Therapeutic Implications of Menin Inhibitors in the Treatment of Acute Leukemia: A Critical Review
Abstract
1. Introduction
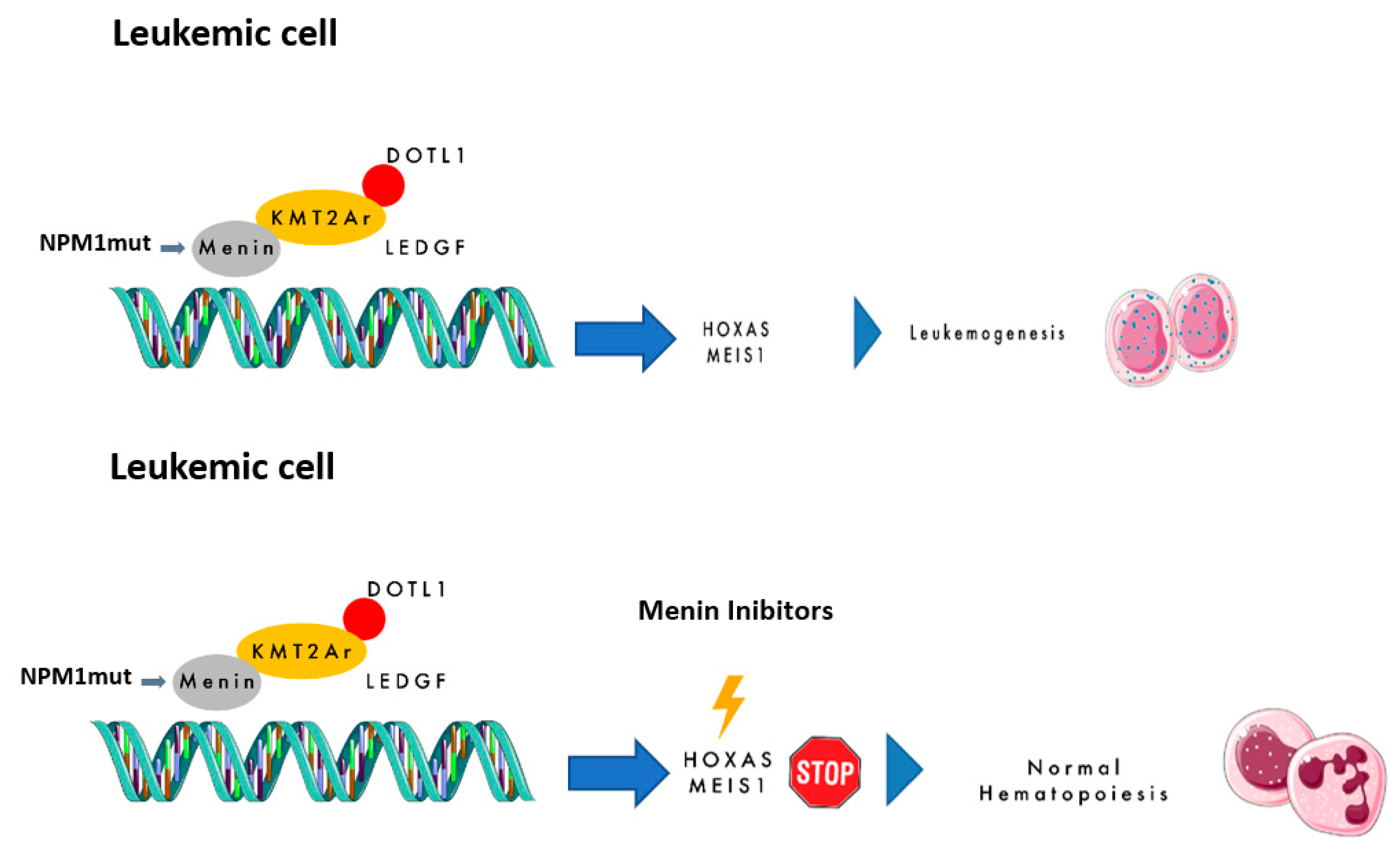
2. Menin Protein: From Physiological Role to Leukemogenesis
2.1. Revumenib
2.1.1. Revumenib Single Agent
2.1.2. Revumenib in Combination with Other Agents
2.1.3. Considerations on Revumenib
2.2. Ziftomenib
2.2.1. Ziftomenib Single Agent
2.2.2. Ziftomenib in Combination with Other Agents
2.2.3. Considerations on Ziftomenib
2.3. Bleximenib
2.3.1. Bleximenib Single Agent
2.3.2. Bleximenib in Combination with Other Agents
2.3.3. Considerations on Bleximenib
2.4. Enzomenib
Consideration of Enzomenib
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Döhner, H.; Campbell, P.J. Genomic classification in acute myeloid leukemia. N. Engl. J. Med. 2016, 375, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Zarka, J.; Sasaki, K.; Short, N.J.; Patel, K.P.; Loghavi, S.; Routbort, M.; Kantarjian, H.; Luthra, R.; Wang, S.A.; et al. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J. 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Marcucci, G.; Maharry, K.; Radmacher, M.D.; Mrózek, K.; Margeson, D.; Whitman, S.P.; Wu, Y.Z.; Schwind, S.; Paschka, P.; et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, M.; Köhnke, T.; Hoster, E.; Schneider, S.; Dufour, A.; Zellmeier, E.; Hartmann, L.; Fischer, L.; Bentz, M.; Denk, D.; et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica 2014, 99, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.H.; Pratz, K.W.; Carroll, M.P. Targeting Menin and CD47 to address unmet needs in acute myeloid leukemia. Cancers 2022, 14, 5906. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Buhl, S.; Mueller, D.; García-Cuéllar, M.P.; Maethner, E.; Slany, R.K. Leukemogenic transformation by HOXA cluster genes. Blood 2010, 115, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, J.; He, S.; Shi, A.; Purohit, T.; Muntean, A.G.; Sorenson, R.J.; Showalter, H.D.; Murai, M.J.; Belcher, A.M.; Lawlor, E.R.; et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol. 2012, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y. Structure, function and inhibition of critical protein–protein interactions involving mixed lineage leukemia 1 and its fusion oncoproteins. J. Hematol. Oncol. 2021, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Krivtsov, A.V.; Evans, K.; Gadrey, J.Y.; Eschle, B.K.; Hatton, C.; Uckelmann, H.J.; Ross, K.N.; Perner, F.; Olsen, S.N.; Pritchard, T.; et al. A Menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell 2019, 36, 660–673.e11. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute myeloid leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Revumenib: First Approval. Drugs 2025, 85, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Aldoss, I.; Thirman, M.J.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.N.; Perl, A.; Dickens, D.S.; McMahon, C.M.; et al. Menin Inhibition With Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J. Clin. Oncol. 2025, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Guest, S.; Tasian, K.; Breese, E.; Schafer, J.; DiPersio, G.; Issa, G.C.; Silverman, B.; Stieglitz, B.; Pollard, J.; et al. Safety and Activity of Revumenib Combination with Fludarabine/Cytarabine (FLA) in Patients with Relapsed/Refractory Acute Leukemias; EHA: Madrid, Spain, 2024. [Google Scholar]
- Zeidner, J.F.; Lin, T.L.; Welkie, R.L.; Curran, E.; Koenig, K.; Stock, W.; Madanat, Y.F.; Swords, R.; Baer, M.R.; Blum, W.; et al. Azacitidine, Venetoclax, and Revumenib for Newly Diagnosed NPM1-Mutated or KMT2A-Rearranged AML. J. Clin. Oncol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.; Ambinder, A.J.; Xiao, W.; Manalis, S.; Shalek, A.K.; Gondek, L.P.; Kentsis, A.; Short, N.J.; Daver, N.; Ning, J.; et al. A Multi-Site Break Through Cancer Trial: Phase II Study Investigating Dual Inhibition of BCL2 and Menin in AML MRD Using the Combination of Venetoclax and Revumenib: Trial in Progress. In Proceedings of the 2024 American Society of Hematology Annual Meeting, San Diego, CA, USA, 7–10 December 2024. [Google Scholar]
- Issa, G.C.; Aldoss, I.; DiPersio, J.F.; Cuglievan, B.; Stone, R.M.; Arellano, M.L.; Thirman, M.J.; Patel, M.R.; Dickens, D.; Shenoy, S.; et al. The Menin Inhibitor SNDX-5613 (Revumenib) Leads to Durable Responses in Patients with KMT2A-Rearranged or NPM1 Mutant AML: Updated Results of a Phase 1 Study. Blood 2022, 140 (Suppl. S1), 150–152. [Google Scholar] [CrossRef]
- Arellano, M.L.; Thirman, M.J.; DiPersio, J.F.; Heiblig, M.; Stein, E.M.; Schuh, A.C.; Zucenka, A.; De Botton, S.; Grove, C.S.; Mannis, G.N.; et al. Menin Inhibition with Revumenib for NPM1-Mutated Relapsed or Refractory Acute Myeloid Leukemia: The AUGMENT-101 Study. Blood 2025, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Issa, G.C.; Thirman, M.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.; Perl, A.; Dickens, D.; McMahon, C.M.; et al. Revumenib Monotherapy in Patients with Relapsed/Refractory KMT2Ar Acute Leukemia: Topline Efficacy and Safety Results from the Pivotal AUGMENT-101 Phase 2 Study. Blood 2023, 142 (Suppl. S2), LBA-5. [Google Scholar] [CrossRef]
- Aldoss, I.; Issa, G.C.; Blachly, J.S.; Thirman, M.J.; Mannis, G.L.; Arellano, M.L.; DiPersio, J.F.; Traer, E.; Zwaan, C.M.; Shukla, N.; et al. Updated Results and Longer Follow-Up from the AUGMENT-101 Phase 2 Study of Revumenib in All Patients with Relapsed or Refractory (R/R) KMT2Ar Acute Leukemia. Blood 2024, 144 (Suppl. S1), 211. [Google Scholar] [CrossRef]
- Lauw, M.I.; Qi, Z.; Eversmeyer, L.; Prakash, S.; Wen, K.W.; Yu, J.; Monaghan, S.A.; Aggarwal, N.; Wang, L. Distinct Pathologic Feature of Myeloid Neoplasm with t(v;11p15); NUP98 Rearrangement. Hum. Pathol. 2022, 123, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Issa, G.C.; Erba, H.P.; Altman, J.K.; Montesinos, P.; De Botton, S.; Walter, R.B.; Pettit, K.; Savona, M.R.; Shah, M.V.; et al. Ziftomenib in Relapsed or Refractory Acute Myeloid Leukaemia (KOMET-001): A Multicentre, Open-Label, Multi-Cohort, Phase 1 Trial. Lancet Oncol. 2024, 25, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Issa, G.C.; Wang, E.S.; Erba, H.; Altman, J.K.; Balasubramanian, S.K.; Gail, J.; Roboz, G.J.; Schiller, M.C.; Palmisiano, N.D.; et al. Ziftomenib Combined with Venetoclax/Azacitidine in Relapsed/Refractory NPM1-M or KMT2A-R Acute Myeloid Leukemia: Interim Phase 1a Results from KOMET-007. Blood 2024, 144 (Suppl. S1), 2880. [Google Scholar] [CrossRef]
- Erba, H.; Wang, E.; Fathi, A.; Roboz, G.; Madanat, Y.; Strickland, S.; Balasubramanian, S.; Mangan, J.; Pratz, K.; Advani, A.; et al. Ziftomenib Combined with Intensive Induction (7+3) in Newly Diagnosed NPM1-M or KMT2A-R Acute Myeloid Leukemia: Interim Phase 1a Results from KOMET-007. Abstract 4159213. In Proceedings of the EHA 2025 Congress, Milan, Italy, 12–15 June 2025; EHA Library: Milan, Italy, 2025. [Google Scholar]
- Goldberg, A.D.; Corum, D.; Ahsan, J.; Nie, K.; Kozlek, T.; Leoni, M.; Dale, S. KOMET-008: A Phase 1 Study to Determine the Safety and Tolerability of Ziftomenib Combinations for the Treatment of KMT2A-Rearranged or NPM1-Mutant Relapsed/Refractory Acute Myeloid Leukemia. Blood 2023, 142 (Suppl. S1), 1553. [Google Scholar] [CrossRef]
- Kwon, M.C.; Thuring, J.W.; Querolle, O.; Dai, X.; Verhulst, T.; Pande, V.; Marien, A.; Goffin, D.; Wenge, D.V.; Yue, H.; et al. Preclinical Efficacy of the Potent, Selective Menin-KMT2A Inhibitor JNJ-75276617 (Bleximenib) in KMT2A- and NPM1-Altered Leukemias. Blood 2024, 144, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.C.; Querolle, O.; Dai, X.; Thuring, J.W.; Verhulst, T.; Marien, A.; Goffin, D.; Cai, W.; Keersmaekers, V.; Eyassu, F.; et al. Pharmacological Characterization of JNJ-75276617, a Menin-KMT2A Inhibitor, as Targeted Treatment for KMT2A-Altered and NPM1-Mutant Acute Leukemia. Blood 2022, 140, 5928–5929. [Google Scholar] [CrossRef]
- Jabbour, E.; Searle, E.; Abdul-Hay, M.; Abedin, S.; Aldoss, I.; Piérola, A.A.; Alonso-Dominguez, J.M.; Chevallier, P.; Cost, C.; Daskalakis, N.; et al. A First-in-Human Phase 1 Study of the Menin-KMT2A (MLL1) Inhibitor JNJ-75276617 in Adult Patients with Relapsed/Refractory Acute Leukemia Harboring KMT2A or NPM1 Alterations. Blood 2023, 142, 57. [Google Scholar] [CrossRef]
- Searle, E.; Recher, C.; Abdul-Hay, M.; Abedin, S.; Aldoss, I.; Pierola, A.A.; Alonso-Dominguez, J.M.; Chevallier, P.; Cost, C.; Daskalakis, N.; et al. Bleximenib Dose Optimization and Determination of RP2D from a Phase 1 Study in Relapsed/Refractory Acute Leukemia Patients with KMT2A and NPM1 Alterations. Blood 2024, 144 (Suppl. S1), 212. [Google Scholar] [CrossRef]
- Recher, C.; O’Nions, J.; Aldoss, I.; Pierola, A.A.; Allred, A.; Alonso-Dominguez, J.M.; Barreyro, L.; Bories, P.; Curtis, M.; Daskalakis, N.; et al. Phase 1b Study of Menin-KMT2A Inhibitor Bleximenib in Combination with Intensive Chemotherapy in Newly Diagnosed Acute Myeloid Leukemia with KMT2Ar or NPM1 Alterations. Blood 2024, 144 (Suppl. S1), 215. [Google Scholar] [CrossRef]
- Wei, A.H.; Searle, E.; Aldoss, I.; Pierola, A.A.; Alonso-Dominguez, J.M.; Curtis, M.; Daskalakis, N.; Della Porta, M.G.; Döhner, H.; D’Souza, A.; et al. A Phase 1b Study of the Menin-KMT2A Inhibitor JNJ-75276617 in Combination with Venetoclax and Azacitidine in Relapsed/Refractory Acute Myeloid Leukemia with Alterations in KMT2A or NPM1. Abstract 422237; EHA Library: Madrid, Spain, 2024. [Google Scholar]
- Eguchi, K.; Shimizu, T.; Kato, D.; Furuta, Y.; Kamioka, S.; Ban, H.; Ymamoto, S.; Yokoyama, A.; Kitabayashi, I. Preclinical evaluation of a novel orally bioavailable menin-MLL interaction inhibitor, DSP-5336, for the treatment of acute leukemia patients with MLL-rearrangement or NPM1 mutation. Blood 2021, 138, 3339. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Yuda, J.; Watts, J.M.; Levis, M.J.; Erba, H.P.; Fukushima, K.; Shima, T.; Palmisiano, N.D.; Wang, E.S.; Borate, U.; et al. Phase 1 results: First-in-human phase 1/2 study of the menin-MLL inhibitor enzomenib (DSP-5336) in patients with relapsed or refractory acute leukemia. Blood 2024, 144 (Suppl. S1), 213. [Google Scholar] [CrossRef]
- Numata, M.; Haginoya, N.; Shiroishi, M.; Hirata, T.; Sato-Otsubo, A.; Yoshikawa, K.; Takata, Y.; Nagase, Y.; Kashimoto, Y.; Suzuki, Y.; et al. A novel menin-MLL1 inhibitor, DS-1594a, prevents the progression of acute leukemia with rearranged MLL1 or mutated NPM1. Cancer Cell Int. 2023, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.; Resetca, D.; Redel, C.; Lin, P.; MacDonald, A.S.; Ciaccio, R.; Kenney, T.M.G.; Wei, Y.; Andrews, D.W.; Sunnerhagen, M.; et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer 2021, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.; Ravandi, F.; Montesinos, P.; Barrientos, J.C.; Badar, T.; Alegre, A.; Bashey, A.; Burgues, J.M.B.; Brunetti, L.; Curran, E.K.; et al. Covalent menin inhibitor BMF-219 in patients with relapsed or refractory (R/R) acute leukemia: Preliminary phase 1 data from the COVALENT-101 study. Blood 2023, 142, 2916. [Google Scholar] [CrossRef]
- Perner, F.; Stein, E.M.; Van Wenge, D.; Singh, S.; Kim, J.; Apazidis, A.; Rahnamoun, H.; Anand, D.; Marinaccio, C.; Hatton, C.; et al. MEN1 mutations mediate clinical resistance to menin inhibition. Nature 2023, 615, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Cutler, J.A.; Bourgeois, W.; Donovan, K.A.; Gu, S.; Hatton, C.; Perlee, S.; Perner, F.; Rahnamoun, H.; Theall, A.C.P.; et al. IKAROS and MENIN coordinate therapeutically actionable leukemogenic gene expression in MLL-r acute myeloid leukemia. Nat. Cancer 2022, 3, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, W.; Cutler, J.A.; Aubrey, B.J.; Wenge, D.V.; Perner, F.; Martucci, C.; Henrich, J.A.; Klega, K.; Nowak, R.P.; Donovan, K.A.; et al. Mezigdomide is effective alone and in combination with menin inhibition in preclinical models of KMT2A-r and NPM1c AML. Blood 2024, 143, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
| Compound | Setting | Clinical Trial (NCT) | Study Population (N) | Findings | Safety | Ref. |
|---|---|---|---|---|---|---|
| Revumenib | Monotherapy | AUGMENT-101 NCT04065399 | R/R NPM1m and KMT2Ar acute leukemias efficacy evaluation N = 97 safety evaluation N = 116 | ORR 53% CR + CRh 30% MRD negativity 78% | QTc 53% DS 16% | [16] |
| Combination with FLA | AUGMENT-102 NCT05326516 | R/R NPM1m, KMT2Ar or NUP98r acute leukemias N = 27 | CRc 51% MRD negativity 71% | TEAEs 40% | [17] | |
| Combination with venetoclax and azacitidine | BEAT-AML NCT03013998 | newly diagnosed AML patients aged >60 years with KMT2Ar or NPM1m N = 26 | CRc 96% MRD negativity 92% | DS 15% QTc 46% | [18,19] |
| Compound | Setting | Clinical Trial (NCT) | Study Population | Findings | Safety | Ref. |
|---|---|---|---|---|---|---|
| (N) | ||||||
| Ziftomenib | Monotherapy | KOMET-001 NCT04067336 | R/R KMT2Ar and NPM1m AML | CR + CRi 9/36 (25%) at 600 mg | Pneumonia G3 at 400 mg DS G4 at 1000 mg | [25] |
| Combination with VEN-AZA Combination with 3 + 7 | KOMET-007 NCT05735184 | Newly diagnosed or R/R NPM1m or KMT2Ar AML N = 54 (ven-aza) N = 51 (3 + 7) | VEN-AZA arm ORR 68% CRc 50% 3 + 7 arm Median CR and OS were not achieved | DS 8% TEAEs > 20% TAEA > 30% | [26,27] | |
| Combination with gilteritinib, FLAG-IDA, LDAC NPM1m AML | KOMET-008 NCT06001788 | R/R NPM1-m or KMT2A-r AML | NA | NA | [28] |
| Compound | Setting | Clinical Trial (NCT) | Study Population | Findings | Safety | Ref |
|---|---|---|---|---|---|---|
| (N) | ||||||
| Bleximenib | Monotherapy | cAMeLot-1 | R/R AML | ORR 50% (10/20) | TRAE 58% | [31,32] |
| NCT 04811560 | KMT2Ar NPM1m NUP98-214 N = 58 | At both 90/100 mg BID and 150 mg BID, 39% (5/13) at 45 mg BID | DS 13% | |||
| Combination with 3 + 7 | Ale1002 | Newly diagnosed AML | ORR 93% | 95% (21/22) > 1 TAEA 17/22; (77%) diarrhea and 15/22; (68%) thrombocytopenia | [33] | |
| NCT 05453903 | N = 22 | |||||
| Combination with VEN-AZA | Ale1002 | R/R AML KMT2Ar NPM1m | ORR: 86% | Grade ≤ 2 GI events (7%) no DS or QTc | [34] | |
| NCT 05453903 | N = 45 pts | CRc: 48% |
| Compound | Setting | Clinical Trial (NCT) | Study Population | Findings | Safety | Ref. |
|---|---|---|---|---|---|---|
| (N) | ||||||
| Enzomenib | monotherapy | NCT04988555 | R/R AML NPM1m | CR + CRh: | No QTc prolongation | [36] |
| KMT2Ar and other HOXA9/MEIS1 | KMT2A 7/23 (30%) | DS 11% | ||||
| 84 pts | NPM1 8/17 (47%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canichella, M.; Papayannidis, C.; Mazzone, C.; de Fabritiis, P. Therapeutic Implications of Menin Inhibitors in the Treatment of Acute Leukemia: A Critical Review. Diseases 2025, 13, 227. https://doi.org/10.3390/diseases13070227
Canichella M, Papayannidis C, Mazzone C, de Fabritiis P. Therapeutic Implications of Menin Inhibitors in the Treatment of Acute Leukemia: A Critical Review. Diseases. 2025; 13(7):227. https://doi.org/10.3390/diseases13070227
Chicago/Turabian StyleCanichella, Martina, Cristina Papayannidis, Carla Mazzone, and Paolo de Fabritiis. 2025. "Therapeutic Implications of Menin Inhibitors in the Treatment of Acute Leukemia: A Critical Review" Diseases 13, no. 7: 227. https://doi.org/10.3390/diseases13070227
APA StyleCanichella, M., Papayannidis, C., Mazzone, C., & de Fabritiis, P. (2025). Therapeutic Implications of Menin Inhibitors in the Treatment of Acute Leukemia: A Critical Review. Diseases, 13(7), 227. https://doi.org/10.3390/diseases13070227








