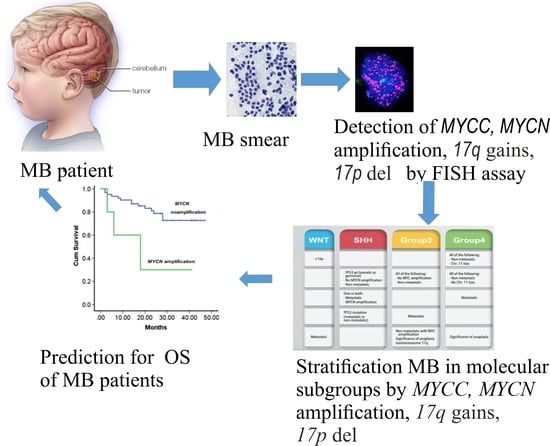
The Prognostic Value of Amplification of the MYCC and MYCN Oncogenes in Russian Patients with Medulloblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Characteristics of MB Patients
2.2. Preparation of Tumor Smears
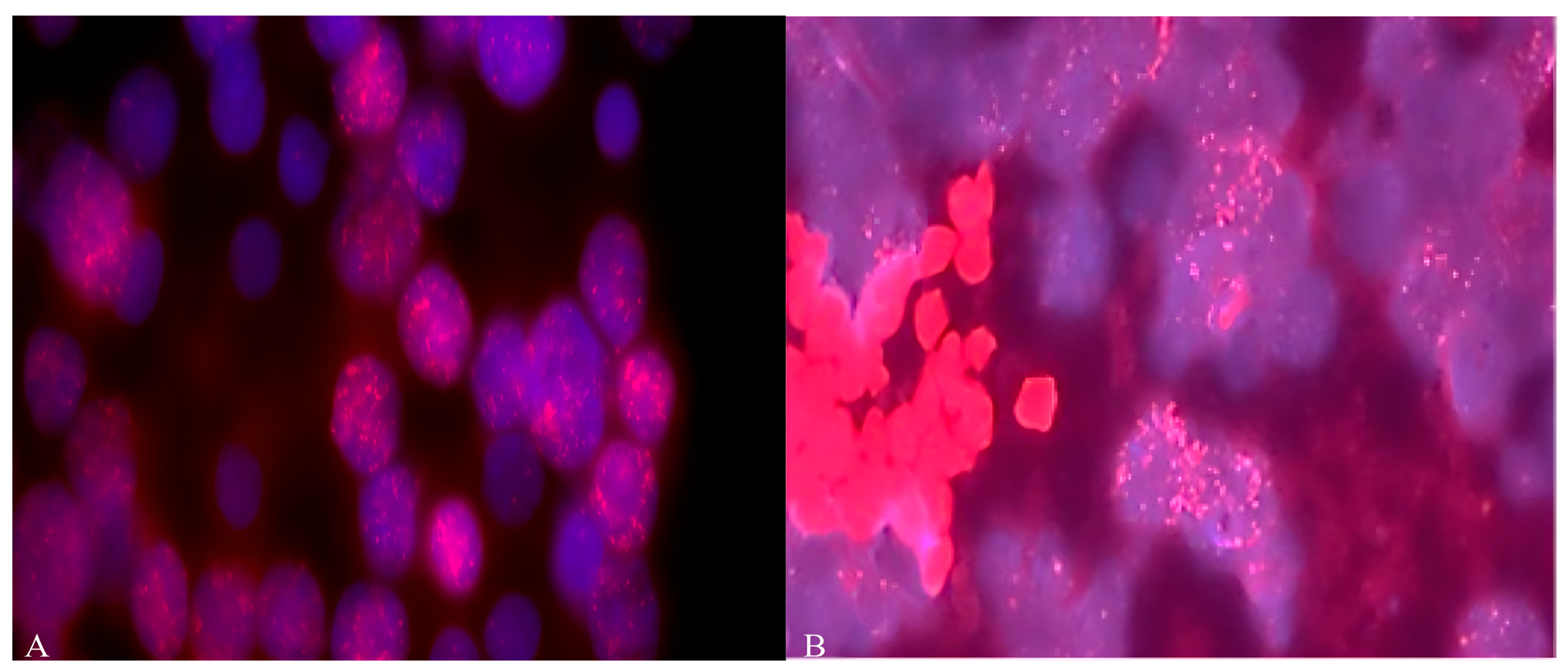
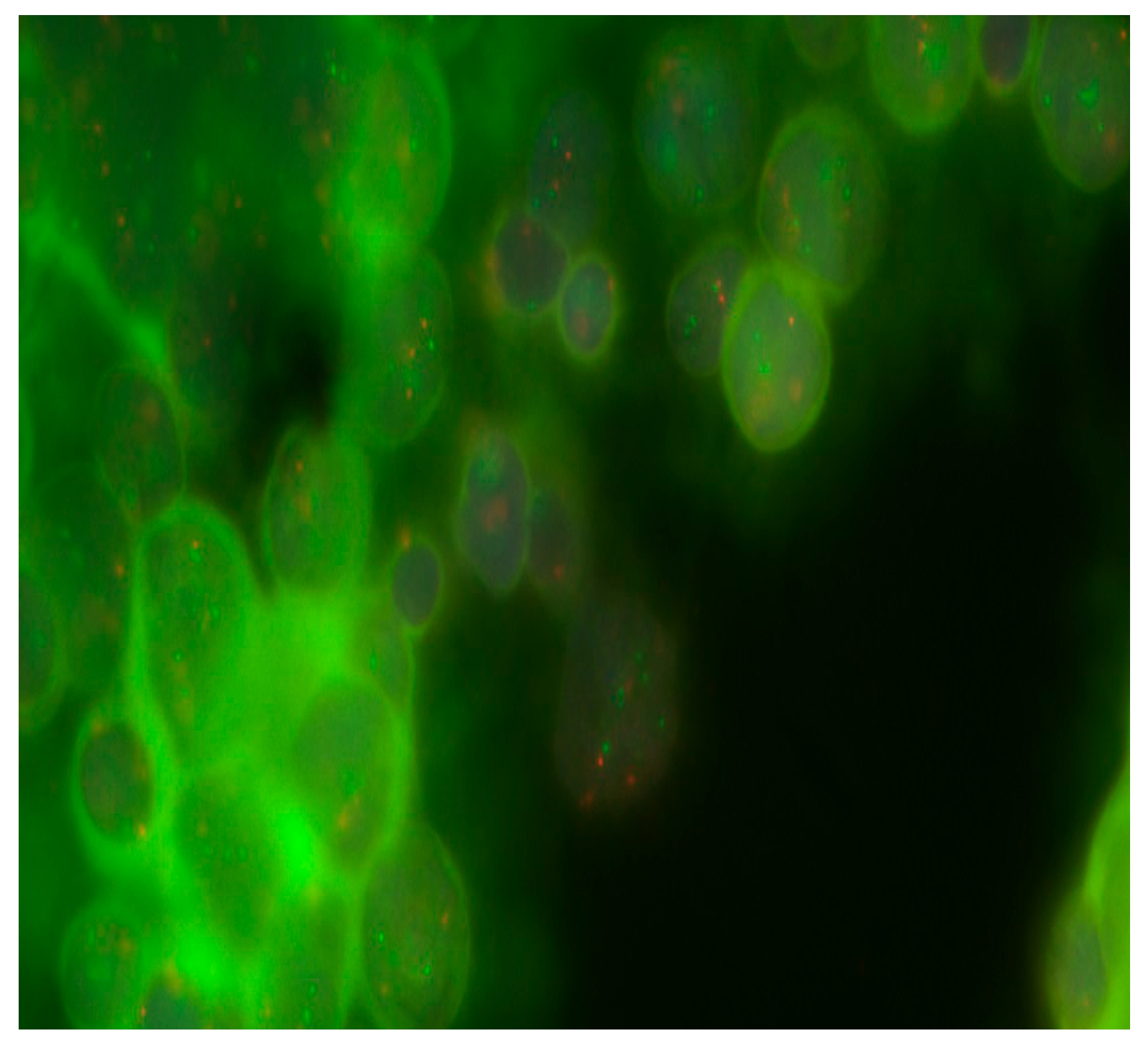
2.3. Determination of Cytogenetic Aberrations by FISH Analysis
2.3.1. Preparation of Samples for FISH Staining
2.3.2. Staining of Interphase Nuclei by FISH
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef]
- Choi, J.Y. Medulloblastoma: Current Perspectives and Recent Advances. Brain Tumor Res. Treat. 2023, 11, 28–38. [Google Scholar] [CrossRef]
- Komori, T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022, 39, 47–50. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Poppiti, R.J. Medulloblastoma cancer stem cells: Molecular signatures and therapeutic targets. J. Clin. Pathol. 2020, 73, 243–249. [Google Scholar] [CrossRef]
- Manoranjan, B.; Venugopal, C.; McFarlane, N.; Doble, B.W.; Dunn, S.E.; Scheinemann, K.; Singh, S.K. Medulloblastoma stem cells: Modeling tumor heterogeneity. Cancer Lett. 2013, 338, 23–31. [Google Scholar] [CrossRef]
- Kijima, N.; Kanemura, Y. Molecular Classification of Medulloblastoma. Neurol. Med. Chir. 2016, 56, 687–697. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef]
- Zou, H.; Poore, B.; Broniscer, A.; Pollack, I.F.; Hu, B. Molecular Heterogeneity and Cellular Diversity: Implications for Precision Treatment in Medulloblastoma. Cancers 2020, 12, 643. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Shao, Y.; Tang, L.; Gong, J. Multi-omics analysis of intertumoral heterogeneity within medulloblastoma uncharted-pathway subtypes. Brain Tumor Pathol. 2021, 38, 234–242. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef]
- Gold, M.P.; Ong, W.; Masteller, A.M.; Ghasemi, D.R.; Galindo, J.A.; Park, N.R.; Huynh, N.C.; Donde, A.; Pister, V.; Saurez, R.A.; et al. Developmental basis of SHH medulloblastoma heterogeneity. Nat. Commun. 2024, 15, 270. [Google Scholar] [CrossRef]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef]
- Williamson, D.; Schwalbe, E.C.; Hicks, D.; Aldinger, K.A.; Lindsey, J.C.; Crosier, S.; Richardson, S.; Goddard, J.; Hill, R.M.; Castle, J.; et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022, 40, 111162. [Google Scholar] [CrossRef]
- De Bortoli, M.; Castellino, R.C.; Lu, X.-Y.; Deyo, J.; Sturla, L.M.; Adesina, A.M.; Perlaky, L.; Pomeroy, S.L.; Lau, C.C.; Man, T.K.; et al. Medulloblastoma outcome is adversely associated with overexpression of EEF1D, RPL30, and RPS20 on the long arm of chromosome 8. BMC Cancer 2006, 6, 223. [Google Scholar] [CrossRef]
- McCabe, M.G.; Bäcklund, L.M.; Leong, H.S.; Ichimura, K.; Collins, V.P. Chromosome 17 alterations identify good-risk and poor-risk tumors independently of clinical factors in medulloblastoma. Neuro-Oncology 2011, 13, 376–383. [Google Scholar] [CrossRef]
- Bailey, S.; Jacobs, S.; Kourti, M.; Massimino, M.; Andre, N.; Doz, F.; Dufouri, C.; Vennarini, S.; Padovani, L.; Aquilina, K.; et al. Medulloblastoma therapy: Consensus treatment recommendations from SIOP-Europe and the European Reference Network. EJC Paediatr. Oncol. 2025, 5, 100205. [Google Scholar] [CrossRef]
- Ryan, S.L.; Schwalbe, E.C.; Cole, M.; Lu, Y.; Lusher, M.E.; Megahed, H.; O’Toole, K.; Nicholson, S.L.; Bognar, L.; Garami, M.; et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol. 2012, 123, 501–513. [Google Scholar] [CrossRef]
- Aldosari, N.; Bigner, S.H.; Burger, P.C.; Becker, L.; Kepner, J.L.; Friedman, H.S.; McLendon, R.E. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2002, 126, 540–544. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Danilenko, M.; Hill, R.M.; Crosier, S.; Ryan, S.L.; Williamson, D.; Castle, J.; Hicks, D.; Kool, M.; et al. Molecular and clinical heterogeneity within MYC-family amplified medulloblastoma is associated with survival outcomes: A multicenter cohort study Open Access. Neuro-Oncology 2025, 27, 222–236. [Google Scholar] [CrossRef]
- Kaur, K.; Kakkar, A.; Kumar, A.; Mallick, S.; Julka, P.K.; Gupta, D.; Suri, A.; Suri, V.; Sharma, M.C.; Sarkar, C. Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. 2015, 26, 334–343. [Google Scholar] [CrossRef]
- Pan, E.; Pellarin, M.; Holmes, E.; Smirnov, I.; Misra, A.; Eberhart, C.G.; Burger, P.C.; Biegel, J.A.; Feuerstein, B.G. Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin. Cancer Res. 2005, 11, 4733–4740. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.W.; Schlanstein, M.; Northcott, P.A.; Cho, Y.-J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Ramos, J.M. Fluorescent In Situ Hybridization (FISH). In Immunohistochemistry and Immunocytochemistry; Valle, L.D., Ed.; Springer Science Business Media: Berlin/Heidelberg, Germany, 2022; pp. 179–189. [Google Scholar]
- Zitterbart, K.; Filkova, H.; Tomasikova, L.; Necesalova, E.; Zambo, I.; Kantorova, D.; Slamova, I.; Vranova, V.; Zezulkova, D.; Pesakova, M.; et al. Low-level copy number changes of myc genes have a prognostic impact in medulloblastoma. J. Neuro-Oncol. 2011, 102, 25–33. [Google Scholar] [CrossRef]
- Hastings, R.J.; Moore, S.; Chia, N. ISCN 2024: An International System for Human Cytogenetic Nomenclature; Karger Publishers: Basel, Switzerland, 2024; p. 140. [Google Scholar] [CrossRef]
- van Belle, G.; Fisher, L.D.; Heagerty, P.J.; Lumley, T. Biostatistics: A Methodology for the Health Sciences; Fisher, L.D., van Belle, G., Eds.; Jonh Wiley and Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pfister, S.; Remke, M.; Benner, A.; Mendrzyk, F.; Toedt, G.; Felsberg, J.; Wittmann, A.; Devens, F.; Gerber, N.U.; Joos, S.; et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J. Clin. Oncol. 2009, 27, 1627–1636. [Google Scholar] [CrossRef]
- Moreno, D.A.; da Silva, L.S.; Zanon, M.F.; Bonatelli, M.; de Paula, F.E.; de Medeiros Matsushita, M.; Teixeira, G.R.; Santana, I.V.V.; Saggioro, F.; Neder, L.; et al. Single nCounter assay for prediction of MYCN amplification and molecular classification of medulloblastomas: A multicentric study. J. Neuro-Oncol. 2022, 157, 27–35. [Google Scholar] [CrossRef]
- Ryzhova, M.V.; Snigireva, G.P.; Golanov, A.V.; Zheludkova, O.G.; Trunin, Y.Y.; Antipina, N.A. Correct use of Kreatech DNA probes to detect MYC gene amplification in medulloblastomas by fluorescence in situ hybridization. Arkh Patol. 2019, 81, 66–72. [Google Scholar] [CrossRef]
- Park, A.K.; Lee, S.J.; Phi, J.H.; Wang, K.C.; Kim, D.G.; Cho, B.K.; Haberler, C.; Fattet, S.; Dufour, C.; Puget, S.; et al. Prognostic classification of pediatric medulloblastoma based on chromosome 17p loss, expression of MYCC and MYCN, and Wnt pathway activation. Neuro-Oncology 2012, 14, 203–214. [Google Scholar] [CrossRef]
- Park, A.K.; Lee, J.Y.; Cheong, H.; Ramaswamy, V.; Park, S.-H.; Kool, M.; Phi, J.H.; Choi, S.A.; Cavalli, F.; Taylor, M.D.; et al. Subgroup-specific prognostic signaling and metabolic pathways in pediatric medulloblastoma. BMC Cancer 2019, 19, 571. [Google Scholar] [CrossRef]
- Vriend, J.; Liu, X.-Q. Survival-Related Genes on Chromosomes 6 and 17 in Medulloblastoma. Int. J. Mol. Sci. 2024, 25, 7506. [Google Scholar] [CrossRef]
- Fuller, C.E.; Perry, A. Fluorescence in situ hybridization (FISH) in diagnostic and investigative neuropathology. Brain Pathol. 2002, 12, 67–86. [Google Scholar] [CrossRef]


| Patient No. | MYCN Amp | MYCC Amp | 17p Del | 17q Gain |
|---|---|---|---|---|
| 1 | - | - | + | + |
| 2 | - | - | + | - |
| 3 | - | - | - | - |
| 4 | - | - | - | + |
| 5 | - | - | n.d. | n.d. |
| 6 | - | - | - | + |
| 7 | amp | - | - | + |
| 8 | - | - | - | _ |
| 9 | - | - | - | + |
| 10 | - | - | n.d. | n.d. |
| 11 | - | - | - | - |
| 12 | - | - | n.d. | - |
| 13 | amp | - | n.d. | n.d. |
| 14 | - | amp | + | + |
| 15 | - | - | n.d. | n.d. |
| 16 | - | - | n.d. | n.d. |
| 17 | - | amp | - | - |
| 18 | - | - | n.d. | - |
| Clinical Criteria | Changes in the 17th Chromosome | No Changes in the 17th Chromosome | p Value |
|---|---|---|---|
| Patients | 7 | 6 | |
| Gender | 0.1643 | ||
| boys | 6 | 3 | |
| girls | 1 | 3 | |
| Age | |||
| younger than 3 years | 2 | 1 | 0.6115 |
| older than 3 years | 5 | 5 | |
| Histological type | |||
| desmoplastic | 1 | 1 | 0.9056 |
| classic | 6 | 5 | 0.9056 |
| anaplastic | - | ||
| Metastasis | |||
| yes | 3 | 1 | 0.3077 |
| no | 4 | 5 | 0.3077 |
| Tumor resection: | |||
| total | 2 | 2 | 0.8529 |
| subtotal | 5 | 4 | 0.8529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernov, A.; Batotsyrenova, E.; Zheregelya, S.; Pyurveev, S.; Kashuro, V.; Ivanov, D.; Galimova, E. The Prognostic Value of Amplification of the MYCC and MYCN Oncogenes in Russian Patients with Medulloblastoma. Diseases 2025, 13, 238. https://doi.org/10.3390/diseases13080238
Chernov A, Batotsyrenova E, Zheregelya S, Pyurveev S, Kashuro V, Ivanov D, Galimova E. The Prognostic Value of Amplification of the MYCC and MYCN Oncogenes in Russian Patients with Medulloblastoma. Diseases. 2025; 13(8):238. https://doi.org/10.3390/diseases13080238
Chicago/Turabian StyleChernov, Alexander, Ekaterina Batotsyrenova, Sergey Zheregelya, Sarng Pyurveev, Vadim Kashuro, Dmitry Ivanov, and Elvira Galimova. 2025. "The Prognostic Value of Amplification of the MYCC and MYCN Oncogenes in Russian Patients with Medulloblastoma" Diseases 13, no. 8: 238. https://doi.org/10.3390/diseases13080238
APA StyleChernov, A., Batotsyrenova, E., Zheregelya, S., Pyurveev, S., Kashuro, V., Ivanov, D., & Galimova, E. (2025). The Prognostic Value of Amplification of the MYCC and MYCN Oncogenes in Russian Patients with Medulloblastoma. Diseases, 13(8), 238. https://doi.org/10.3390/diseases13080238