Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses
Abstract
1. Introduction
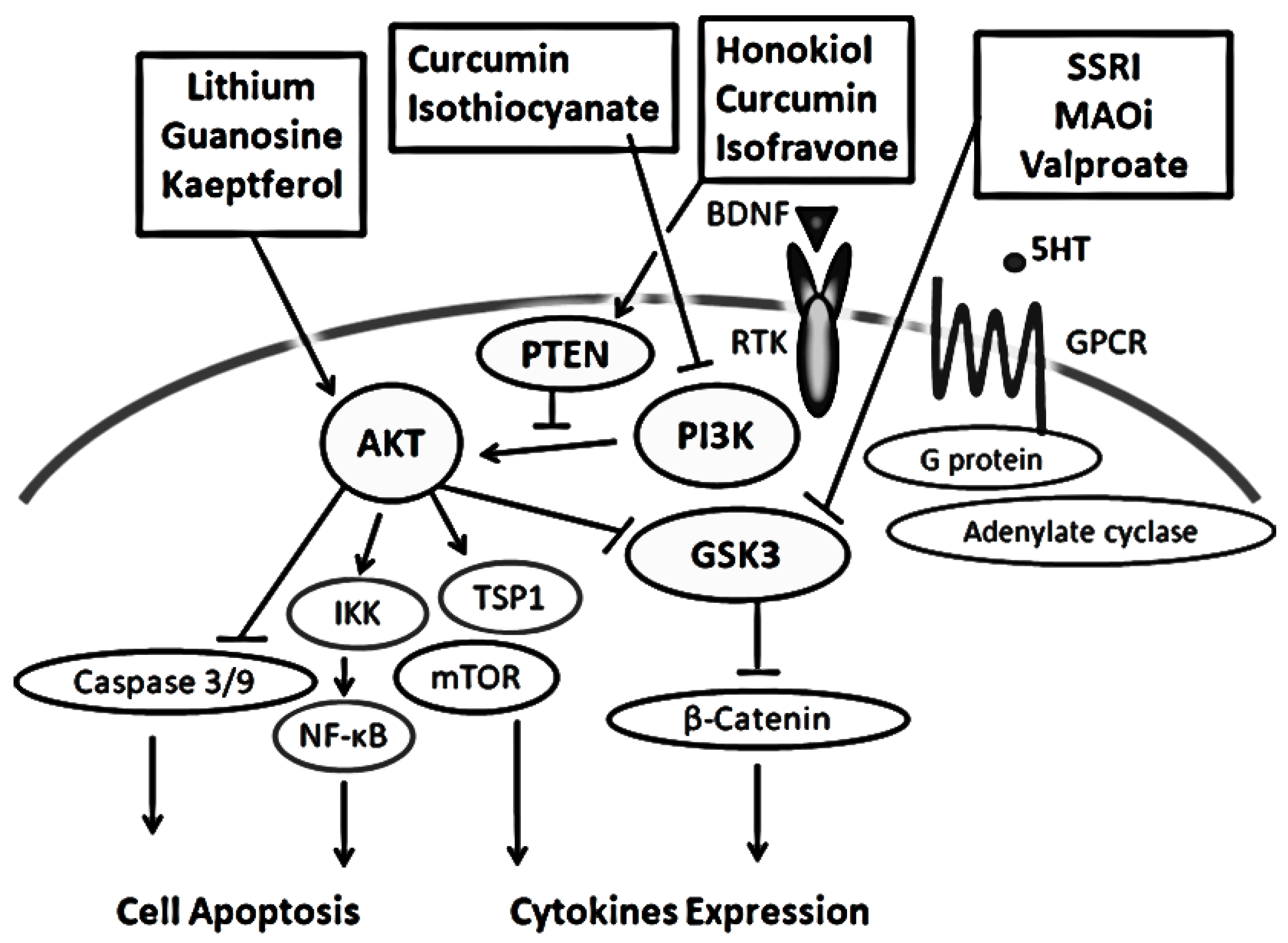
2. Characterization of the PI3K/AKT/GSK3 Signaling Pathway in the Pathogenesis of Psychiatric Illnesses
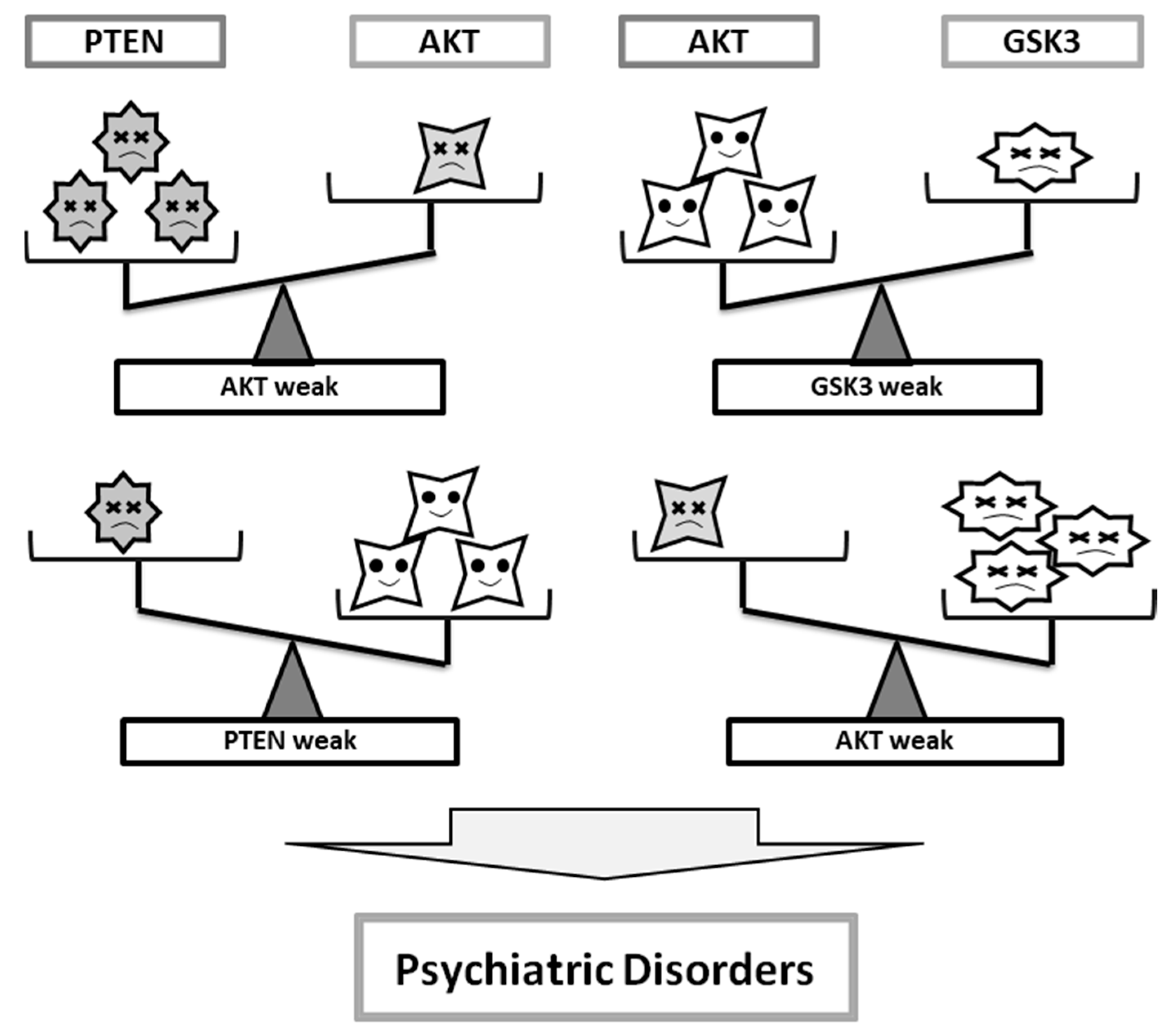
3. Some Diagnostic Clues for Psychiatric Illnesses at the Molecules Involved in PI3K/AKT/GSK3 Pathway
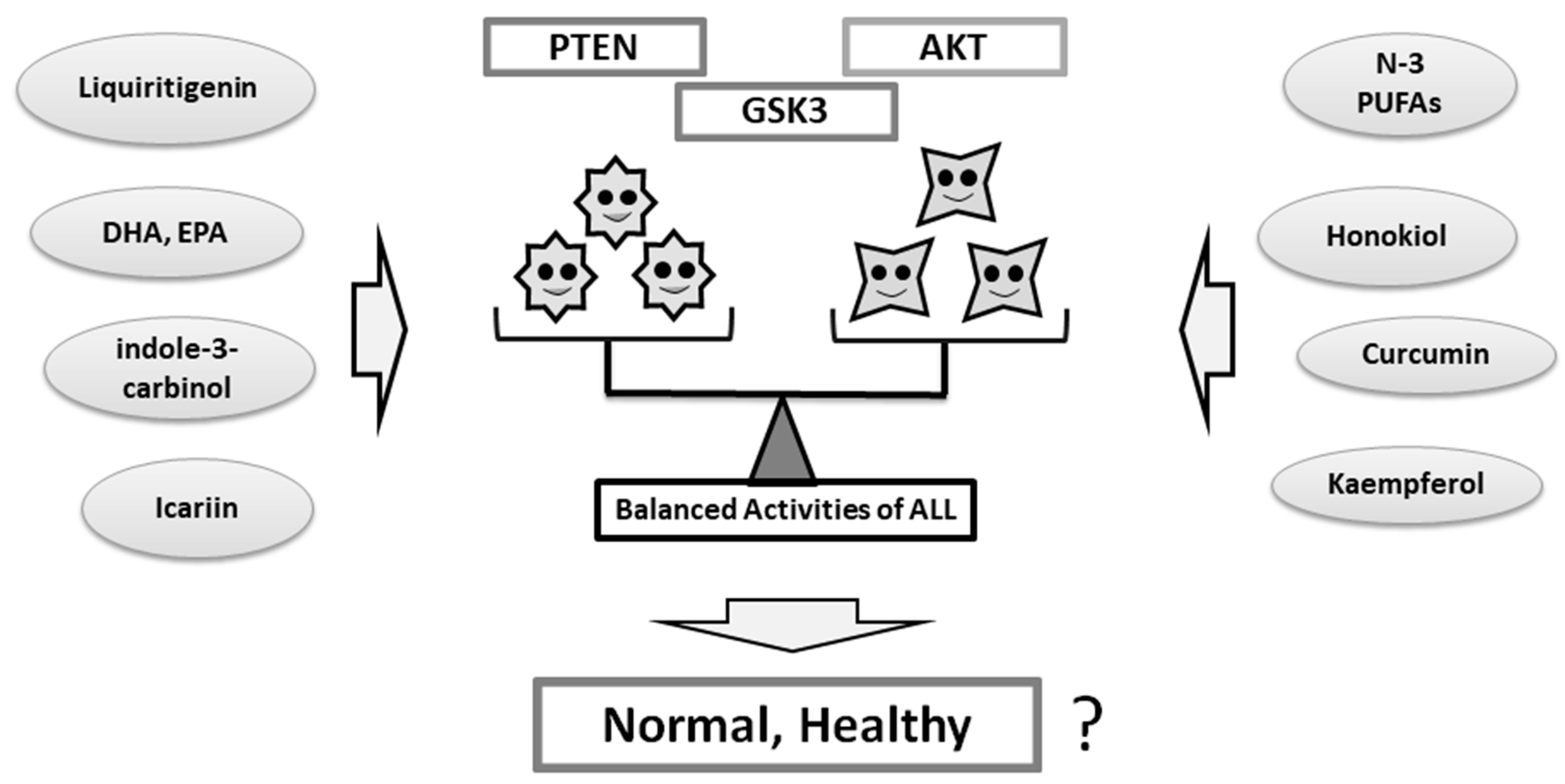
4. Some Diets With Phytocompounds May Contribute to the Neuro-protection in the Psychiatric Diseases via the Modulation of PI3K/AKT/GSK3 Signaling
5. So What Next in Perspectives?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| DHA | docosahexaenonic acids |
| EPA | eicosopentaenoic acid |
| GSK3 | Glycogen synthase kinase 3 |
| 5-HT | 5-hydroxytryptamine, serotonin |
| mTOR | mammalian target of rapamycin |
| PIP3 | phosphatidylinositol 3,4,5-triphosphate |
| PI3K | phosphatidylinositol-3 kinase |
| PPARγ | Peroxisome Proliferator-Activated Receptor γ |
| PTEN | Phosphatase and tensin homolog on chromosome 10 |
| ROS | reactive oxygen species |
| SSRIs | selective serotonin reuptake inhibitors |
References
- Vriend, C. The neurobiology of impulse control disorders in Parkinson’s disease: From neurotransmitters to neural networks. Cell Tissue Res. 2018, 373, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S.; Castner, S.A.; Svensson, T.H.; Siever, L.J.; Williams, G.V. Targeting the dopamine D1 receptor in schizophrenia: Insights for cognitive dysfunction. Psychopharmacology 2004, 174, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J. Psychiatry Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Swift, J.L.; Godin, A.G.; Doré, K.; Freland, L.; Bouchard, N.; Nimmo, C.; Sergeev, M.; De Koninck, Y.; Wiseman, P.W.; Beaulieu, J.M. Quantification of receptor tyrosine kinase transactivation through direct dimerization and surface density measurements in single cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7016–7021. [Google Scholar] [CrossRef]
- Freland, L.; Beaulieu, J.M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front. Mol. Neurosci. 2012, 5, 14. [Google Scholar] [CrossRef]
- Kang, H.; Schneider, H.; Rudd, C.E. Phosphatidylinositol 3-kinase p85 adaptor function in T-cells. Co-stimulation and regulation of cytokine transcription independent of associated p110. J. Biol. Chem. 2002, 277, 912–921. [Google Scholar] [CrossRef]
- He, W.; Yuan, Q.H.; Zhou, Q. Histamine H3 receptor antagonist Clobenpropit protects propofol-induced apoptosis of hippocampal neurons through PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8013–8020. [Google Scholar]
- Zhou, X.; Cordon-Barris, L.; Zurashvili, T.; Bayascas, J.R. Fine-tuning the intensity of the PKB/Akt signal enables diverse physiological responses. Cell Cycle 2014, 13, 3164–3168. [Google Scholar] [CrossRef]
- Diez, H.; Garrido, J.J.; Wandosell, F. Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons. PLoS ONE 2012, 7, e32715. [Google Scholar] [CrossRef] [PubMed]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Tschopp, O.; Hemmings-Mieszczak, M.; Feng, J.; Brodbeck, D.; Perentes, E.; Hemmings, B.A. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 2003, 278, 32124–32131. [Google Scholar] [CrossRef]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Leibrock, C.; Ackermann, T.F.; Hierlmeier, M.; Lang, F.; Borgwardt, S.; Lang, U.E. Akt2 deficiency is associated with anxiety and depressive behavior in mice. Cell. Physiol. Biochem. 2013, 32, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Poduri, A.; Evrony, G.D.; Cai, X.; Elhosary, P.C.; Beroukhim, R.; Lehtinen, M.K.; Hills, L.B.; Heinzen, E.L.; Hill, A.; Hill, R.S.; et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 2012, 74, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, Y.; Bureau, G.; Laurier-Laurin, M.É.; Asselin, E.; Massicotte, G.; Cyr, M. Genetic Deletion of Akt3 Induces an Endophenotype Reminiscent of Psychiatric Manifestations in Mice. Front. Mol. Neurosci. 2017, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.R.; Floyd, K.; Law, A.J. PKBγ/AKT3 loss-of-function causes learning and memory deficits and deregulation of AKT/mTORC2 signaling: Relevance for schizophrenia. PLoS ONE 2017, 12, e0175993. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Chen, Y.; Dai, W. Chromatin PTEN is involved in DNA damage response partly through regulating Rad52 sumoylation. Cell Cycle 2013, 12, 3442–3447. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Ho, J.; Srikumar, T.; Dowling, R.J.; Gorrini, C.; Miller, S.J.; Mak, T.W.; Neel, B.G.; Raught, B.; Stambolic, V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 2013, 341, 395–399. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases 2018, 6, E28. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, S.; Wang, Z.; Wang, Z.; Sun, C.; Zhang, Y. Inhibition of PTEN Attenuates Endoplasmic Reticulum Stress and Apoptosis via Activation of PI3K/AKT Pathway in Alzheimer’s Disease. Neurochem. Res. 2017, 42, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Knafo, S.; Esteban, J.A. PTEN: Local and Global Modulation of Neuronal Function in Health and Disease. Trends Neurosci. 2017, 40, 83–91. [Google Scholar] [CrossRef]
- Hobert, J.A.; Embacher, R.; Mester, J.L.; Frazier, T.W.; Eng, C. Biochemical screening and PTEN mutation analysis in individuals with autism spectrum disorders and macrocephaly. Eur. J. Hum. Genet. 2014, 22, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.A.; Kinstrie, R.; Sibbet, G.; Rawjee, T.; Morrice, N.; Cleghon, V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell 2006, 24, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.; Cohen, P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994, 338, 37–42. [Google Scholar] [CrossRef]
- Besing, R.C.; Rogers, C.O.; Paul, J.R.; Hablitz, L.M.; Johnson, R.L.; McMahon, L.L.; Gamble, K.L. GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus 2017, 27, 890–898. [Google Scholar] [CrossRef]
- Law, A.J.; Wang, Y.; Sei, Y.; O’Donnell, P.; Piantadosi, P.; Papaleo, F.; Straub, R.E.; Huang, W.; Thomas, C.J.; Vakkalanka, R.; et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc. Natl. Acad. Sci. USA 2012, 109, 12165–12170. [Google Scholar] [CrossRef]
- Desrivières, S.; Krause, K.; Dyer, A.; Frank, J.; Blomeyer, D.; Lathrop, M.; Mann, K.; Banaschewski, T.; Laucht, M.; Schumann, G. Nucleotide sequence variation within the PI3K p85 alpha gene associates with alcohol risk drinking behaviour in adolescents. PLoS ONE 2008, 3, e1769. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Li, G.; Anderson, R.E.; Tomita, H.; Adler, R.; Liu, X.; Zack, D.J.; Rajala, R.V. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 2007, 27, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.; Clayton-Smith, J.; Woo, V.G.; McKee, S.; Manson, F.D.; Medne, L.; Zackai, E.; Swanson, E.A.; Fitzpatrick, D.; Millen, K.J.; et al. Mapping of deletion and translocation breakpoints in 1q44 implicates the serine/threonine kinase AKT3 in postnatal microcephaly and agenesis of the corpus callosum. Am. J. Hum. Genet. 2007, 81, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Rivière, J.B.; Mirzaa, G.M.; O’Roak, B.J.; Beddaoui, M.; Alcantara, D.; Conway, R.L.; St-Onge, J. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 2012, 44, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Qiao, X.; Wu, H.; Yin, F.; Zhang, J.; Ji, Y.; Wei, S.; Lai, J. An Association Study Between Genetic Polymorphisms in Functional Regions of Five Genes and the Risk of Schizophrenia. J. Mol. Neurosci. 2016, 59, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Carlson, G.C.; Talbot, K.; Kazi, H.; Hill-Smith, T.E.; Easton, R.M.; Birnbaum, M.J.; Lucki, I. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus 2012, 22, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; La Harpe, R.; Malafosse, A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol. Psychiatry 2007, 61, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.H.; Mazei-Robison, M.; Iñiguez, S.D.; Ables, J.L.; Vialou, V.; Berton, O.; Ghose, S.; Covington, H.E.; Wiley, M.D.; et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol. Psychiatry 2008, 64, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Sotnikova, T.D.; Marion, S.; Lefkowitz, R.J.; Gainetdinov, R.R.; Caron, M.G. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Sze, Y.; Chang, C.C.; Lee, J.; Zhang, X. Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3β pathway. Transl. Psychiatry 2015, 5, e528. [Google Scholar] [CrossRef]
- Valencia, A.; Reeves, P.B.; Sapp, E.; Li, X.; Alexander, J.; Kegel, K.B.; Chase, K.; Aronin, N.; DiFiglia, M. Mutant huntingtin and glycogen synthase kinase 3-beta accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington’s disease. J. Neurosci. Res. 2010, 88, 179–190. [Google Scholar] [CrossRef]
- Li, X.; Jope, R.S. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 2010, 35, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Wnt and GSK3 Signaling Pathways in Bipolar Disorder: Clinical and Therapeutic Implications. Clin. Psychopharmacol. Neurosci. 2017, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Polter, A.; Beurel, E.; Yang, S.; Garner, R.; Song, L.; Miller, C.A.; Sweatt, J.D.; McMahon, L.; Bartolucci, A.A.; Li, X.; et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 2010, 35, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Vogt, D.; Cho, K.K.A.; Lee, A.T.; Sohal, V.S.; Rubenstein, J.L.R. The parvalbumin/somatostatin ratio is increased in Pten mutant mice and by human PTEN ASD alleles. Cell Rep. 2015, 11, 944–956. [Google Scholar] [CrossRef]
- Tilot, A.K.; Bebek, G.; Niazi, F.; Altemus, J.B.; Romigh, T.; Frazier, T.W.; Eng, C. Neural transcriptome of constitutional Pten dysfunction in mice and its relevance to human idiopathic autism spectrum disorder. Mol. Psychiatry 2016, 21, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.L.; Liu, G.; Xiao, D.; Zhang, B.H.; Guo, C.C.; Ye, X.G.; Liu, Y.; Zhang, N.; Wang, M.; Han, Y.J.; et al. Association between miR-137 polymorphism and risk of schizophrenia: A meta-analysis. Genet. Mol. Res. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.T.; Anderson, B.R.; Shah, N.; Zimmer, S.E.; Hawkins, D.; Valdez, A.N.; Gu, Q.; Bassell, G.J. Inhibition of the Schizophrenia-Associated MicroRNA miR-137 Disrupts Nrg1α Neurodevelopmental Signal Transduction. Cell Rep. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Z.; Liu, J.; Wang, J.; Qu, C. MicroRNA-137 regulates hypoxia-induced retinal ganglion cell apoptosis through Notch1. Int. J. Mol. Med. 2018, 41, 1774–1782. [Google Scholar] [CrossRef]
- Murphy, C.P.; Li, X.; Maurer, V.; Oberhauser, M.; Gstir, R.; Wearick-Silva, L.E.; Viola, T.W.; Schafferer, S.; Grassi-Oliveira, R.; Whittle, N.; et al. MicroRNA-Mediated Rescue of Fear Extinction Memory by miR-144-3p in Extinction-Impaired Mice. Biol. Psychiatry 2017, 81, 979–989. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Acar, Ş.; Coşkun, S.; Güneş, M.; Güneş, S.; Yılmaz, M.F.; Görür, A.; Tamer, L. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J. Psychiatr. Res. 2015, 69, 67–71. [Google Scholar] [CrossRef]
- Olivieri, F.; Ahtiainen, M.; Lazzarini, R.; Pöllänen, E.; Capri, M.; Lorenzi, M.; Fulgenzi, G.; Albertini, M.C.; Salvioli, S.; Alen, M.J.; et al. Hormone replacement therapy enhances IGF-1 signaling in skeletal muscle by diminishing miR-182 and miR-223 expressions: A study on postmenopausal monozygotic twin pairs. Aging Cell 2014, 13, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Naughton, M.E.; Scott, K.A.; O’Connor, R.M.; Moloney, G.; Clarke, G.; Dowling, J.; Walsh, A.; Ismail, F.; Shorten, G.; et al. MicroRNAs as biomarkers for major depression: A role for let-7b and let-7c. Transl. Psychiatry 2016, 6, e862. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.P.; Rushlow, W.J. The effects of neuropsychiatric drugs on glycogen synthase kinase-3 signaling. Neuroscience 2011, 199, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Xu, M.; Hu, L.; Yan, T.; He, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandrin rescues depressive-like behaviors induced by chronic unpredictable mild stress via GDNF/ERK1/2/ROS and PI3K/AKT/NOX signaling pathways in mice. Psychiatry Res. 2017, 257, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Competition between Li+ and Mg2+ in metalloproteins. Implications for lithium therapy. J. Am. Chem. Soc. 2011, 133, 9506–9515. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Ghadirivasfi, M.; Barati, M.; Ghasemzadeh, M.R.; Narimani, S.; Mousavi-Behbahani, Z.; Joghataei, M.; Soleimani, M.; Taban, M.; Mehrabi, S.; et al. Methamphetamine-induced psychosis is associated with DNA hypomethylation and increased expression of AKT1 and key dopaminergic genes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 1180–1189. [Google Scholar] [CrossRef]
- He, Y.; Wang, P.; Wei, P.; Feng, H.; Ren, Y.; Yang, J.; Rao, Y.; Shi, J.; Tian, J. Effects of curcumin on synapses in APPswe/PS1dE9 mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 217–225. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, L.; Zhou, C.; Wang, Q.; Li, X.; Zhou, M.; Wang, H. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: Autophagy and the PI3K/AKT pathways as a potential mechanism. J. Surg. Res. 2016, 206, 67–76. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [PubMed]
- Barry, R.L.; Byun, N.E.; Williams, J.M.; Siuta, M.A.; Tantawy, M.N.; Speed, N.K.; Saunders, C.; Galli, A.; Niswender, K.D.; Avison, M.J. Brief exposure to obesogenic diet disrupts brain dopamine networks. PLoS ONE 2018, 13, e0191299. [Google Scholar] [CrossRef] [PubMed]
- Figlewicz, D.P.; Jay, J.; West, C.H.; Zavosh, A.; Hampe, C.S.; Radtke, J.R.; Raskind, M.A.; Peskind, E.R. Effect of dietary palmitic and stearic acids on sucrose motivation and hypothalamic and striatal cell signals in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R191–R200. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Freeman, M.P. Omega-3 fatty acids in psychiatry. Psychiatr. Clin. N. Am. 2013, 36, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Kim, G. Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutr. Res. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Chia, S.C.; Henry, J.; Mok, Y.M.; Honer, W.G.; Sim, K. Fatty acid and vitamin interventions in adults with schizophrenia: A systematic review of the current evidence. J. Neural Transm. 2015, 122, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef]
- Yang, J.; Zou, Y.; Jiang, D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR-21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int. J. Mol. Med. 2018, 41, 1845–1854. [Google Scholar] [CrossRef]
- Aronchik, I.; Kundu, A.; Quirit, J.G.; Firestone, G.L. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol. Cancer Res. 2014, 12, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.; Hashem, R.M.; Rashed, L.A.; El-Razek, S.M. Comparative study between the effect of the peroxisome proliferator activated receptor-alpha ligands fenofibrate and n-3 polyunsaturated fatty acids on activation of 5’-AMP-activated protein kinase-alpha1 in high-fat fed rats. J. Pharm. Pharmacol. 2009, 61, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.H.; Goswami, D.; Griffin, P.R.; Noy, N.; Ortlund, E.A. Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor β/δ (FABP5-PPARβ/δ) signaling pathway. J. Biol. Chem. 2014, 289, 14941–14954. [Google Scholar] [CrossRef]
- Ghosh-Choudhury, T.; Mandal, C.C.; Woodruff, K.; St Clair, P.; Fernandes, G.; Choudhury, G.G.; Ghosh-Choudhury, N. Fish oil targets PTEN to regulate NFkappaB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res. Treat. 2009, 118, 213–228. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. https://doi.org/10.3390/diseases7010022
Matsuda S, Ikeda Y, Murakami M, Nakagawa Y, Tsuji A, Kitagishi Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases. 2019; 7(1):22. https://doi.org/10.3390/diseases7010022
Chicago/Turabian StyleMatsuda, Satoru, Yuka Ikeda, Mutsumi Murakami, Yukie Nakagawa, Ai Tsuji, and Yasuko Kitagishi. 2019. "Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses" Diseases 7, no. 1: 22. https://doi.org/10.3390/diseases7010022
APA StyleMatsuda, S., Ikeda, Y., Murakami, M., Nakagawa, Y., Tsuji, A., & Kitagishi, Y. (2019). Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases, 7(1), 22. https://doi.org/10.3390/diseases7010022




