, 17, 50–68. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Bok, R.A.; Small, E.J. Bloodborne biomolecular markers in prostate cancer development and progression. Nat. Rev. Cancer 2002, 2, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Giacona, M.B.; Ruben, G.C.; Iczkowski, K.A.; Roos, T.B.; Porter, D.M.; Sorenson, G.D. Cell-free DNA in human blood plasma: Length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Wang, B.G.; Huang, H.Y.; Chen, Y.C.; Bristow, R.E.; Kassauei, K.; Cheng, C.C.; Roden, R.; Sokoll, L.J.; Chan, D.W.; Shih, I.-M. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003, 63, 3966–3968. [Google Scholar]
- Beheshti, B.; Park, P.C.; Sweet, J.M.; Trachtenberg, J.; Jewett, M.A.; Squire, J.A. Evidence of chromosomal instability in prostate cancer determined by spectral karyotyping (SKY) and interphase FISH analysis. Neoplasia 2001, 3, 62–69. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef]
- Cahill, D.P.; Lengauer, C.; Yu, J.; Riggins, G.J.; Willson, J.K.; Markowitz, S.D.; Kinzler, K.W.; Vogelstein, B. Mutations of mitotic checkpoint genes in human cancers. Nature 1998, 392, 300–303. [Google Scholar] [CrossRef]
- Hashad, D.; Sorour, A.; Ghazal, A.; Talaat, I. Free circulating tumor DNA as a diagnostic marker for breast cancer. J. Clin. Lab. Anal. 2012, 26, 467–472. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Eichelser, C.; Kropidlowski, J.; Janni, W.; Rack, B.; Pantel, K. Loss of heterozygosity at tumor suppressor genes detectable on fractionated circulating cell-free tumor DNA as indicator of breast cancer progression. Clin. Cancer Res. 2012, 18, 5719–5730. [Google Scholar] [CrossRef] [PubMed]
- Dorjgochoo, T.; Zheng, Y.; Gao, Y.T.; Ma, X.; Long, J.; Bao, P.; Zhang, B.; Wen, W.; Lu, W.; Zheng, W.; et al. No association between genetic variants in angiogenesis and inflammation pathway genes and breast cancer survival among Chinese women. Cancer Epidemiol. 2013, 37, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Wrzeszczynski, K.O.; Varadan, V.; Kamalakaran, S.; Levine, D.A.; Dimitrova, N.; Lucito, R. Integrative prediction of gene function and platinum-free survival from genomic and epigenetic features in ovarian cancer. Methods Mol. Biol. 2013, 1049, 35–51. [Google Scholar] [PubMed]
- Catarino, R.; Ferreira, M.M.; Rodrigues, H.; Coelho, A.; Nogal, A.; Sousa, A.; Medeiros, R. Quantification of free circulating tumor DNA as a diagnostic marker for breast cancer. DNA Cell Biol. 2008, 27, 415–421. [Google Scholar] [CrossRef]
- Allen, D.; Butt, A.; Cahill, D.; Wheeler, M.; Popert, R.; Swaminathan, R. Role of cell-free plasma DNA as a diagnostic marker for prostate cancer. Ann. N. Y. Acad. Sci. 2004, 1022, 76–80. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Davilas, E.; Sotiriou, V.; Koliopanos, A.; Aggelakis, F.; Dardoufas, K.; Agnanti, N.J.; Karydas, I.; Nasioulas, G. Cell-free DNA and RNA in plasma as a new molecular marker for prostate cancer. Oncol. Res. 2004, 14, 439–445. [Google Scholar] [CrossRef]
- Feng, J.; Gang, F.; Li, X.; Jin, T.; Houbao, H.; Yu, C.; Uuorong, L. Plasma cell-free DNA and its DNA integrity as biomarker to distinguish prostate cancer from benign prostatic hyperplasia in patients with increased serum prostate-specific antigen. Int. Urol. Nephrol. 2013, 45, 1023–1028. [Google Scholar] [CrossRef]
- Chun, F.K.; Muller, I.; Lange, I.; Friedrich, M.G.; Erbersdobler, A.; Karakiewicz, P.I.; Graefen, M.; Pantel, K.; Huland, H.; Schwarzenbach, H. Circulating tumour-associated plasma DNA represents an independent and informative predictor of prostate cancer. BJU Int. 2006, 98, 544–548. [Google Scholar] [CrossRef]
- Wroclawski, M.L.; Serpa-Neto, A.; Fonseca, F.L.; Castro-Neves-Neto, O.; Pompeo, A.S.; Machado, M.T.; Pompeo, A.C.L.; Del Giglio, A. Cell-free plasma DNA as biochemical biomarker for the diagnosis and follow-up of prostate cancer patients. Tumor Biol. 2013, 34, 2921–2927. [Google Scholar] [CrossRef]
- Zhong, X.-Y.; von Mühlenen, I.; Li, Y.; Kang, A.; Gupta, A.K.; Tyndall, A.; Holzgreve, W.; Hahn, S.; Hasler, P. Increased concentrations of antibody-bound circulatory cell-free DNA in rheumatoid arthritis. Clin. Chem. 2007, 53, 1609–1614. [Google Scholar] [CrossRef]
- Umetani, N.; Giuliano, A.E.; Hiramatsu, S.H.; Amersi, F.; Nakagawa, T.; Martino, S.; Hoon, D.S. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J. Clin. Oncol. 2006, 24, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Butch, A.W.; Crary, D.; Yee, M. Analytical performance of the Roche total and free PSA assays on the Elecsys 2010 immunoanalyzer. Clin. Biochem. 2002, 35, 143–145. [Google Scholar] [CrossRef]
- Xue, X.; Teare, M.D.; Holen, I.; Zhu, Y.M.; Woll, P.J. Optimizing the yield and utility of circulating cell-free DNA from plasma and serum. Clin. Chim. Acta 2009, 404, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bubp, J.; Jen, M.; Matuszewski, K. Caring for glucose-6-phosphate dehydrogenase (G6PD)—Deficient patients: Implications for pharmacy. Pharm. Ther. 2015, 40, 572. [Google Scholar]
- Sunami, E.; Shinozaki, M.; Higano, C.S.; Wollman, R.; Dorff, T.B.; Tucker, S.J.; Martinez, S.R.; Mizuno, R.; Singer, F.R.; Hoon, D.S. Multi-marker circulating DNA assay for assessing blood of prostate cancer patients. Clin. Chem. 2009, 55, 559–567. [Google Scholar] [CrossRef]
- Altimari, A.; Grigioni, A.D.E.; Benedettini, E.; Gabusi, E.; Schiavina, R.; Martinell, A.; Morselli-Labate, A.M.; Martorana, G.; Grigioni, W.F.; Fiorentino, M. Diagnostic role of circulating free plasma DNA detection in patients with localized prostate cancer. Am. J. Clin. Pathol. 2008, 129, 756–762. [Google Scholar] [CrossRef]
- Cherepanova, A.V.; Tamkovich, S.N.; Bryzgunova, O.E.; Vlassov, V.V.; Aktionov, P.P. Deoxyribonuclease activity and circulating DNA concentration in blood plasma of patients with prostate tumors. Ann. N. Y. Acad. Sci. 2008, 1137, 218–221. [Google Scholar] [CrossRef]
- Ellinger, J.; Bastian, P.J.; Haan, K.I.; Heukamp, L.C.; Buettner, R.; Fimmers, R.; Mueller, S.C.; Von Ruecker, A. Noncancerous PTGS2 DNA fragments of apoptotic origin in sera of prostate cancer patients qualify as diagnostic and prognostic indicators. Int. J. Cancer 2008, 122, 138–143. [Google Scholar] [CrossRef]
- Wu, T.-L.; Zhang, D.; Chia, J.-H.; Tsao, K.-C.; Sun, C.-F.; Wu, J.T. Cell-free DNA: Measurement in various carcinomas and establishment of normal reference range. Clin. Chim. Acta 2002, 321, 77–87. [Google Scholar] [CrossRef]
- Fawzy, A.; Sweify, K.M.; El-Fayoumy, H.M.; Nofal, N. Quantitative analysis of plasma cell-free DNA and its DNA integrity in patients with metastatic prostate cancer using ALU sequence. J. Egypt Natl. Cancer Inst. 2016, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Hosseini, J.; Mirfakhraie, R.; Habibi, M.; Azargashb, E.; Pouresmaeili, F. The value of the plasma circulating cell-free DNA concentration and integrity index as a clinical tool for prostate cancer diagnosis: A prospective case-control cohort study in an Iranian population. Cancer Manag. Res. 2019, 11, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Arko-Boham, B.; Aryee, N.A.; Blay, R.M.; Owusu, E.; Tagoe, E.A.; Shackie, E.-S.D.; Debrah, A.B.; Adu-Aryee, A.N. Circulating cell-free DNA integrity as a diagnostic and prognostic marker for breast and prostate cancers. Cancer Genet. 2019, 235–236, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hanley, R.; Rieger-Christ, K.M.; Canes, D.; Emara, N.R.; Shuber, A.P.; Boynton, K.A.; Libertino, J.A.; Summerhayes, I.C. DNA integrity assay: A plasma-based screening tool for the detection of prostate cancer. Clin. Cancer Res. 2006, 12, 4569–4574. [Google Scholar] [CrossRef]
- Abate-Shen, C.; Shen, M.M. Molecular genetics of prostate cancer. Genes Dev. 2000, 14, 2410–2434. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Iwata, T.; Koh, C.M.; Jenkins, R.B.; Lan, F.; Dang, C.V.; Hicks, J.L.; Morgan, J.; Cornish, T.C.; Sutcliffe, S.; et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol. 2008, 21, 1156–1167. [Google Scholar] [CrossRef]
- Elo, J.P.; Visakorpi, T. Molecular genetics of prostate cancer. Ann. Med. 2001, 33, 130–141. [Google Scholar] [CrossRef]
- Nodouzi, V.; Nowroozi, M.; Hashemi, M.; Javadi, G.; Mahdian, R. Concurrent down-regulation of PTEN and NKX3.1 Expression in Iranian patients with prostate cancer. Int. Braz. J. Urol. 2015, 41, 898–905. [Google Scholar] [CrossRef]
- Arnold, A. Primary hyperparathyroidism: Molecular genetic insights and clinical implications. Presented at Society for Endocrinology BES 2017, Harrogate, UK. Endocr. Abstr. 2017, 50, PL1. [Google Scholar]
- Coller, H.A.; Grandori, C.; Tamayo, P.; Colbert, T.; Lander, E.S.; Eisenman, R.N.; Golub, T.R. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc. Natl. Acad. Sci. USA 2000, 97, 3260–3265. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tafvizi, F.; Moslemi, E. Evaluation of c-Myc mRNA expression level in benign prostatic hyperplasia and prostatic adenocarcinoma tissues and its correlation with clinicopathological characteristics. Int. J. Med. Lab. 2018, 5, 103–112. [Google Scholar]
- Fleming, W.H.; Hamel, A.; Dodd, J.G.; Matusik, R.J.; MacDonald, R.; Ramsey, E.; Pettiarew, N.M.; Johnston, B. Expression of the c-myc protooncogene in human prostatic carcinoma and benign prostatic Hyperplasia. Cancer Res. 1986, 46, 1535–1538. [Google Scholar] [PubMed]
- Martignano, F.; Gurioli, G.; Salvi, S.; Calistri, D.; Costantini, M.; Gunelli, R.; De Giorgi, U.; Foca, F.; Casadio, V. GSTP1 methylation and protein expression in prostate cancer: Diagnostic implications. Dis. Mark. 2016, 2016, 4358292. [Google Scholar]
- Siddiqi, M.A.; Syeed, N.; Sameer, A.S.; Hamid, A.; Shah, Z.A.; Afroze, D.; Rasool, R. Promoter methylation profile of GSTP1 and RASSF1A in benign hyperplasia and metastatic prostate cancer patients in a Kashmiri population. Mol. Med. Rep. 2010, 3, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Alix-Panabières, C.; Müller, I.; Letang, N.; Vendrell, J.-P.; Rebillard, X.; Pantel, K. Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer. Clin. Cancer Res. 2009, 15, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Stephan, C.; Lewandowski, M.; Klotzek, S.; Jung, M.; Kristiansen, G.; Lein, M.; Loening, S.A.; Schnorr, D. Increased cell-free DNA in plasma of patients with metastatic spread in prostate cancer. Cancer Lett. 2004, 205, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.O.; Alves, B.C.; Gehrke Fde, S.; Kuniyoshi, R.K.; Wroclavski, M.L.; Del Giglio, A.; Fonseca, F.L.A. Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumor Biol. 2013, 34, 983–986. [Google Scholar] [CrossRef]
- Lee, T.H.; Montalvo, L.; Chrebtow, V.; Busch, M.P. Quantitation of genomic DNA in plasma and serum samples: Higher concentrations of genomic DNA found in serum than in plasma. Transfusion 2001, 41, 276–282. [Google Scholar] [CrossRef]
- Fong, S.L.; Zhang, J.T.; Lim, C.K.; Eu, K.W.; Liu, Y. Comparison of seven methods for extracting cell-free DNA from serum samples of colorectal cancer patients. Clin. Chem. 2009, 55, 587–589. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Wroclawski, M.L.; Pinto, J.L.; Marsicano, S.R.; Delgado, P.O.; Coelho, P.G. Methodological standardization for the extraction of free DNA in plasma of peripheral blood. J. Cancer Sci. 2012, 1, 1–5. [Google Scholar]
- Ramachandran, K.; Speer, C.G.; Fiddy, S.; Reis, I.M.; Singal, R. Free circulating DNA as a biomarker of prostate cancer: Comparison of quantitation methods. Anticancer Res. 2013, 33, 4521–4529. [Google Scholar] [PubMed]
- Agostini, M.; Pucciarelli, S.; Enzo, M.V.; Del Bianco, P.; Briarava, M.; Bedin, C.; Maretto, I.; Friso, M.L.; Lonardi, S.; Mescoli, C.; et al. Circulating cell-free DNA: A promising marker of pathologic tumor response in rectal cancer patients receiving preoperative chemoradiotherapy. Ann. Surg. Oncol. 2011, 18, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Mead, R.; Duku, M.; Bhandari, P.; Cree, I.A. Circulating tumour markers can define patients with normal colons, benign polyps, and cancers. Br. J. Cancer 2011, 105, 239–245. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, S.F.; Eid, M.A.; El-Sourogy, H.A.; Attia, G.F.; Ezzat, S.A. Evaluation of serum DNA integrity as a screening and prognostic tool in patients with hepatitis C virus-related hepatocellular carcinoma. Int. J. Biol. Mark. 2010, 25, 79–86. [Google Scholar] [CrossRef]
- Deligezer, U.; Eralp, Y.; Akisik, E.E.; Akisik, E.Z.; Saip, P.; Topuz, E.; Dalay, N. Size distribution of circulating cell-free DNA in sera of breast cancer patients in the course of adjuvant chemotherapy. Clin. Chem. Lab. Med. 2008, 46, 311–317. [Google Scholar] [CrossRef]
- Umetani, N.; Kim, J.; Hiramatsu, S.; Reber, H.A.; Hines, O.J.; Bilchik, A.J.; Hoon, D.S. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: Direct quantitative PCR for ALU repeats. Clin. Chem. 2006, 52, 1062–1069. [Google Scholar] [CrossRef]
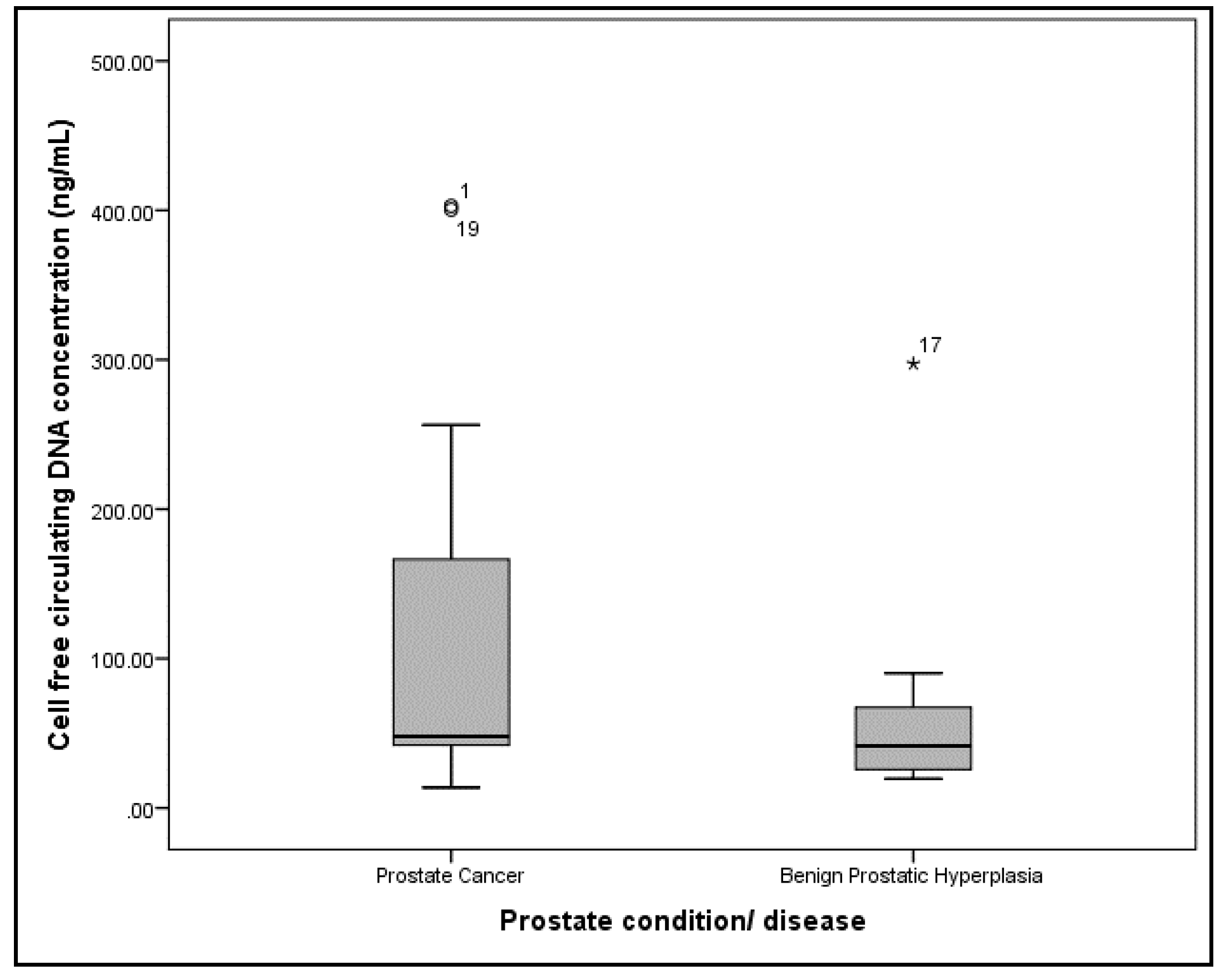
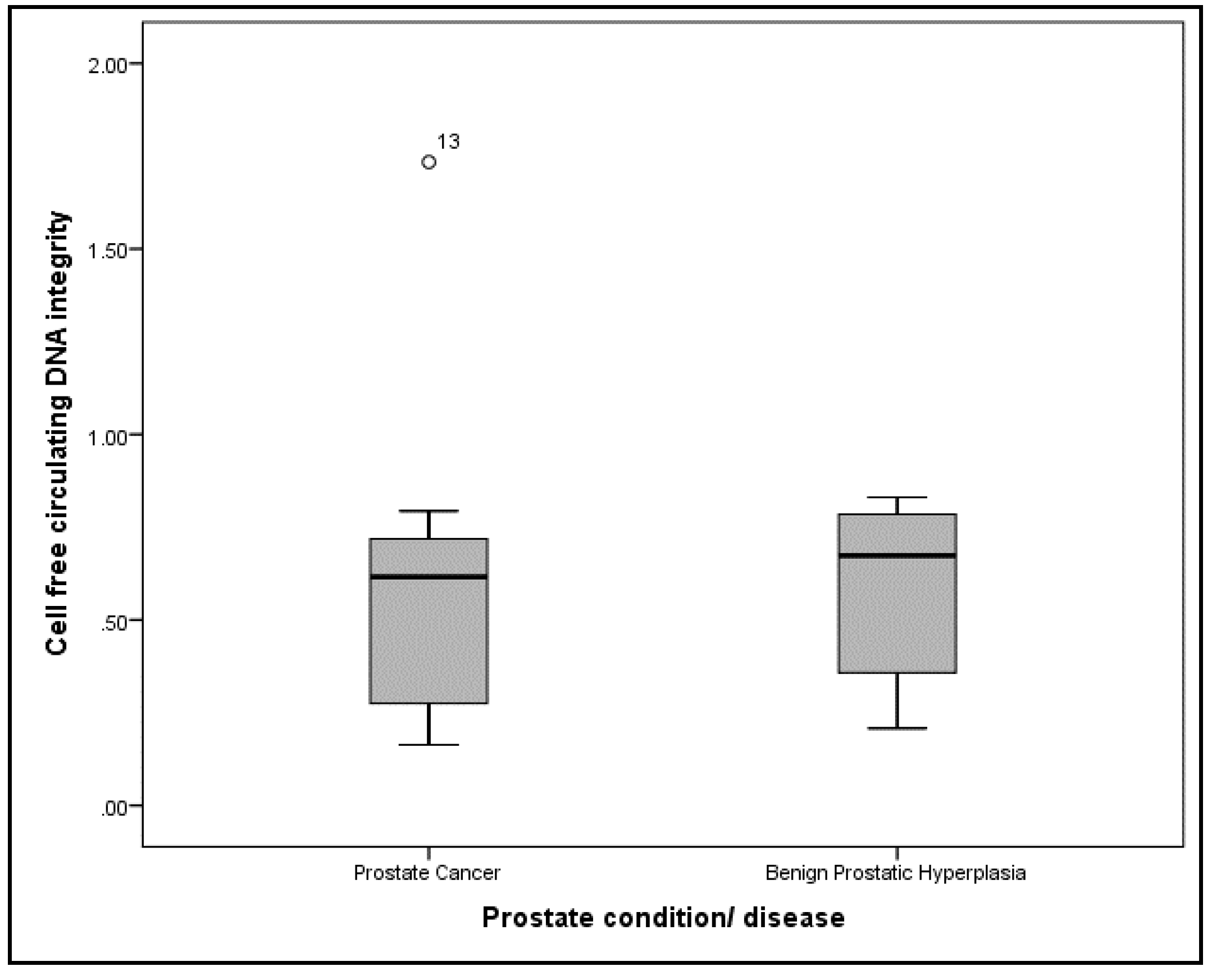
| Characteristics | PCa (n = 11) | BPH (n = 9) | p |
|---|---|---|---|
| Age in years, median (IQR) | 68 (7) | 65 (8) | 0.236 |
| Type of comorbidities, n (%) | |||
| Hypertension | 5 (45.5%) | 2 (22.2%) | - |
| Diabetes | 3 (27.3%) | 2 (22.2%) | - |
| Renal Calculi | 1 (9.1%) | 2 (22.2%) | - |
| Other | 2 (18.2%) | 1 (11.1%) | - |
| Serum total PSA in ng/mL, median (IQR) | 24.2 (152.4) | 8.2 (5.05) | 0.138 |
| Serum total PSA range, n (%) | |||
| ≥4 ng/mL | 11 (100%) | 8 (88.9%) | 0.450 |
| <4 ng/mL | 0 (0.0%) | 1 (11.1%) | |
| Prostate volume in cc, median (IQR) | 54 (28.3) *a | 113.8 (125.1) *b | 0.011 |
| Gleason Score, median (IQR) | 7 (3) | - | - |
| Gleason score, n (%) | |||
| <7 | 4 (36.4%) | - | - |
| =7 | 3 (27.3%) | - | - |
| >7 | 4 (36.4%) | - | - |
| Variables | n | Cell Free Circulating DNA Concentration | Cell Free Circulating DNA Integrity | ||
|---|---|---|---|---|---|
| rs | p | rs | p | ||
| Age | 11 | −0.110 | 0.747 | −0.303 | 0.395 |
| Serum total PSA | 11 | 0.200 | 0.555 | −0.455 | 0.160 |
| Gleason score | 11 | −0.119 | 0.728 | −0.052 | 0.879 |
| Total prostate volume | 11 | −0.179 | 0.702 | 0.071 | 0.879 |
| Number of comorbidities | 11 | −0.162 | 0.633 | 0.249 | 0.461 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condappa, A.; McGrowder, D.; Aiken, W.; McLaughlin, W.; Gossell-Williams, M. Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study. Diseases 2020, 8, 34. https://doi.org/10.3390/diseases8030034
Condappa A, McGrowder D, Aiken W, McLaughlin W, Gossell-Williams M. Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study. Diseases. 2020; 8(3):34. https://doi.org/10.3390/diseases8030034
Chicago/Turabian StyleCondappa, Andrew, Donovan McGrowder, William Aiken, Wayne McLaughlin, and Maxine Gossell-Williams. 2020. "Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study" Diseases 8, no. 3: 34. https://doi.org/10.3390/diseases8030034
APA StyleCondappa, A., McGrowder, D., Aiken, W., McLaughlin, W., & Gossell-Williams, M. (2020). Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study. Diseases, 8(3), 34. https://doi.org/10.3390/diseases8030034






