Abstract
Background. Medulloblastoma (MB) prognosis and response to therapy depend largely on genetic changes in tumor cells. Many genes and chromosomal abnormalities have been identified as prognostic factors, including amplification of MYC oncogenes, gains in 1q and 17q, deletions in 10q and 21p, or isochromosomes 17 (i(17)(q10)). The frequency of these abnormalities varies greatly between ethnic populations, but the frequency of specific abnormalities, such as MYCC and MYCN amplification, 17q gain, and deletions, in the Russian population is unknown. Objective: The aim is to study the frequency of MYCC and MYCN amplifications, 17q gain, and 17p deletion and determine their prognostic value in Russian patients with MB. Methods. This study was performed on MB cells obtained from 18 patients (12 boys and 6 girls, aged between 3 months and 17 years, with a median age of 6.5 years). Determination of cytogenetic aberrations was carried out using FISH assays with MYCC-SO, MYCN-SO, and MYCN-SG/cen2 probes, as well as cen7/p53 dual color probes and PML/RARα dual color probes (Abbott Molecular, USA). One-way ANOVA and Fisher’s F-test were used to compare the two groups. The differences were considered significant when p < 0.05. Results. In 77.7% of patients (14/18), the classical type of MB was present; in 16.7% (3/18), desmoplastic type; and in 5.6% (1/18), nodular desmoplasic types of neoplasms. Amplification of MYC genes was detected in 22.2% of Russian patients (n = 4 out of 18). Patients with MYC amplification had the worst overall survival (OS: 0% vs. 68%, p = 0.0004). Changes on the 17th chromosome were found in 58.3% of patients. Deletion of 17p occurred in 23.1%, and gain of 17q occurred in 46.2%. There were no significant differences in OS, clinical signs, or the presence of additional 17q material or 17p deletion among patients with MB. Conclusions: Amplification of the MYC gene is a predictor of poor overall survival to therapy and a high risk of metastatic relapse. This allows us to more accurately stratify patients into risk groups in order to determine the intensity and duration of therapy.
1. Introduction
Cancer is currently one of the most common and fatal diseases among humans. The main treatments for cancer include surgery, radiation therapy and chemotherapy [1]. However, radiotherapy and chemotherapy have low specificity, leading to damage to both tumor and healthy tissue, resulting in a low survival rate for treated patients due to drug resistance, tumor relapse, and metastasis. Recently, new targeted immunotherapy methods have been developed, including antibody-drug conjugates (ADCs), chimeric antigen receptor (CAR)-T therapy, and bispecific T-cell engaged therapy (BiTE) [1].
The most common (15.0–20.0%) malignant brain tumor in children is medulloblastoma (MB) [2]. Currently, there is increasing evidence that prognosis and response to therapy depend largely on the molecular and genetic features of tumor cells. According to WHO recommendations, at least four different histological types of malignant brain tumors (MBs) are recognized. Based on morphological criteria for histologic classification, these MBs can be divided into four categories: classic (CLA), desmoplastic-nodular (DN), malignant with extensive nodularity (MBE), and large-cell anaplastic (LCA) [3].
MBs are localized in the cerebellum and arise from neural progenitor stem cells (NSCs) [4]. These cells represent a small population of slowly growing cells that accumulate mutations and are resistant to treatment. If therapy has a damaging effect on a tumor, NSCs begin to proliferate and differentiate into other types of cancer cells, creating cellular and molecular diversity [5]. Histologically, MBs may arise from cells of the inferior rhomboid fossa of the brainstem, granule cell precursors in the external granular layer of the cerebellum, or unipolar brush cells of the superior rhomboidal fossa and cells from glutamatergic nuclei in the cerebellar cortex. Molecular genetic studies using transcriptome analysis and gene methylation profiling have found that these cells differ in their protein and gene expression, leading to the identification of four molecular subtypes: WNT (inferior), SHH (granule cell precursor), and groups 3 and 4 formed from cells of the superior rhomboidal fossa, and cells of the glutamatergic nuclei of the cerebellum [6,7]. Each subgroup has different genetic drivers, clinical signs, and prognoses. The WNT subtype of MB is observed in 10% of patients who have the best prognosis. Their survival rate reaches 90% [8]. Tumors classified as the WNT subtype contain mutations in the WNT pathway, including WNTα, WNT β, CTNNB1, deletion of chromosome 6, and nuclear staining for β-catenin [9,10]. The SHH subtype occurs in 30% of MB patients, including young children (<3 years) and adults (>16 years) [10,11]. It is characterized by activation or mutations in the SHH signaling pathway, such as PTCH1, SMO, and SUFU, as well as amplification of GLI1 and GLI2. Patients with tumors from this subgroup have an intermediate prognosis. Cytogenetic analysis of tumor cells from young children reveals deletions of chromosome 10q and amplification of the MYCN gene. In adult MB patients, deletions of chromosomes 10, 2, and 17 and gain of 17q and/or amplification of the GLI2 gene are observed, which is associated with a poorer prognosis than in children [11]. Group 3 MB patients have worse clinical prognosis, with a 5-year survival rate ranging from 39% to 58%, depending on the patient’s age. Metastasis is recorded in 50% of these cases [12]. Cytogenetic markers in this patient group include an isochromosome i17q in 40–50% of cases; deletions of chromosomes 8 and 10q; and gains of 1q, 7, and 18. MYCC and MYCN amplification, SMARCA4 mutations, and GABRA5 gene overexpression are also present [12]. MB patients in group 4 account for 35–40% of all MBs. Driver mutations in this group include overexpression of PRDM6 and GFI1/GFI1B, KDM6A, KMT2C, and ZMYM3, as well as amplification of MYCN, OTX 2, and CDK6 [13]. In cytogenetic analysis, the most common (80%) aberration in group 4 tumors is a gain of chromosome 17q, and other mutations include gains of chromosomes 7 and 18q, as well as deletions of chromosomes 8q, 8p, 11p, and X [13].
A lot of these genetic and cytogenetic alterations are used in clinical laboratory diagnostics as biomarkers for stratifying patients into molecular subtypes and clinically favorable and unfavorable risk groups. In molecular groups 3 and 4, which are characterized by the worst clinical prognosis, the most common cytogenetic changes include amplification of the MYCC (8q24.12-q24.13) and MYCN (2p24) genes, as well as deletion or gain in isochromosome i(17)(10) [14,15]. Amplification of the MYCC oncogene was adopted by the International Society for Pediatric Oncology and the Primitive Neuroectodermal Tumour Committee of the European Society of Oncology (SIOP-Europe-PNET) as an official molecular stratification factor for MB in children [16]. For MB, a relationship has been established between the risk of patient mortality and the number of MYCC and MYCN oncogene copies. The probability of death increases 3.5-fold with an increase of 10 MYCC copies and 1.7-fold with each additional MYCN copy [17]. On the other hand, the frequency of MYCC and MYCN amplification in isochromosome 17 (i(17)(q10)) varies in different ethnic populations. For example, in the USA, the frequency of amplification of MYCC is 5.2% (4 out of 77 tumors) [18]; in Europe, the frequency for MYCC is 3–6% [19]; and in India, it reaches 11% (7 out of 62 cases) [20]. The frequency of i(17)(q10) in MB patients from the USA occurred was 14% (5/35) [21]. Kool et al. conducted an international study involving 550 patients with MB, in which the frequencies of 17p deletions and 17q gains in group 3 were 42% and 62%, respectively, and 73% in group 4 [22]. At the same time, the frequencies of MYCC, MYCN amplification, 17q gains and 17p deletions, and i(17)(q10), in the Russian population remain unknown. In addition, although the impact of MYCC and MYCN amplifications, 17q gains, and 17p deletions on the OS of MB patients has been proven, it is still necessary to explore this relationship with the frequencies of MYCC, MYCN, and i(17)(q10) in the context of the Russian population.
Objective: The aim is to study the frequency of MYCC and MYCN amplification, 17q gains, and 17p deletion and determine their prognostic value in Russian MB patients.
2. Materials and Methods
2.1. Clinical Characteristics of MB Patients
This study was conducted on MB cells obtained from biopsies of 18 patients (12 boys and 6 girls), aged between 3 months and 17 years (median age 6.5 years), who were treated in the children’s neurosurgery department of the Almazov National Medical Research Centre, Ministry of Health, Saint Petersburg, Russia, between 2019 and 2024.
2.2. Preparation of Tumor Smears
To conduct this study, smear-imprints of different tumor sections were prepared. Different fragments of the same neoplasm were applied to defatted slides several times so that the cells remained on them. The quality of the material was checked under a light microscope with a magnification of ×100 times. The slides were then dried and left for 24 h at room temperature. They were then fixed in 400 µL of Carnoy’s solution (3:1 methanol/acetic acid, Sigma-Aldrich, St. Louis, MO, USA) overnight [23].
2.3. Determination of Cytogenetic Aberrations by FISH Analysis
2.3.1. Preparation of Samples for FISH Staining
The slides treated with fixative were dried for 2 h at room temperature and then transferred to a thermostat at 56 °C for 2–3 h. After cooling to room temperature, the area with the greatest accumulation of cells was identified under a light microscope. The slide was then treated with RNase (10 µg/mL for 15 min at 37 °C) and proteinase K (also 10 µg/mL for another 30 min at the same temperature), followed by two washes in distilled water and drying at room temperature. Following this treatment, the slides were immersed in sodium citrate buffer (0.6 M chloride and 0.06 M sodium citrate at pH 7.2 from Sigma-Aldrich, USA) for 30 min at 38 °C; then, dehydrated using successive concentrations of alcohol (70%, 80%, and 96%) for 2 min each; and finally, dried at ambient temperature for 15–20 min.
2.3.2. Staining of Interphase Nuclei by FISH
DNA probes (7 μL Vysis LSI/WSP buffer, 2 μL water, and 1 μL DNA probe) were applied to slides with cell samples and covered with a coverslip (22 × 22 nm). To determine the amplification of MYC (MYCC and MYCN genes) (copy count > 40–50 copies), we used 0.5 μL locus-specific Vysis LSI DNA probes: MYCC (8q24.12-q24.3) and MYCN (2p24). These DNA probes were labeled with Spectrum Orange fluorescence (Abbott Molecular, Des Plaines, IL USA). We also used cen17/p53 Dual Color chromosome 17 centromeric probes (Abbott Molecular, USA) to visualize gains or deletions of the TP53 gene on chromosome 17. After that, we performed DNA denaturation at 73 °C for 5 min, and then, hybridization was carried out in a humidified atmosphere at 37 °C for 16–18 h in a hybridizer (Dako, Carpinteria, CA, USA). Slides were immersed in 0.4× sodium citrate buffer saline solution with 0.3% nonylphenoxypolyethoxyethanol NP40 detergent (Thermo Scientific, Waltham, MA, USA) and heated to 73 °C for 2 min. Slides were then transferred to a sodium citrate-buffered saline solution (0.6 M chloride, 0.06 M citrate, Sigma-Aldrich, USA) containing 0.1% NP-40 for 10 min. Samples were then dehydrated in a series of increasing alcohol strengths (70%, 80%, and 96%) and dried at room temperature for 15–20 min in the dark. Finally, a working solution of DAPI (1.5 μg/mL) was applied to the dried slides and left to stain for 10–15 min, after which a cover glass was placed over the stained area [23,24]. The preparations were analyzed using a Leica DMLB fluorescence microscope (Germany), with filters for DAPI, Texas Red, and FITC, at a total magnification of 1000 (100× oil immersion objective, 10× eyepiece). At least 200 cells per field were counted, and their number was recorded for each set of oncogenes. A total of 24 slides were analyzed, containing 4800 cells. The samples were registered according to the International Nomenclature for Human Cytogenetics (ISCN) 2024 [25].
2.4. Statistical Analysis
The results were presented as the arithmetic mean plus/minus the standard error of the mean for the sample (M ± m). One-way ANOVA and the F-test (Fisher) were used to compare the two groups. The differences were considered reliable at a significance level of p < 0.05 [26]. Descriptive statistics, ANOVA tests, and OS analyses were performed using GraphPad Prism version 8.01 (21 September 2020) from San Diego, CA, USA.
3. Results
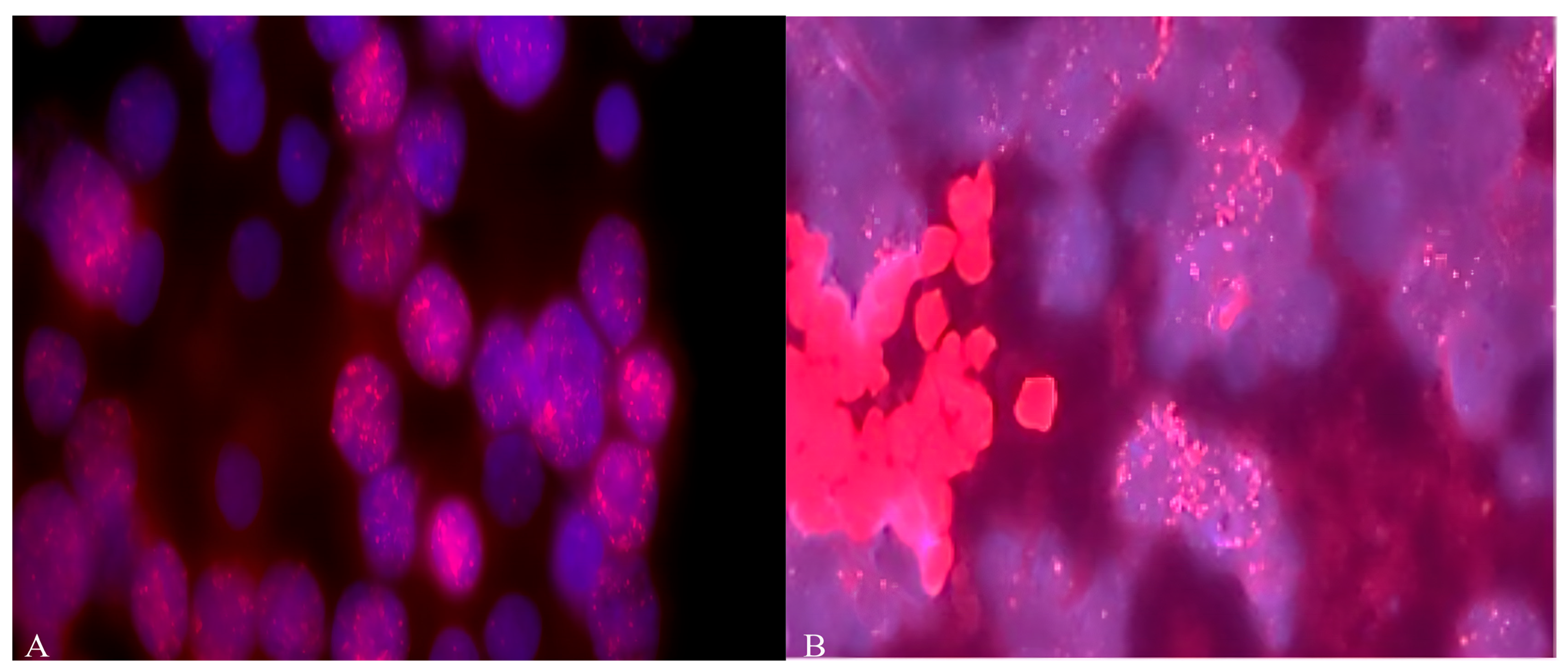
For 77.7% (14/18) of the patients, the classic MB type was found. In 16.7% (3/18), the desmoplastic type was detected, and in 5.6%, a desmoplasic-nodular neoplasm was found [27]. As shown in Figure 1 and Table 1, diffuse amplification of the MYCN and MYCC genes was detected in more than 22% (4 out of 18) of MB samples from Russian patients, with more than 40–50 copies.
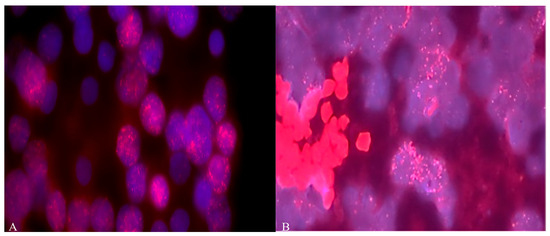
Figure 1.
(A) Amplification of MYCN and (B) MYCC (copy number more than 60) oncogenes in MB cells from Russian patients. Objective magnification ×100. Blue color indicates DAPI nuclei, red dots indicate staining with MYCC (8q24.12-q24.3) and MYCN (2p24) probes.

Table 1.
Amplification of MYCC and MYCN oncogenes, and 17p del and 17q gain aberrations in MB cells from Russian patients.
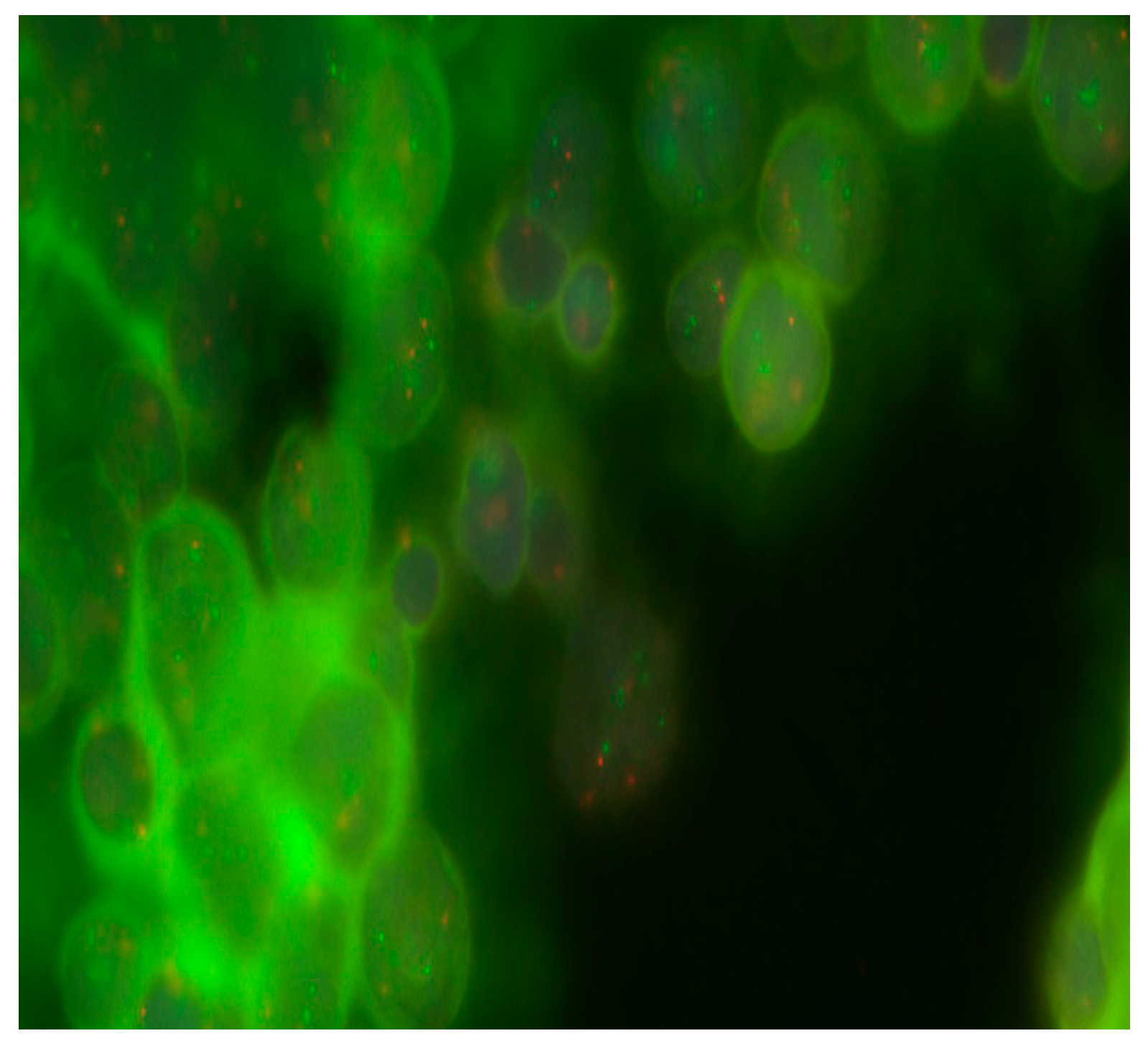
Aberrations in the 17th chromosome were detected in 58.3% (7/13) of patients. Deletions in 17p were detected in 23.1% (3/13), and 17q gains were found in 46.2% (6/13). Table 1 and Figure 2 show that in one case, the presence of i(17)(q10) was identified.
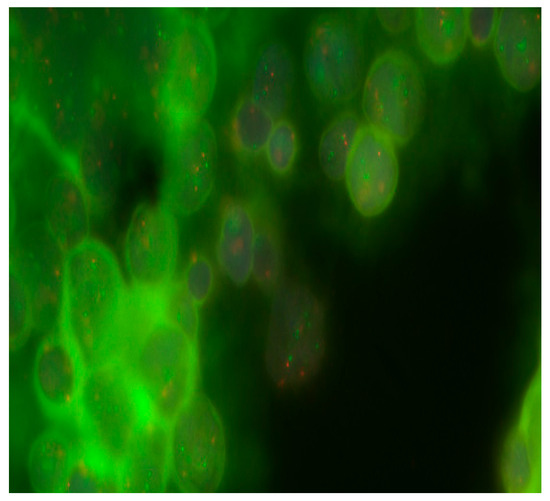
Figure 2.
Detection of 17q gain in MB cells from Russian patients. Objective magnification ×100. Green dots are cen17/p53 Dual Color chromosome 17 centromeric probes.
We did not find statistically significant differences between the presence of the 17q and 17p chromosome abbreviations and clinical symptoms in MB patients (Table 2).

Table 2.
Dependence between 17q and 17p chromosome abbreviations and the clinical signs of patients with MB from Russia (n = 13).
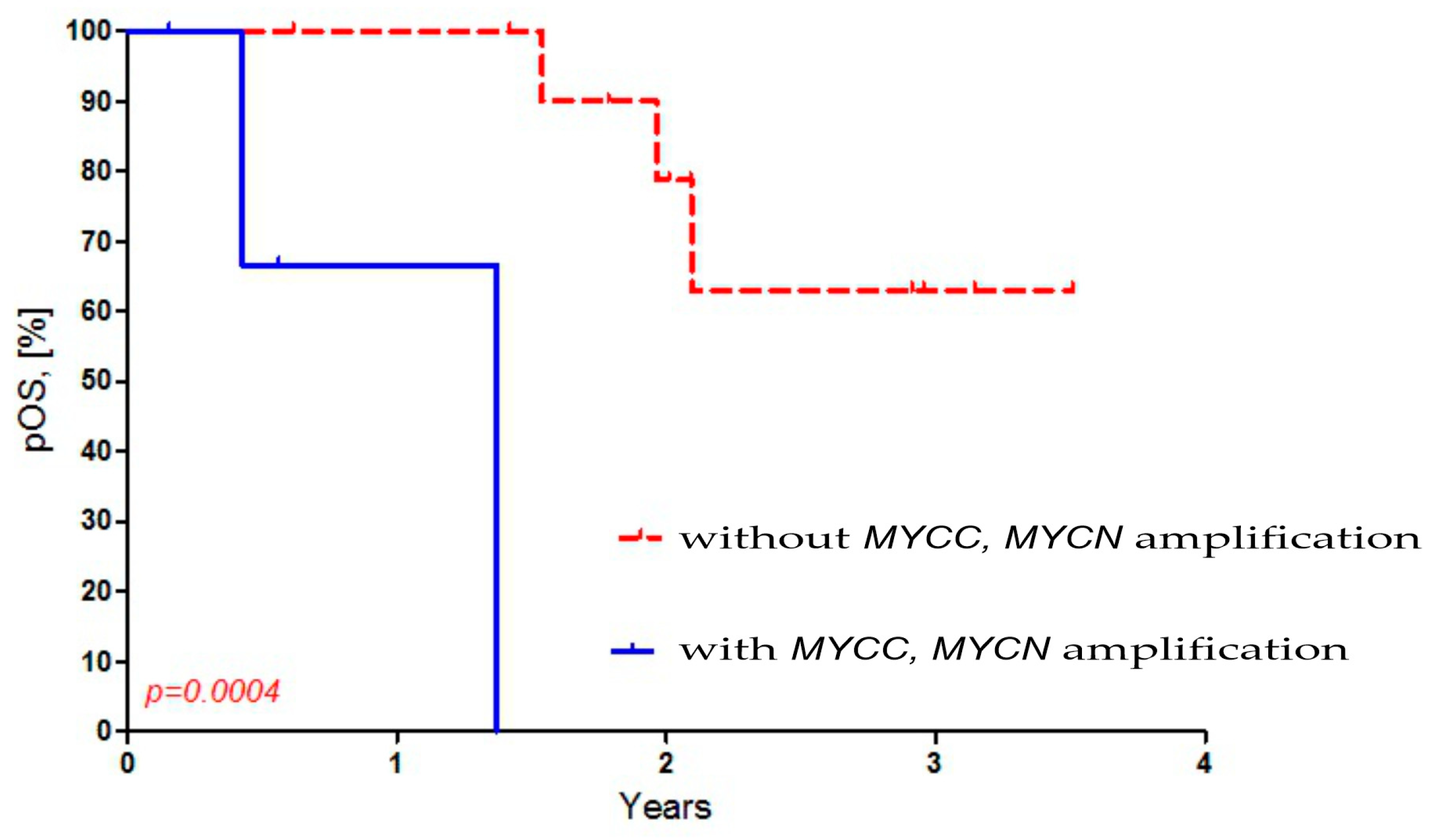
Then, we compared the presence of MYCC and MYCN amplification in MB cells to the overall survival (OS) in Russian patients. We observed a worse OS in Russian MB patients with both MYCC and amplification (p = 0.0004). The OS for Russian MB patients who had amplification was 0%, while it was 68% for those without amplification (Figure 3).
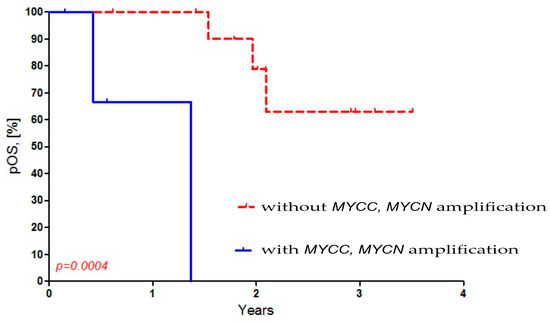
Figure 3.
Dependence of overall survival of Russian patients on MYCC and MYCN amplification in MB cells.
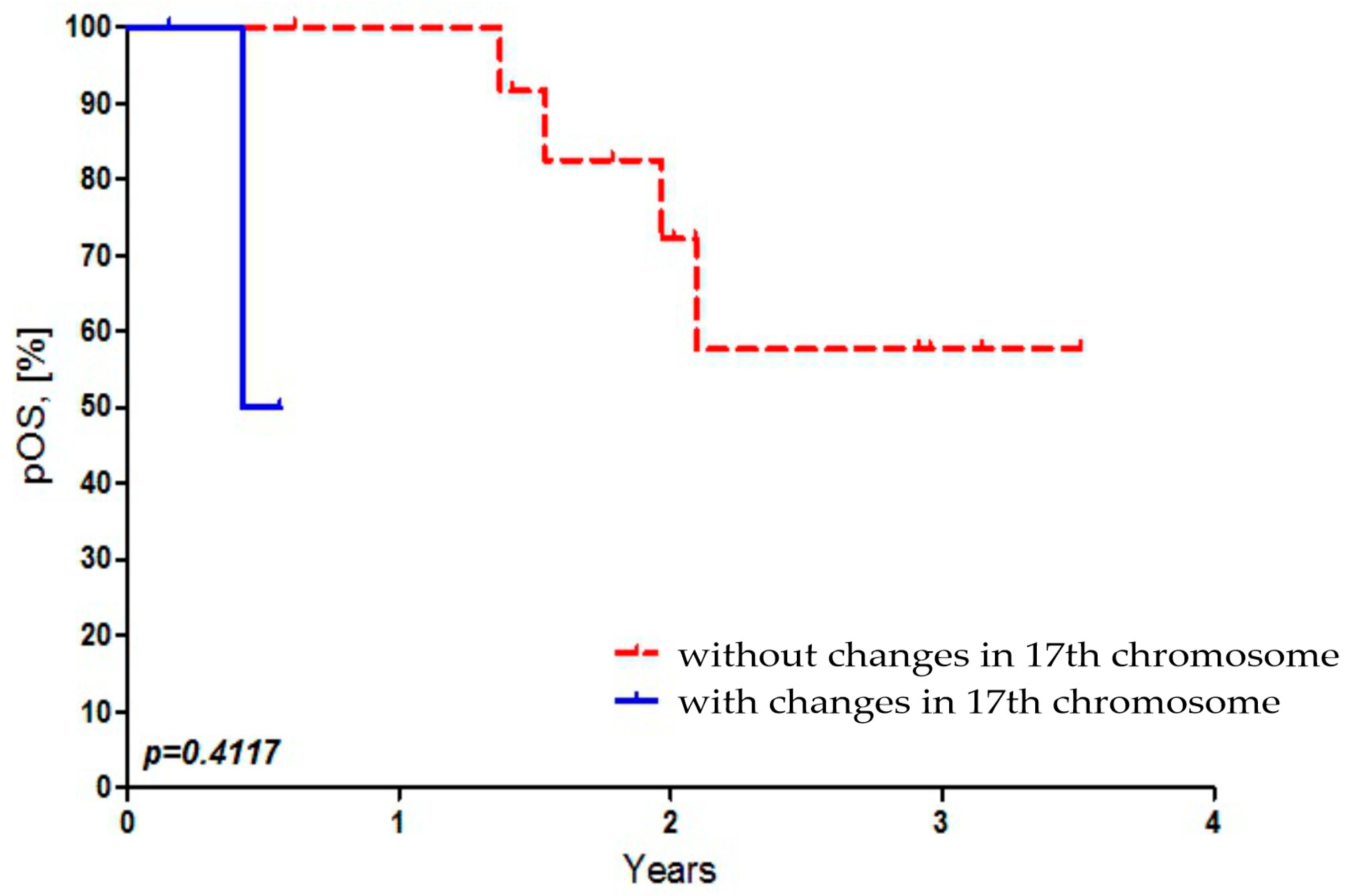
There was no statistically significant dependence found between OS of Russian patients and the presence of 17p and 17q abbreviations in MB cells (Figure 4).
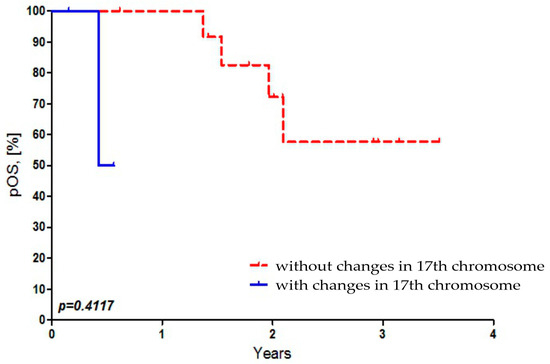
Figure 4.
Dependence of overall survival of Russian patients on the presence of 17p and 17q abbreviations in MB cells.
4. Discussion
Our results revealed MYCC and MYCN amplification in 22.2% of Russian MB patients of Slavic origin, which was associated with low OS. Figure 1 and Figure 3 show this association, as does Table 1. Pfister et al. demonstrated that the amplification of MYCN and MYCC is detected in 4% (3/75) and 6% (5/75), respectively, of German MB cases and is associated with high tumor malignancy and a low (13%) five-year OS for patients. The absence of amplification in these cases is associated with a high (73.0%) five-year survival rate for the patients [28]. In contrast, Moreno et al., using FISH, found MYCN amplification in 3.2% (3/92) of SHH subtype MBs in Brazilian patients. The authors also successfully used an nCounter analysis to stratify MBs into molecular subgroups and predict MYCN amplification [29]. Aldosari et al. detected MYCN amplification by FISH in 5.2% (4/77) of MBs from US patients [18]. A recent study conducted by Schwalbe et al. on 1600 patients with MB characterized the clinical and molecular features of the disease, including amplification of MYCC and MYCN oncogenes [19]. The amplification frequencies were 4% (64/1600) for the MYCC oncogene and 6% (95/1, 600) for the MYCN oncogene. The third molecular subtype, represented by MYC-amplified SHH-MB, was obtained from patients aged over three. These patients often had metastases, associated with a five-year progression-free survival rate of 11% [19]. There is only one publication on the Internet by Ryzhova et al. (2019), in which the authors used FISH probes to amplify MYCC in four Russian patients with MB [30]. However, in this publication, the authors used only MYCC probes, not MYCN or 17p or 17q. The number of patients was too small to estimate the frequency of amplification of these oncogenes among the Russian population. Therefore, our study is the first to illustrate the frequency of MYCC and MYCN amplification among MB patients in Russia. In addition, our findings confirm that the frequency of both MYCC and MYCN amplification in Russian patients with MB is associated with worse OS.
Our data coincide with the results of Pfister et al. (2019) [29], who established the presence of deletions in the 17th chromosome in 53% (42/80) of patients. We did not find an association between the deletion of 17p or gain of 17q and OS in Russian MB patients (Figure 4). In contrast, Park et al. (2020) established this association between the deletion of 17p and poor OS among 30 MB patients [31]. However, this association was not confirmed in independent data cohorts (n = 100). The authors suggest that this discrepancy may be due to the interaction between loss of tumor suppressor genes (chromosome 17p) and high expression of MYCC or MYCN oncogenes. Stratifying patients based on these two factors revealed different survival rates between subgroups: the 5-year OS probability ranged from 19% to 81%. Park et al. showed that age is a significant prognostic factor, after adjusting for chromosome 17, MYCC status, and MYCN expression. The reduction in OS at less than 3 years is more significant in patients with high expression of either MYCC, MYCN, or 17p del [31]. The same authors in a later study indicate that poor OS in the third subgroup of MB is associated with high expression of MYC and increased expression of genes such as PDGFRA, IGF1R, and FGF2, as well as the i(17)(q10), PI3K/AKT, and MAPK/ERK pathways [32]. It is possible that the differences between our data and those of Park et al. on chromosome 17 [31] may be due to the small number of patients (n = 6) in whom these changes were detected (Table 1). Interestingly, in a recent study (2024), Vriend and Liu identified 967 genes associated with the OS of MB patients using a gene expression dataset and a Cox proportional hazards regression model [33]. These genes were localized to chromosomes 6 and 17 in MB cells. They include the oncogene high-mobility group protein HMG-I/HMG-Y (HMGA1) on chromosome 6, whose high expression is associated with poor OS in patients. Genes on chromosomes 17p del and 17q gain that are associated with telomere lengthening are linked to low OS in MB patients [33].
FISH analysis is the “gold standard” in cytogenetics because it allows for quantitative detection of aberrant changes with high sensitivity and specificity, even in very small numbers of cells among cells with a normal karyotype, using fresh and frozen tissue, cytological preparations, and formalin-fixed paraffin embedded tissue. In this respect, validation of this method is generally not required [23,34].
The present pilot study has several limitations. First, it has a small sample size. It is possible that, with an increased number of MB patients, the frequency of MYCC and MYCN amplifications, and 17p and 17q abbreviations in MB will change during clinical trials. Second, the retrospective design of this study may lead to changes in OS for patients. Third, MB is a heterogeneous tumor that includes four or more cellular and molecular subtypes. These characteristics affect OS patients, which, given their small sample, may affect the frequency of MYCC and MYCN amplification, as well as chromosome 17 abnormalities, and a different life expectancy. Also, FISH analysis does not allow for the detection of large genomic changes. It is characterized by signal attenuation, a limited number of commercial probes, and cytological artifacts [34].
5. Conclusions
The obtained results of this pilot study indicate for the first time a high frequency of detection of MYCC and MYCN amplification, and 17p del/17q gain abbreviations in a small cohort of MB patients from Russia. However, an increase in the number of MB patients due to multicenter collaboration will allow us to correct and verify the frequency of cytogenetic abnormalities in Russian patients with MB. In this group of people from Russia, the association between MYCC, MYCN, and OS of patients was confirmed. Detection of MYCC and MYCN amplification by i-FISH analysis allows for the classification of patients’ tumors into three or four molecular subtypes associated with an unfavorable clinical prognosis. This allows for the correction of treatment protocols (drugs, regimens, and doses) before its start and thereby increases therapy effectiveness.
Author Contributions
Conceptualization, A.C. and E.G.; Software, A.C. and S.P.; Formal analysis, E.B., S.P., S.Z., and V.K.; Investigation, A.C., S.P., and E.G.; Statistical analysis, A.C., S.Z., and S.P.; Writing—original draft, A.C.; Writing—review and editing, A.C., E.B., S.P., S.Z., E.G., V.K., and D.I.; Supervision, E.G.; Project administration, E.G., V.K., and D.I.; Funding acquisition, D.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation for the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences State assignments, 075-00263-25-00.
Institutional Review Board Statement
This investigation was completed in accordance with the Helsinki declaration and approved by the Ethics committee of the institute of experimental medicine (No. 6/20, from 21 October 2020).
Informed Consent Statement
Informed consent was obtained from all patients of this study.
Data Availability Statement
Experimental data can be provided by the authors upon request.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef]
- Choi, J.Y. Medulloblastoma: Current Perspectives and Recent Advances. Brain Tumor Res. Treat. 2023, 11, 28–38. [Google Scholar] [CrossRef]
- Komori, T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022, 39, 47–50. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Poppiti, R.J. Medulloblastoma cancer stem cells: Molecular signatures and therapeutic targets. J. Clin. Pathol. 2020, 73, 243–249. [Google Scholar] [CrossRef]
- Manoranjan, B.; Venugopal, C.; McFarlane, N.; Doble, B.W.; Dunn, S.E.; Scheinemann, K.; Singh, S.K. Medulloblastoma stem cells: Modeling tumor heterogeneity. Cancer Lett. 2013, 338, 23–31. [Google Scholar] [CrossRef]
- Kijima, N.; Kanemura, Y. Molecular Classification of Medulloblastoma. Neurol. Med. Chir. 2016, 56, 687–697. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef]
- Zou, H.; Poore, B.; Broniscer, A.; Pollack, I.F.; Hu, B. Molecular Heterogeneity and Cellular Diversity: Implications for Precision Treatment in Medulloblastoma. Cancers 2020, 12, 643. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Shao, Y.; Tang, L.; Gong, J. Multi-omics analysis of intertumoral heterogeneity within medulloblastoma uncharted-pathway subtypes. Brain Tumor Pathol. 2021, 38, 234–242. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef]
- Gold, M.P.; Ong, W.; Masteller, A.M.; Ghasemi, D.R.; Galindo, J.A.; Park, N.R.; Huynh, N.C.; Donde, A.; Pister, V.; Saurez, R.A.; et al. Developmental basis of SHH medulloblastoma heterogeneity. Nat. Commun. 2024, 15, 270. [Google Scholar] [CrossRef]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef]
- Williamson, D.; Schwalbe, E.C.; Hicks, D.; Aldinger, K.A.; Lindsey, J.C.; Crosier, S.; Richardson, S.; Goddard, J.; Hill, R.M.; Castle, J.; et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022, 40, 111162. [Google Scholar] [CrossRef]
- De Bortoli, M.; Castellino, R.C.; Lu, X.-Y.; Deyo, J.; Sturla, L.M.; Adesina, A.M.; Perlaky, L.; Pomeroy, S.L.; Lau, C.C.; Man, T.K.; et al. Medulloblastoma outcome is adversely associated with overexpression of EEF1D, RPL30, and RPS20 on the long arm of chromosome 8. BMC Cancer 2006, 6, 223. [Google Scholar] [CrossRef]
- McCabe, M.G.; Bäcklund, L.M.; Leong, H.S.; Ichimura, K.; Collins, V.P. Chromosome 17 alterations identify good-risk and poor-risk tumors independently of clinical factors in medulloblastoma. Neuro-Oncology 2011, 13, 376–383. [Google Scholar] [CrossRef]
- Bailey, S.; Jacobs, S.; Kourti, M.; Massimino, M.; Andre, N.; Doz, F.; Dufouri, C.; Vennarini, S.; Padovani, L.; Aquilina, K.; et al. Medulloblastoma therapy: Consensus treatment recommendations from SIOP-Europe and the European Reference Network. EJC Paediatr. Oncol. 2025, 5, 100205. [Google Scholar] [CrossRef]
- Ryan, S.L.; Schwalbe, E.C.; Cole, M.; Lu, Y.; Lusher, M.E.; Megahed, H.; O’Toole, K.; Nicholson, S.L.; Bognar, L.; Garami, M.; et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol. 2012, 123, 501–513. [Google Scholar] [CrossRef]
- Aldosari, N.; Bigner, S.H.; Burger, P.C.; Becker, L.; Kepner, J.L.; Friedman, H.S.; McLendon, R.E. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2002, 126, 540–544. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Danilenko, M.; Hill, R.M.; Crosier, S.; Ryan, S.L.; Williamson, D.; Castle, J.; Hicks, D.; Kool, M.; et al. Molecular and clinical heterogeneity within MYC-family amplified medulloblastoma is associated with survival outcomes: A multicenter cohort study Open Access. Neuro-Oncology 2025, 27, 222–236. [Google Scholar] [CrossRef]
- Kaur, K.; Kakkar, A.; Kumar, A.; Mallick, S.; Julka, P.K.; Gupta, D.; Suri, A.; Suri, V.; Sharma, M.C.; Sarkar, C. Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. 2015, 26, 334–343. [Google Scholar] [CrossRef]
- Pan, E.; Pellarin, M.; Holmes, E.; Smirnov, I.; Misra, A.; Eberhart, C.G.; Burger, P.C.; Biegel, J.A.; Feuerstein, B.G. Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin. Cancer Res. 2005, 11, 4733–4740. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.W.; Schlanstein, M.; Northcott, P.A.; Cho, Y.-J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Ramos, J.M. Fluorescent In Situ Hybridization (FISH). In Immunohistochemistry and Immunocytochemistry; Valle, L.D., Ed.; Springer Science Business Media: Berlin/Heidelberg, Germany, 2022; pp. 179–189. [Google Scholar]
- Zitterbart, K.; Filkova, H.; Tomasikova, L.; Necesalova, E.; Zambo, I.; Kantorova, D.; Slamova, I.; Vranova, V.; Zezulkova, D.; Pesakova, M.; et al. Low-level copy number changes of myc genes have a prognostic impact in medulloblastoma. J. Neuro-Oncol. 2011, 102, 25–33. [Google Scholar] [CrossRef]
- Hastings, R.J.; Moore, S.; Chia, N. ISCN 2024: An International System for Human Cytogenetic Nomenclature; Karger Publishers: Basel, Switzerland, 2024; p. 140. [Google Scholar] [CrossRef]
- van Belle, G.; Fisher, L.D.; Heagerty, P.J.; Lumley, T. Biostatistics: A Methodology for the Health Sciences; Fisher, L.D., van Belle, G., Eds.; Jonh Wiley and Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pfister, S.; Remke, M.; Benner, A.; Mendrzyk, F.; Toedt, G.; Felsberg, J.; Wittmann, A.; Devens, F.; Gerber, N.U.; Joos, S.; et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J. Clin. Oncol. 2009, 27, 1627–1636. [Google Scholar] [CrossRef]
- Moreno, D.A.; da Silva, L.S.; Zanon, M.F.; Bonatelli, M.; de Paula, F.E.; de Medeiros Matsushita, M.; Teixeira, G.R.; Santana, I.V.V.; Saggioro, F.; Neder, L.; et al. Single nCounter assay for prediction of MYCN amplification and molecular classification of medulloblastomas: A multicentric study. J. Neuro-Oncol. 2022, 157, 27–35. [Google Scholar] [CrossRef]
- Ryzhova, M.V.; Snigireva, G.P.; Golanov, A.V.; Zheludkova, O.G.; Trunin, Y.Y.; Antipina, N.A. Correct use of Kreatech DNA probes to detect MYC gene amplification in medulloblastomas by fluorescence in situ hybridization. Arkh Patol. 2019, 81, 66–72. [Google Scholar] [CrossRef]
- Park, A.K.; Lee, S.J.; Phi, J.H.; Wang, K.C.; Kim, D.G.; Cho, B.K.; Haberler, C.; Fattet, S.; Dufour, C.; Puget, S.; et al. Prognostic classification of pediatric medulloblastoma based on chromosome 17p loss, expression of MYCC and MYCN, and Wnt pathway activation. Neuro-Oncology 2012, 14, 203–214. [Google Scholar] [CrossRef]
- Park, A.K.; Lee, J.Y.; Cheong, H.; Ramaswamy, V.; Park, S.-H.; Kool, M.; Phi, J.H.; Choi, S.A.; Cavalli, F.; Taylor, M.D.; et al. Subgroup-specific prognostic signaling and metabolic pathways in pediatric medulloblastoma. BMC Cancer 2019, 19, 571. [Google Scholar] [CrossRef]
- Vriend, J.; Liu, X.-Q. Survival-Related Genes on Chromosomes 6 and 17 in Medulloblastoma. Int. J. Mol. Sci. 2024, 25, 7506. [Google Scholar] [CrossRef]
- Fuller, C.E.; Perry, A. Fluorescence in situ hybridization (FISH) in diagnostic and investigative neuropathology. Brain Pathol. 2002, 12, 67–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).