Abstract
Background/Objectives: Acetazolamide is widely used for acute mountain sickness (AMS) prophylaxis. Whilst generally safe, acute kidney injury (AKI) is a rare but serious adverse event. We present a case of anuric AKI following minimal exposure to acetazolamide, contributing to the limited literature on its nephrotoxicity at prophylactic doses. Methods: A 54-year-old previously healthy male ingested 250 mg/day of oral acetazolamide for two days. He developed acute anuria and lumbar pain. Diagnostic evaluation included laboratory tests, imaging, microbiological cultures, autoimmune panels, and diuretic response. No signs of infection, urinary tract obstruction, or systemic disease were found. Results: The patient met KDIGO 2012 criteria for stage 3 AKI, with peak serum creatinine of 10.6 mg/dL and metabolic acidosis. Imaging confirmed non-obstructive nephrolithiasis. Conservative treatment failed; intermittent hemodialysis was initiated. Renal function recovered rapidly, with the normalization of serum creatinine and urinary output by day 4. Conclusions: This case represents the lowest cumulative dose of acetazolamide reported to cause stage 3 AKI. The findings support a pathophysiological mechanism involving sulfonamide-induced crystalluria and intratubular obstruction. Physicians should consider acetazolamide in the differential diagnosis of AKI, even with short-term prophylactic use.
1. Introduction
Acetazolamide (ACZ) is a sulfonamide-derived drug that acts as a non-competitive inhibitor of carbonic anhydrase, primarily in the proximal tubule, increasing renal excretion of bicarbonate, sodium, and water, leading to alkaline diuresis and normal anion gap metabolic acidosis. It is not metabolized and is excreted unchanged in the urine [1].
ACZ is commonly used for the treatment of various clinical conditions, including glaucoma, decompensated heart failure, pulmonary hypertension, pseudotumor cerebri, seizures, metabolic alkalosis, periodic paralysis, hypochloremia, and prophylaxis against altitude sickness [2]. The most common adverse events associated with ACZ use include paresthesia, dysgeusia, nausea, polyuria, and fatigue [3].
Renal complications associated with acetazolamide use occur in the following two distinct forms, with different mechanisms and timelines: [1] acute kidney injury (AKI) due to acute exposure and [2] nephrolithiasis due to long-term use [4]. AKI is a potentially severe but rare complication, with a mechanism which is primarily explained by crystalluria, intratubular obstruction, and retrograde urinary flow [5]. The absence of histological diagnosis in most of the relevant literature is attributed to the rapid recovery of renal function in most cases.
Acute mountain sickness (AMS) affects more than 25% of individuals ascending to 3500 m and over 50% of those reaching altitudes above 6000 m. AMS can progress from nonspecific symptoms to life-threatening high-altitude cerebral edema in less than 1% of cases [6]. The first descriptions of AMS date back to late 19th-century observations by European travelers to the Andes Mountains in South America, notably Edward Whymper [7].
Regarding pathophysiology, during high-altitude ascent susceptible individuals may develop relative hypoventilation and hypoxia, triggering multiple pathological responses that contribute to disease onset. Increased capillary permeability, driven by a complex interaction between fluid mechanics (regional hyperperfusion and elevated hydrostatic pressure) and biohumoral responses (inflammatory mediator release), along with impaired compensatory mechanisms (e.g., reduced ability to mitigate intracranial volume fluctuations), leads to cerebral vasogenic edema [8].
Prophylactic management consists of ACZ 125 mg every 12 h, initiated the day before ascent and continued for two to four days after reaching the target altitude. For AMS treatment, a dose of 250 mg every 12 h is recommended [6].
We present the case of anuric AKI in a man with no significant medical history, following ACZ use as AMS prophylaxis.
2. Case Report
A 54-year-old male with no significant medical history ingested ACZ 250 mg/day orally for two doses as prophylaxis for AMS due to a planned trip to the city of Huancavelica, Peru (3676 m above sea level). Twelve hours after the second dose of ACZ, he developed severe right lumbar pain and a reduced urinary output that progressed to anuria. He was subsequently transferred to Lima (at sea level), where anuria and pain persisted. Upon admission, the patient was in fair general condition, with adequate hydration, lucid, and oriented in time, space, and person. His vital signs were as follows: blood pressure (BP) 117/70 mmHg, heart rate (HR) 97 bpm, and oxygen saturation of 97%. He presented with severe low back pain, while the rest of the physical examination was non-contributory. Bladder catheterization confirmed anuria.
Laboratory tests revealed hemoglobin (Hb) 15.8 g/dL, leukocytes 17,600/μL 17.6 × 109/L without left shift, C-reactive protein (CRP) 280 g/dL, serum creatinine (sCr) 5.84 mg/dL, and urea 77 mg/dL. The remaining laboratory results are shown in Table 1.

Table 1.
Laboratory findings.
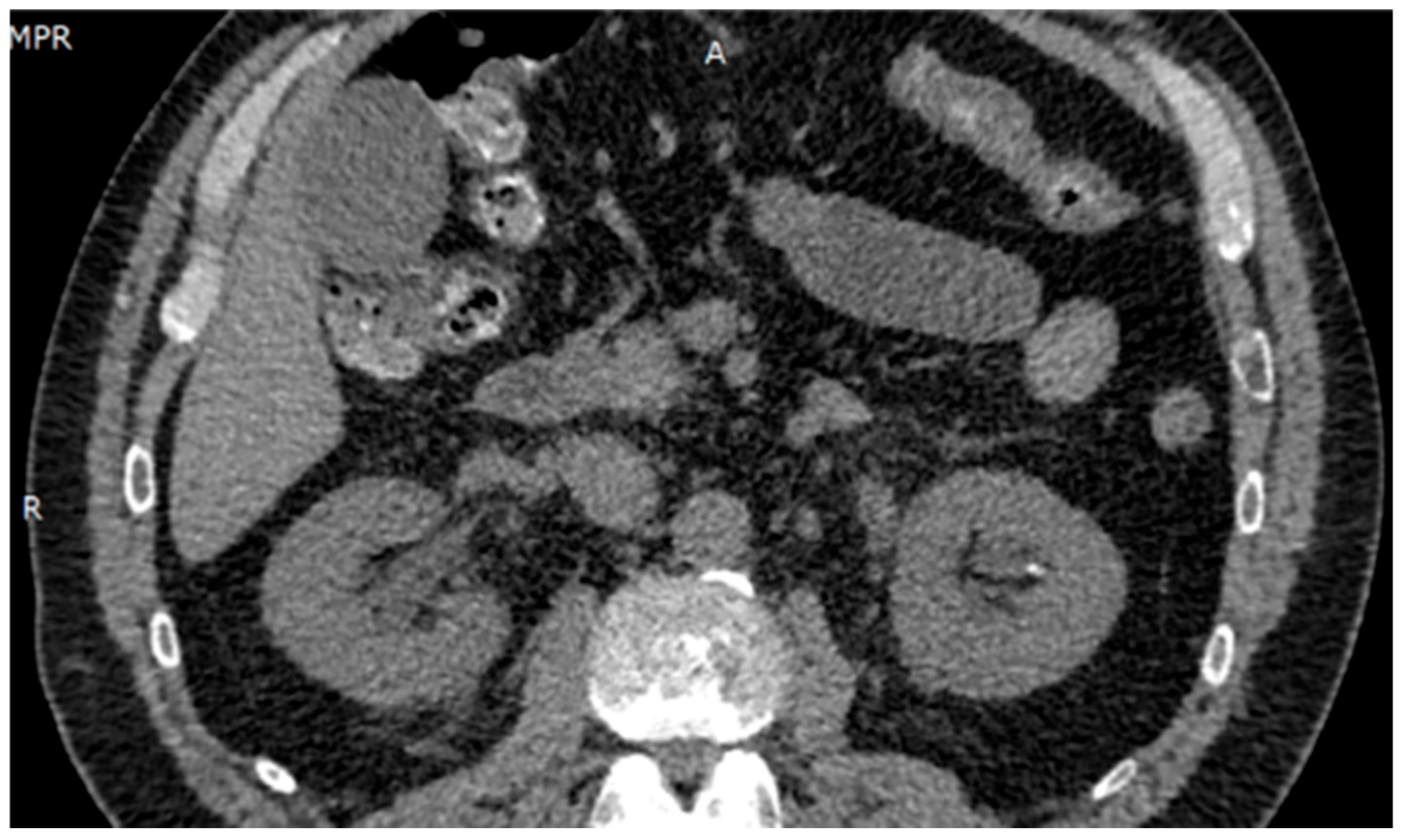
Ultrasound imaging showed a 3.5 mm calculus in the lower left calyx, with no evidence of obstruction. Renal Doppler ultrasound revealed no abnormalities. Urotomography (UROTAC) confirmed left renal lithiasis without obstructive effect and an empty bladder (Figure 1).
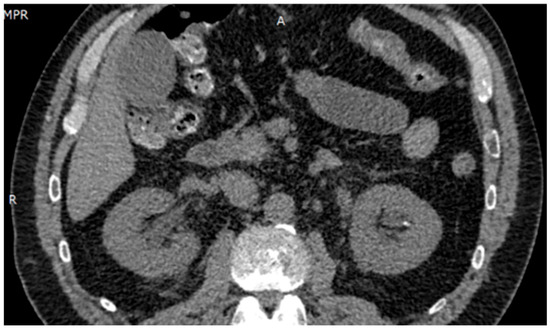
Figure 1.
UROTAC showing uncomplicated nephrolithiasis.
Negative blood and urine cultures were obtained, along with negative infectious and autoimmune serologies, including hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus-1 antibody, venereal disease research laboratory (VDRL) test, complement levels, antinuclear antibody, antineutrophil cytoplasmic antibody, anti-streptolysin O antibody, and rheumatoid factor. Creatine phosphokinase levels were normal.
Urinalysis revealed a pH of 6.5, dipstick 2+ for albumin, specific gravity of 1010, red blood cells > 100/high-power field (HPF), leukocytes 6–8/HPF, sparse epithelial cells, and no crystals or casts. The 24 h proteinuria was 950 mg.
After ruling out obstructive, infectious, and autoimmune etiologies, ACZ ingestion was suspected as the likely cause of AKI, classified as stage 3 according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 criteria [9].
Venous excess ultrasound score (VExUS) and lung ultrasound score (LUS) assessments showed no signs of systemic or pulmonary vascular congestion. Consequently, intravenous (IV) fluid therapy with 1500 mL of isotonic saline was initiated, followed by IV administration of 60 mg furosemide to induce diuresis; however, no response was observed. Pain management included IV paracetamol and morphine.
At 24 h post-admission, anuria persisted, with an increase in creatinine and urea to 10.1 mg/dL and 110 mg/dL, respectively. Arterial blood gas analysis revealed an arterial pH of 7.29, PCO2 of 26.2 mmHg, HCO3− of 13 mmol/L, and anion gap of 23.7 mmol/L, leading to the decision to initiate hemodialysis (HD), which was performed for two consecutive days.
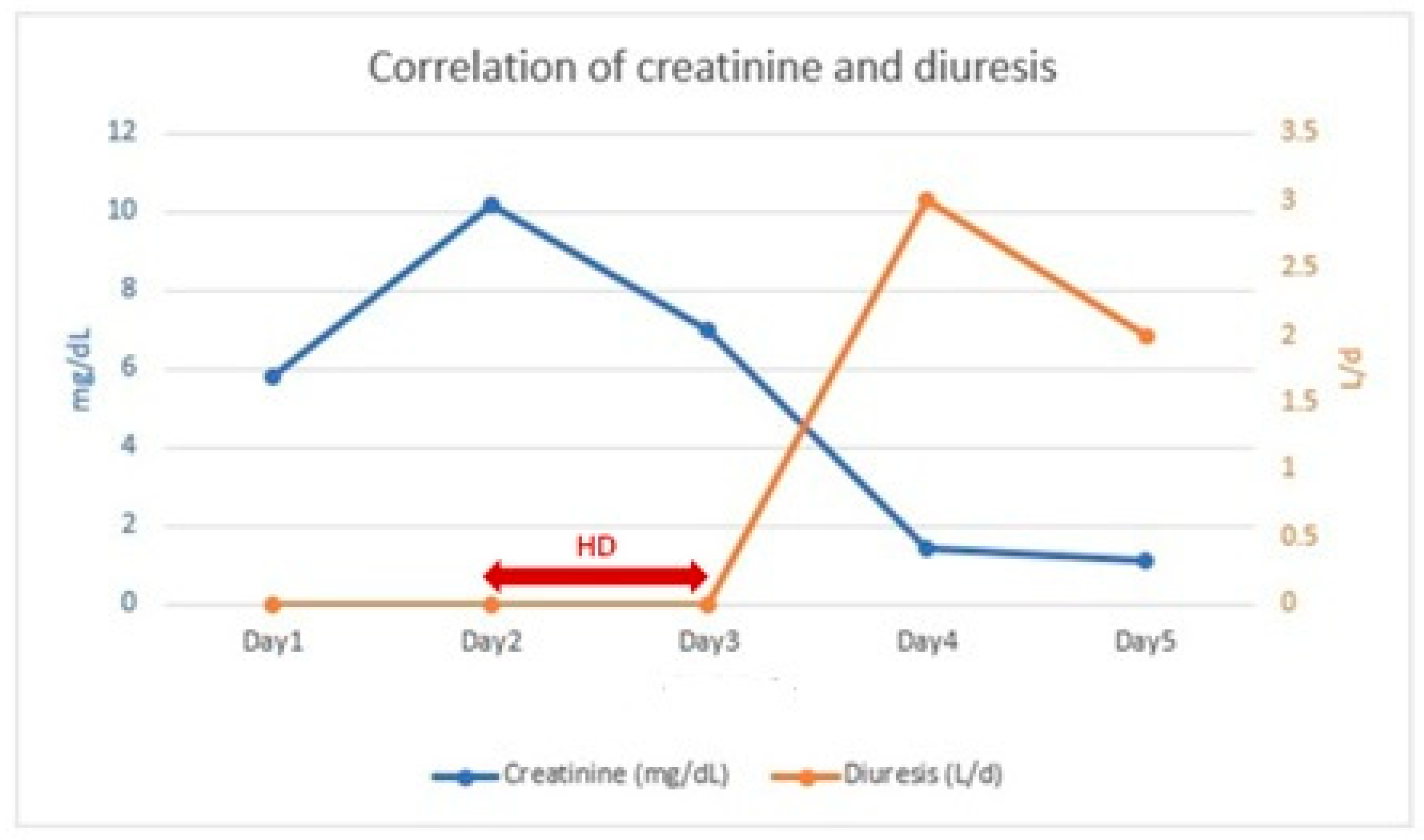
Within 24 h after the second HD session, the patient exhibited a urine output of 3010 mL/24 h and a decrease in serum creatinine to 1.46 mg/dL (Figure 2). In the following 24 h, renal function continued to improve (sCr: 1.19 mg/dL) with a urine output of 2000 mL/24 h.
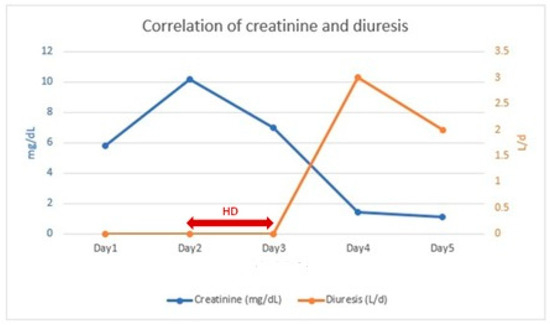
Figure 2.
Correlation of creatinine and diuresis.
On the fifth day post-admission, due to favorable clinical evolution, the patient was discharged. One week later, during outpatient follow-up, laboratory results showed 24 h proteinuria of 180 mg, serum creatinine of 0.8 mg/dL, and a normal urinalysis.
3. Discussion
We present an unusual case of anuric AKI in a patient who ingested ACZ as prophylaxis for AMS. AMS is caused by cerebral edema associated with hypobaric hypoxia, which affects individuals who ascend too rapidly to high altitudes beyond their current level of acclimatization. ACZ is recommended as a preventive therapy due to its ability to enhance the poikilocapnic hypoxic ventilatory response, leading to increased partial pressure of arterial oxygen. Additionally, it reduces cerebrospinal fluid production, which may contribute to its beneficial effects [6]. To our knowledge, this is the first reported case of stage 3 AKI, according to KDIGO 2012 criteria [9], associated with the lowest documented cumulative dose of acetazolamide in the literature.
ACZ is a sulfonamide derivative and a carbonic anhydrase inhibitor, primarily acting in the proximal tubule. It is excreted unchanged in the urine, and in acidic urine it forms crystals within the renal tubules. AKI has been recognized as a possible complication of its use; however, this effect remains relatively unknown [2]. In our patient, AKI occurred with a total ACZ dose of 500 mg (250 mg every 24 h for two days), which is even lower than the previously lowest documented cumulative dose in the literature. López-Romero et al. reported a similar case in a 76-year-old man who developed stage 3 anuric AKI (KDIGO 2012) without urological evidence of obstruction after receiving ACZ after cataract surgery (250 mg three times daily for one day) [10].
Most patients with AKI due to acetazolamide present with symptoms such as unilateral or bilateral low back pain, anuria, nausea, and vomiting. Our patient exhibited predominant right lumbar pain and anuria, similar to the case reported by Liu et al., where a 52-year-old man who took ACZ for bilateral intermittent acute angle closure glaucoma symptoms (450 mg per day for 10 days) developed right-sided low back pain and anuria [5].
In our case, the onset of symptoms occurred 36 h after starting ACZ. A similar timeframe was reported by López-Romero et al., who documented symptom onset 24 h after initiating ACZ [10]. However, longer latency periods have been described, such as the case reported by Higenbottam et al. where a 46-year-old patient developed AKI symptoms three weeks after starting ACZ therapy [11].
Notable laboratory findings in our patient were microscopic hematuria and significant proteinuria. López-Menchero et al. reported a case of a 58-year-old man taking ACZ for serous maculopathy (750 mg per day for four days) who developed low back pain and stage 3 anuric AKI without radiological evidence of obstruction or thrombosis. Urine microscopy showed red blood cells but no casts or crystals [12]. Similarly, in the case reported by Neyra et al. microscopic hematuria and proteinuria (240 mg/24 h) were documented, although the latter was not as pronounced as in our case [13].
The majority of patients with AKI due to acetazolamide experience improvement in renal function upon discontinuation of the drug, intravenous volume expansion with 0.9% saline, and, if necessary, sodium bicarbonate (to increase urinary pH), achieving spontaneous resolution and rarely requiring renal replacement therapy (RRT). However, in our patient, despite conservative management, anuria persisted, and azotemia worsened, necessitating two sessions of HD, similar to the cases reported by López-Romero et al. and Neyra et al. Unlike our case, however, these two patients had received higher doses of ACZ—750 mg and 1250 mg, respectively [10,13].
The most conclusive evidence of ACZ-induced AKI was described by Rossert in 1989. Renal biopsy revealed tubular damage with disruption of the tubular basement membrane, cellular debris, and intratubular crystalluria. Tamm-Horsfall protein was detected in Bowman’s space, suggesting intratubular obstruction and retrograde urine flow due to crystal formation [14]. It is noteworthy that the absence of a histopathological diagnosis in most reported cases is attributed to the rapid recovery of renal function.
Acute interstitial nephritis (AIN) due to acetazolamide (ACZ) remains an important differential diagnosis as sulfonamides along with beta-lactam are among the leading causes of drug-induced AIN. Regarding clinical features, back pain may be present in over 30% of cases [15], likely reflecting the edema-induced distension of the renal capsule, and a rapid improvement in renal function is typically observed, as occurred in our patient. The classical triad of fever, rash, and eosinophilia, although absent in this case, is reported in fewer than 10% of patients. A key laboratory finding is C-reactive protein (CRP), which is frequently elevated in AIN. Although the ACZ dose was low, this does not exclude AIN as the allergic reaction is dose-independent.
On the other hand, drug-induced AIN usually develops several days to weeks after exposure to the offending agent, and the clinical course is generally non-oliguric [16]. Therefore, intratubular crystallization remains the most likely diagnosis. Nonetheless, as a renal biopsy was not performed, the presence of AIN cannot be definitively excluded, and it is even possible that both mechanisms contributed to the pathogenesis of the acute kidney injury (AKI).
On the other hand, drug-induced AIN generally develops several days to weeks after exposure to the offending agent, and the clinical course is typically non-oliguric [16]. Therefore, intratubular crystallization remains the most likely diagnosis. However, since a renal biopsy was not performed, AIN cannot be definitively excluded, and a dual mechanism involving both etiologies may have contributed to the development of AKI.
In our case, the development of AKI following exclusive ACZ exposure and the absence of imaging evidence of urinary obstruction strongly supports a pathogenic mechanism involving intratubular obstruction due to sulfonamide crystalluria induced by ACZ. It is important to highlight that crystalluria findings on urinary microscopy are rarely reported in AKI secondary to ACZ [17]. The clinical presentation of anuria and renal colic is characteristic. Urine microscopic examination frequently reveals red blood cells, although sulfonamide crystals have rarely been observed in most cases of ACZ-induced AKI.
Our case is one of the few reported cases of AKI following ACZ prophylaxis for acute mountain sickness. Intravascular volume expansion with isotonic fluids is recommended for renal recovery, which typically occurs within 96 h after ACZ discontinuation. In some cases, by increasing urinary pH, sodium bicarbonate prevents the precipitation of sulfonamide crystals [18]. The importance of considering ACZ as a potentially nephrotoxic agent and including it in the differential diagnosis of anuric AKI is emphasized.
4. Conclusions
In conclusion, we describe the clinical and laboratory manifestations of an unusual case of stage 3 anuric AKI following the ingestion of 500 mg of ACZ for acute mountain sickness prophylaxis—a rare adverse effect at such a low cumulative dose, with the possibility of AIN due to a dose-independent allergic reaction. Although intratubular sulfonamide crystallization appears to be the most likely cause, AIN cannot be ruled out in the absence of a renal biopsy. Given the widespread use of ACZ in various medical conditions, physicians should be aware of this potential complication and consider it in the differential diagnosis of patients with ARF and a history of its use.
Author Contributions
Conceptualization, M.D.D., R.V.C., C.L.R., and C.R.T.; methodology, R.V.C. and M.D.D.; validation, R.L.M., M.C.T., M.D.D., and E.J.M.; formal analysis, J.C.D.L.F., M.C.T., M.D.D., and C.B.C.; investigation, J.C.D.L.F., M.C.T., M.D.D., C.R.T., and C.B.C.; resources, J.C.D.L.F., R.L.M., and M.D.D.; data curation, M.D.D., C.L.R., M.C.T., and J.A.N.; writing—original draft preparation, E.J.M., C.L.R., and M.D.D.; writing—review and editing, E.J.M., M.C.T., and M.D.D.; visualization, R.V.C., M.D.D., and R.L.M.; supervision, J.C.D.L.F., J.A.N., M.D.D., and M.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not required for this study according to our Institutional Review Board (approval code: 010-2025, acquired on 10 February 2025) due to the nature of our research as a case report in routine clinical practice.
Informed Consent Statement
Written informed consent was obtained from the patient for the publication of this article (including the publication of images).
Data Availability Statement
No new data were created or analyzed in this study. The data used to support the findings of this study are available from the corresponding author on request (contact elena.jimenezm@salud-juntaex.es (E.J.M.)).
Acknowledgments
We would like to express our sincere gratitude to all of the medical staff at Hospital Cayetano Heredia for their involvement in the treatment and care of the patient. We extend special thanks to Marín for his continuous support and active participation in the patient’s day-to-day management.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mincione, F.; Scozzafava, A.; Supuran, C.T. The development of topically acting carbonic anhydrase inhibitors as antiglaucoma agents. Curr. Pharm. Des. 2008, 14, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Shukralla, A.A.; Dolan, E.; Delanty, N. Acetazolamide: Old drug, new evidence? Epilepsia Open 2022, 7, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, C.N.; Owens, R.L.; Orr, J.E.; Edwards, B.A.; Malhotra, A. Side effects of acetazolamide: A systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir. Res. 2020, 7, e000557. [Google Scholar] [CrossRef] [PubMed]
- Paisley, K.E.; Tomson, C.R. Calcium phosphate stones during long-term acetazolamide treatment for epilepsy. Postgrad. Med. J. 1999, 75, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sii, F.; Horsburgh, J.; Shah, P. Anuric acute kidney injury due to low dose oral acetazolamide with hypercrystalluria. Clin. Exp. Ophthalmol. 2017, 45, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Imray, C.; Wright, A.; Subudhi, A.; Roach, R. Acute mountain sickness: Pathophysiology, prevention, and treatment. Prog. Cardiovasc. Dis. 2010, 52, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Whymper, E.; Shipton, E. (Eds.) Travels Amongst the Great Andes of the Equator; Republished; Charles Knight: London, UK, 1972; originally published in London: Murray, 1891–1892. [Google Scholar]
- Savioli, G.; Ceresa, I.F.; Gori, G.; Fumoso, F.; Gri, N.; Floris, V.; Varesi, A.; Martuscelli, E.; Marchisio, S.; Longhitano, Y.; et al. Pathophysiology and Therapy of High-Altitude Sickness: Practical Approach in Emergency and Critical Care. J. Clin. Med. 2022, 11, 3937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, L.C.; Poma, S.F.; Viejo, B.I. Acute kidney injury secondary to post-phacoemulsification treatment with acetazolamide. Rev. Nefrol. Dial. Traspl. 2022, 42, 248–252. [Google Scholar]
- Higenbottam, T.; Ogg, C.S.; Saxton, H.M. Acute renal failure from the use of acetazolamide (Diamox). Postgrad. Med. J. 1978, 54, 127–128. [Google Scholar] [CrossRef] [PubMed]
- López-Menchero, R.; Albero, M.D.; Barrachina, M.D.; Álvarez, L. Fracaso renal agudo anúrico secundario a tratamiento con acetazolamida. Nefrología 2006, 26, 755–756. [Google Scholar] [PubMed]
- Neyra, J.A.; Alvarez-Maza, J.C.; Novak, J.E. Anuric Acute Kidney Injury Induced by Acute Mountain Sickness Prophylaxis With Acetazolamide. J. Investig. Med. High Impact Case Rep. 2014, 2, 2324709614530559. [Google Scholar] [CrossRef] [PubMed]
- Rossert, J.; Rondeau, E.; Jondeau, G.; Ronco, P.; Mougenot, B.; Kanfer, A.; Sraer, J.D. Tamm-Horsfall protein accumulation in glomeruli during acetazolamide-induced acute renal failure. Am. J. Nephrol. 1989, 9, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Perazella, M.A. Treatment of Drug-Induced Acute Tubulointerstitial Nephritis: The Search for Better Evidence. Clin. J. Am. Soc. Nephrol. 2018, 13, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.O.; Merino, D.; Laurain, A.; Cremoni, M.; Andreani, M.; Rocher, F.; Destere, A.; Esnault, V.L.; Sicard, A.; Drici, M.-D. Drug-Induced Tubulointerstitial Nephritis: Insights From the World Health Organization Safety Database. Kidney Int. Rep. 2022, 7, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Abou-Mrad, R.M.; Ibrahim, M.; Osman, N.; Babu, R.S. Anuric acute kidney injury requiring dialysis following acetazolamide use for cataract surgery. Am. J. Case Rep. 2021, 22, e931319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawson, C.; Morris, L.; Wilson, V.; Burns, B., Jr. Hemorrhagic Anuria With Acute Kidney Injury After a Single Dose of Acetazolamide: A Case Study of a Rare Side Effect. Cureus 2020, 12, e10107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).