Abstract
Background: Primary hyperparathyroidism (PHPT) represents a multi-faced disease with a wide spectrum of manifestations. Familial forms of PHPT (affecting up to 10% of the cases) involve a particular category that encompasses a large range of hereditary syndromes, including parathyroid hyper-function, frequently in the setting of a multi-glandular disease. Objective: The aim was to analyze the most recent findings regarding PHPT in multiple endocrine neoplasia type 2 (MEN2) to a better understanding of the timing with respect to the associated ailments, MEN2-related PHPT (MEN2-PHPT) clinical and genetic particularities, optimum diagnostic, and overall management, particularly, surgical outcomes. Methods: This was a PubMed-based compressive review with regard to the latest data published in English from January 2020 until January 2025, using the following keywords: “primary hyperparathyroidism” and “multiple endocrine neoplasia”, “multiple endocrine neoplasia type 2”, “MEN2”, or “MEN2A”. We included original full-length studies of any study design that provided clinically relevant data in MEN2-PHPT and excluded reviews, meta-analysis, and case reports/series. Results: A total of 3783 individuals confirmed with MEN2 or RET pathogenic variants carriers were analyzed across 14 studies that provided data on PHPT. The prevalence of MEN2-PHPT subjects varied between 7.84% and 31.3%, with particularly low rates in non-index patients (3.8%). PHPT was the first syndrome manifestation in 0.9% of MEN2 patients. In terms of gender distribution, females represented 42.85% or 54.9% (similar rates between women and men, and only a single cohort showed a female rate up to 80%). Most subjects were diagnosed with PHPT and underwent surgery in the third or fourth decade of life. The highest median age at MEN2 diagnosis was 42 years. The youngest patients were RET pathogenic variants carriers who underwent (genetic) screening with median ages of 12 or 14 years. RET pathogenic variants analysis (n = 10/14 studies) showed that 16.67% of patients with p.Cys634Arg and 37.5% of those with p.Cys611Tyr had symptomatic PHPT, while those with p.Cys618Phe and p.Leu790Phe were asymptomatic. Timing analysis with respect to the medullary thyroid carcinoma diagnosis showed synchronous PHPT diagnosis in 80% and metachronous in 10% of MEN2 patients; with respect to MEN2-pheochromocytoma, synchronous diagnosis of PHPT was found in 56%, while pheochromocytoma was identified before PHPT in 22% of the cases and after PHPT in 22%. Studies (n = 10/14, N = 156 subjects with MEN2-PHPT) on parathyroidectomy identified that 72.7% to 100% of the individuals underwent surgery, typically performed in adulthood, at ages spanning from a mean of 34.7 to 48.5 years. The post-surgery outcomes varied (e.g., the rate for persistent PHPT was of 0%, 8% to 16.7%; recurrent PHPT of 12.5% to 23%; permanent hypoparathyroidism of 33% to 46%; permanent unilateral vocal cord palsy of 0% up to16.7%). Data regarding the number of involved glands (n = 7, N = 77): the prevalence of multi-glandular disease was pinpointed between 12.5% and 50%. Conclusions: MEN2-PHPT involved unexpected high rates of single-gland involvement (from 33.3% to 87.5%), probably due to an early detection across genetic screening. Traditional female higher prevalence in PHPT was not confirmed in most MEN2 cohorts. As expected, a younger age at PHPT diagnosis and surgery than seen in non-MEN2 patients was identified, being tidily connected with the syndromic constellation of tumors/malignancies. Overall, approximately, one out of ten patients were further confirmed with MEN2 starting with PHPT as the first clinically manifested element.
Keywords:
parathyroid; parathormone; primary hyperparathyroidism; calcium; surgery; gene; multiple endocrine neoplasia; MEN2; MEN2A; RET 1. Introduction
Primary hyperparathyroidism (PHPT) represents a multi-faced disease with a wide spectrum of causes, manifestations, and recently recognized forms such as asymptomatic and normocalcemic PHPT [1,2,3]. Familial forms of PHPT involve a particular category of this disease and encompass a large range of hereditary syndromes that predisposes individuals to parathyroid hyper-function, most frequently in the setting of a multi-glandular disease in addition to other endocrine and non-endocrine ailments [4,5,6,7]. Based on the underlying genetic defect, there are various syndromes, such as multiple endocrine neoplasia type 1 (MEN1) caused by pathogenic variants of MEN1 gene, multiple endocrine neoplasia type 2 (MEN2) due to pathogenic variants of RET oncogene, multiple endocrine neoplasia type 4 (MEN4) harboring CDKN1B/p27 defects, hyperparathyroidism-jaw tumor syndrome due to pathogenic variants of CDC73 gene, as well as familial isolated primary hyperparathyroidism [8,9,10,11,12].
MEN2, an autosomal dominant syndrome [13], used to be further classified as MEN2A and MEN2B. However, more recently, the “MEN2” term was adopted for MEN2A, while MEN2B became known as “MEN3” [14]. RET, a gene located on chromosome 10q11.2 which encodes a tyrosine kinase receptor, is related to cellular proliferation, differentiation, and survival [15,16]. Tumorigenesis in MEN2 typically involves medullary thyroid carcinoma (MTC), as the key finding, associated with pheochromocytoma (PC) and/or PHPT, the endocrine manifestation which presents the lowest prevalence [17,18,19,20].
PHPT may be either by a single-glandular or multi-glandular disease, previously classified as adenoma and hyperplasia [21]. It affects up to one third of individuals confirmed with MEN2, and it may be symptomatic (causing nephrolithiasis/renal failure, osteoporosis and low trauma fractures, gastrointestinal disturbances, cardio-metabolic issues, as well as neuropsychiatric symptoms that overall requires a multidisciplinary team), or it may be identified in asymptomatic patients, through calcium testing or genetic screening protocols [22,23,24,25,26,27]. The single definitive management of PHPT is parathyroidectomy (PTx). The surgical strategy is complex and is based on the number of hyper-functioning glands, therefore imperiously needing preoperative localization via imaging studies such as neck ultrasound, Tc-99m-sesta-Methoxy isobutyl isonitrile scintigraphy, computed tomography, and single-photon emission computed tomography [28,29,30]. PTx may be focused (e.g., for a single gland), subtotal, and minimally invasive or even total with auto-transplantation to a heterotopic site such as the forearm [31]. Another important aspect is the concomitant presence of other endocrine neoplasms/malignancies, and the high probability that PTx is practiced on a previously explored neck for MTC [32]. Due to younger age at presentation, multi-glandular involvement and inheritance risk, a tailored management approach is needed, including (family) genetic counselling, refined screening methods, and a personalized surgical approach provided by a skillful team [33,34].
The aim was to analyze the most recent findings regarding PHPT in MEN2 patients for a better understanding of the timing with respect to the associated ailments amid the syndrome, MEN2-related PHPT clinical and genetic particularities, optimum diagnostic, and overall management, particularly, surgical outcome.
2. Methods
This was a PubMed-based compressive review (of narrative design) with regard to the latest data published in English from January 2020 until January 2025, using the following keywords in different combinations: “primary hyperparathyroidism” and “multiple endocrine neoplasia”, “multiple endocrine neoplasia type 2”, “MEN2”, “MEN2A”. We only included original, full-length studies of any study design that provided clinically relevant data in MEN2-related PHPT and excluded reviews, meta-analysis and case reports/series. A total of 14 papers were finally analyzed [35,36,37,38,39,40,41,42,43,44,45,46,47,48] (Figure 1).
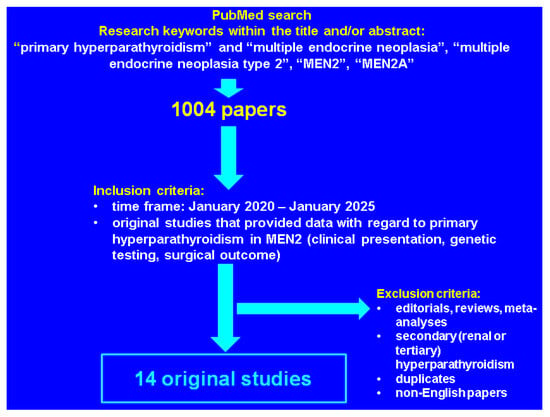
Figure 1.
Flowchart of literature research according to our methods.
3. Results
The 14 studies [35,36,37,38,39,40,41,42,43,44,45,46,47,48] reporting data about PHPT in patients with MEN2 included 218 subjects with PHPT and MEN2, out of a total 3783 individuals confirmed with MEN2 or RET pathogenic variants carriers (N = 7963 subjects across the entire studied population) [35,36,37,38,39,40,41,42,43,44,45,46,47,48] (Table 1).

Table 1.
Included studies with concern to MEN2-related PHPT [35,36,37,38,39,40,41,42,43,44,45,46,47,48] (the display starts with the most recent publication year).
The prevalence of PHPT in MEN2 subjects varied between 7.84% [38] and 31.3% [45], with particularly low prevalence in non-index-patients (3.8%) [39]. Apart from the differences in terms of prevalence observed between index and non-index cases (9% versus 3.8%, p = 0.019) [39], subjects with high-risk pathogenic variants also had a higher prevalence of PHPT compared with moderate-high and low-risk pathogenic variants (11.4% versus 2.4% versus 0.5%, p < 0.001) [40].
PHPT was the first manifestation in 0.9% of patients with MEN2 [47]. MEN2 in subjects confirmed with PHPT was found in 0.12% of them [37], 4.8% [42], and 22.9% [36]. In terms of female-to-male ratio, one cohort reported that 54.9% of the patients with MEN2-related PHPT were females [40], while another found a rate of 42.85%, with no statistically significant difference between males and females (p = 0.090) [43]; another study reported a female prevalence of 80% in MEN2-associated PHPT [37]. Moreover, 80% of PHPT subjects with the parathyroid condition as the first MEN2 manifestation were females [47].
Most patients were diagnosed with PHPT and underwent PTx in the third [35,37,40,47,48] or fourth [36,38,39,40] decade of life. The highest median age at MEN2 diagnosis was 42 years [38]. The youngest patients were RET pathogenic variants carriers who underwent (genetic) screening with median ages of 12 and 14 years [39]. The pattern of inheritance was shown to be of importance in MEN2-PHPT, as follows: a higher risk of developing PHPT in RET carriers was found in those who inherited the condition from the father versus those who inherited it from the mother, according to a hazard ratio (HR) of 3.4 [95% confidence interval (CI) between 1.1 and 10.1, p = 0.029] [44].
3.1. Genetic Findings: RET-Related Primary Hyperparathyroidism
Genetic testing plays a crucial role in familial (hereditary) PHPT, as seen in other endocrine (non-parathyroid) conditions [49,50,51]. Ten (n = 10/14) studies [35,36,38,39,40,41,43,45,47,48] provided the RET pathogenic variants involved in MEN2 or the risk category, according to American Thyroid Association (ATA) criteria [49]. Genetic analysis was reported in a total of 3399 subjects, including 3163 with MEN2, out of whom 177 individuals had MEN2-related PHPT. Holm et al. [38] provided the prevalence of symptoms according to the RET pathogenic variant and found that 16.67% of patients with p.Cys634Arg and 37.5% of those with p.Cys611Tyr had symptomatic PHPT, while those with p.Cys618Phe and p.Leu790Phe were asymptomatic [38]. A retrospective study showed that index cases with MEN2-asociated PHPT as first manifestation of the syndrome had a symptomatic PHPT form in relationship with the following pathogenic variants: p.Cys634Tyr, p.Cys634Arg, p.Cys611Tyr, p.Cys620Arg, p.Glu768Asp, and p.Cys618Phe [47].
In terms of the number of glands involved, Holm et al. [38] reported single-glandular disease in patients harboring the RET mutation at p.Cys634Arg, p.Cys611Tyr, and p.Cys618Phe, and multi-glandular disease in relationship with p.Cys634Arg, p.Cys611Tyr, and p.Leu790Phe [38], while Larsen et al. [47] found multi-gland involvement in subjects with PHPT as first MEN2 manifestation with respect to p.Cys634Tyr and p.Cys634Arg pathogenic variants, and single-gland disease in p.Cys634Tyr, p.Cys634Arg, p.Cys611Tyr, p.Cys620Arg, p.Glu768Asp, and p.Cys618 [47].
Some studies assessed PHPT with regard to the RET pathogenic variant-associated risk category for MTC [39,40]. High-risk variants had a higher prevalence of PHPT compared with moderate-high risk variants and low-risk variants (11.4% versus 2.4% versus 0.5%, p < 0.001). However, the age at PTx did not have a statistically significant difference (p = 0.270) among these mentioned subgroups of analysis. In high-risk variants (for MTC), PHPT prevalence was lower during the recent years (p < 0.001) [40]. Moreover, the age at PTx in index cases versus non-index cases was similar, as similarly found in individuals with high-risk pathogenic variants (p = 0.370) or moderate-high risk (p = 0.980) for MTC [39]. Another cohort from 2023 found a statistically significant increased prevalence of PHPT in patients harboring p.Cys634Arg/Thr/Tyr versus p.Cys618Arg (33.3% versus 3.2%, p = 0.01) [41] (Table 2).

Table 2.
RET pathogenic variants in patients with primary hyperparathyroidism amid MEN2 confirmation [35,36,38,39,40,41,43,45,47,48].
3.2. The Clinical Presentation and Spectrum of Complications in MEN2-Related Primary Hyperparathyroidism
Data regarding symptoms/clinical presentation of PHPT in MEN2 subjects were provided by 5/14 studies (N = 49 patients with MEN2-related PHPT) [36,37,38,46,47]. The most frequent clinical finding/complication was nephrolithiasis with a prevalence of up to 80% [47], followed by osteoporosis with the maximum prevalence of 12.5% [38]. Other symptoms included pancreatitis [46], polyuria [47], and non-specific clinical picture [37], while chronic kidney disease was reported in 9.1% of the individuals in one cohort [36]. Although most studies reported symptomatic PHPT, Holm et al. [38] found that 75% of patients with PHPT were asymptomatic [38]. Of note, apart from PHPT-related symptoms, Larsen et al. [47] also reported the timing of MTC diagnosis, which was synchronous with PHPT diagnosis in 80% and metachronous in 10% of the patients [47] (Table 3).

Table 3.
Clinical picture related to the diagnosis of primary hyperparathyroidism in patients confirmed with MEN2 [36,37,38,46,47].
3.3. Parathyroidectomy in MEN2 Subjects
Ten studies (n = 10/14) reported data regarding PTx (N = 156 subjects with MEN2-related PHPT) [36,37,38,39,40,42,44,46,47,48]. Between 72.7% [36] and 100% [37,39,42,47] of PHPT patients underwent PTx as curative treatment for the underlying parathyroid tumors. PTx was typically performed in adulthood at ages spanning from a mean of 34.7 to 48.5 years [39]. Based on the specific RET pathogenic variant, there were no statistically significant differences regarding the age at PTx (p = 0.270) [40]. When index and non-index patients were compared, the age at PTx was similar, as well [39]. The parent who transmitted the gene, however, influenced the age at PTx, as was shown by Machens et al. [44] who revealed that subjects who inherited the pathogenic variant from the father had younger age at PTx compared to those who inherited from the mother (Plog-rank = 0.018) [44].
Furthermore, in recent years, PTx was performed at younger ages compared to the past as shown by a retrospective study (e.g., the age at PTx decreased from 43.5 years in ≤1950 to 16.5 years in the 1991–2000 birth cohorts with high-risk pathogenic variants) [40], while a cross-sectional study confirmed the same outcome [age at PTx, median (IQR) by birth cohort: 1922–1950 versus 1951–1960 versus 1961–1970 versus 1971–1980 versus 1981–1990 versus 1991–2000 versus 2001–2010: 46 (39.5–55) versus 42 (31–45.5) versus 31 (23–36) versus 26 (26–26) versus 12 (12–12) y, p = 0.008] [48].
With respect to the surgical technique (n = 4/14 studies, N = 32 patients who underwent PTx), selective PTx was performed most commonly [36]. Subtotal PTx was the second most used technique, with the highest prevalence of 69% in a population-based study by Holm et al. [38]. Other procedures included subtotal PTx with auto-transplantation of the parathyroid tissue in the forearm [38] and bilateral neck exploration [37].
The outcomes of PTx varied (only three studies reported the postoperative outcomes), for example, the post-surgery rates of persistent PHPT was of 0% [36], 8% [38], and 16.7% [48]; recurrent PHPT was of 12.5% [36] and 23% [38]; permanent hypoparathyroidism was reported in 46% [38] and 33.3%, respectively [44]; permanent unilateral vocal cord palsy was not identified in one study [38], but another reported it in 16.7% of the subjects [44] (Table 4).

Table 4.
Parathyroidectomy-related findings in surgery candidates amid the confirmation of MEN2-associated primary hyperparathyroidism [36,37,38,39,40,42,44,46,47,48].
3.4. Histological Analysis of the Parathyroid Tumors
Data regarding the number of glands affected were reported by seven studies (N = 77 patients who underwent PTx) [36,37,38,39,42,46,47]. The prevalence of multi-glandular disease ranged between 12.5% [36] and 50% [42,46], while the rate of uni-glandular disease was of 33.3% [42] and 87.5% [36], respectively. In terms of parathyroid tumor size, two studies provided these specific data [37,39]: Gasior et al. [37] found a median (IQR) tumor size of 0.7 (0.55–0.9) cm, with a median (IQR) tumor mass of 118 (56.3–302) mg [37], while Machens et al. [39] reported similar tumor diameters in index and non-index cases of 3.72 (2.98–4.47) and 4.07 (3.39–4.75) cm, respectively (p = 0.505) [39] (Table 5).

Table 5.
Histological features in patients with primary hyperparathyroidism and MEN2 [36,37,38,39,42,46,47].
3.5. Imaging Assessment in Patients with MEN2-Associated Primary Hyperparathyroidism
Three studies [37,42,46] reported imaging findings from 16 patients with PHPT due to MEN2, including preoperative localization [37,46] and intraoperative imaging [42]. Gasior et al. [37] reported that preoperative localization as follows: 40% of patients had ultrasound assessment, and 40% underwent Tc-98m Sestamibi scans [37]. However, preoperative localization was not always successful as it was shown by Diwaker et al. [46] who reported a successful rate in 29% of patients [46]. Berber et al. [46] investigated auto-fluorescence signals during PTx, a more recent technique in PHPT, including MEN2 patients, and reported a median auto-fluorescence intensity of 1.8 and a median heterogeneity index of 0.11 [46].
3.6. Primary Hyperparathyroidism in MEN2 Versus Other Familial Syndromes
Figueiredo et al. [36] investigated differences among different familial forms of PHPT in a retrospective analysis on 48 subjects with familial PHPT, including 11 individuals with PHPT in the setting of MEN2. When PHPT in MEN2 was compared with MEN1, there was no statistically significant difference in the prevalence of PHPT as first manifestation of the syndrome (p = 0.13). However, serum parathormone (PTH) was lower (median of 108.0 versus 196.9 pg/mL, p = 0.01), serum calcium levels were lower (mean ± SD: 10.6 ± 1.1 versus 11.7 ± 1.2 mg/dL, p = 0.03), and less parathyroid glands were affected [median ± standard deviation (SD): 1.1 ± 0.3 versus 2.7 ± 0.9, p < 0.001] in MEN2 compared to MEN1 [36].
In MEN2, PHPT was the first manifestation less frequently compared to hyperparathyroidism-jaw tumor syndrome (0% versus 85%, p = 0.001), while serum PTH (median: 108.0 versus 383.5 pg/mL, p = 0.01) and serum total calcium (mean ± SD: 10.6 ± 1.1 versus 12.9 ± 1.8 mg/dL, p < 0.001) levels were lower, and nephrolithiasis occurred less often (18.2% versus 65%, p = 0.02). The number of parathyroid glands was similar (1.1 ± 0.3 versus 1.6 ± 1.1, p = 0.23) [36].
3.7. Medullary Thyroid Carcinoma in MEN2 Patients (The Data According to the Studies That Also Provided an Analysis of the Primary Hyperparathyroidism)
Ten studies (n = 10/14; N = 3760 patients) analyzed the features of MTC amid MEN2 confirmation [35,38,39,40,41,43,44,46,47,48]; the highest prevalence of MTC reached 100% [47] and varied according to the RET pathogenic variant, with maximum rate in p.Met918Thr (100%) and p.Cys634Phe/Gly/Arg/Ser/Trp/Tyr (88.9%) [35]. Machens et al. [40] reported a higher prevalence in high-risk pathogenic variants (75% versus 65.2% versus 63.2%, p = 0.016) and younger age at thyroidectomy (17 versus 29 versus 39 years, p < 0.001) [40]. A multicenter study showed that RET pathogenic variants correlated with the tumor size; variants affecting codon C634 being associated with larger tumors compared with those involving codon C618 (1.85 ± 1.11 versus 0.89 ± 0.67 cm, p = 0.004) and with higher calcitonin levels as well (333.9 ± 314.5 versus 84.5 ± 201.9 ng/mL, p = 0.030) [41]. The prevalence of C-cell hyperplasia versus MTC confirmation was reported by Holm et al. [38] at 31% versus 69% [38]; similar findings were also revealed by a study on p.Cys634 carriers (76.5% versus 21.6%) [48].
MEN2 individuals who were diagnosed through screening protocols were younger compared with those with hereditary MTC (28.3 ± 19.8 versus 30.15 ± 15.3 years, p < 0.05); they also had smaller tumors compared with sporadic MTC and MEN2 index cases (2.9 ± 0.85 versus 3.14 ± 1.43 versus 2.96 ± 1.38 cm) [46]. Index cases had a higher prevalence of MTC (97.4% versus 57.0%, p < 0.001), as well as larger tumors (1.95 versus 0.79 cm, p < 0.001], higher rates of lymph nodes metastases (71.5% versus 29.5%, p < 0.001), and lower rates of biochemical cure (34.1% versus 74.8%, p < 0.001) [39]. Similar MTC prevalence were highlighted in subjects who inherited the RET variant from the mother or the father [43,44], while lymph node metastases were more frequent in patients who received the gene pathogenic variant from the father compared with the mother (45% versus 19%, p = 0.006 and 43% versus 29%, p = 0.029) [43,44] (Table 6).

Table 6.
Findings regarding medullary thyroid carcinoma in MEN2 patients (the data according to the studies that also provided an analysis of the primary hyperparathyroidism) [35,38,39,40,41,43,44,46,47,48].
3.8. MEN2-Associated Pheochromocytoma (The Data According to the Studies That Also Provided an Analysis of the Primary Hyperparathyroidism)
MEN2-related PC analysis was provided by eleven studies (n = 11/14, N = 4026 patients) [35,38,39,40,41,43,44,45,46,47,48]. High-risk RET pathogenic variants had the highest prevalence of PC, of 55.6% (in p.Cys634Phe/Gly/Arg/Ser/Trp/Tyr) and of 50% (in p.Met918Thr) [35]. These findings are supported by another study that reported a higher prevalence of PC in high-risk pathogenic variants compared with moderate-high and low-risk groups (32.1% versus 16.4% versus 3%, p < 0.001) [40]. Moreover, p.Cys634Arg/Thr/Tyr also had a higher prevalence of PC compared with p.Cys618Arg (53.3% versus 6.5%, p = 0.001) [41]. Milcevic et al. [45] found that, while RET pathogenic variants such as p.Cys634Phe/Gly/Arg/Ser/Trp/Tyr and p.Cys618Phe/Arg/Ser had a PC prevalence of 70.6% and 4.5%, respectively, PC did not occur in other variants such as p.Leu790Phe, p.Val804Met, p.Ser891Ala, and p.Met918Thr [45].
Synchronous diagnosis of PHPT and PC was found in 56%, while PC was identified before PHPT in 22% of the cases, respectively; PC was confirmed after PHPT in 22% [38]. Index cases had a higher prevalence of PC compared to non-index cases (30.1% versus 13.2%, p < 0.001), but similar ages at adrenalectomy (p = 0.431) [39]. PC had the same prevalence in subjects who inherited the variant from the mother compared with the father origin (19% versus 33%, p = 0.051 [43] and 13% versus 19%, p = 0.094 [44]). However, there was a higher prevalence of bilateral PC in those who inherited the pathogenic variant from the father (24% versus 10%, p = 0.021) [43]). Other MEN2-related PC findings included a prevalence of 48.64% [46] and a PC rate in the second adrenal gland in 18.8% of MEN2 patients [48] (Table 7).

Table 7.
Findings regarding pheochromocytoma in MEN2 patients (the data according to the studies that also provided an analysis of the primary hyperparathyroidism) [35,38,39,40,41,43,44,45,46,47,48].
4. Discussion
4.1. Inherited Forms of Primary Hyperparathyroidism
Syndromic (genetic or hereditary) combinations of endocrine ailments, either involving autoimmune or tumor/cancer features, still represents a multidisciplinary challenge nowadays [52,53,54,55]. A total of 5–10% of PHPT patients may have a familial (monogenic) form of disease [56]. Hereditary PHPT occurs either as the sole endocrine condition (as found in familial isolated PHPT), or as a syndromic type. Genetic syndromes that promote the development of PHPT have an autosomal dominant inheritance pattern and include MEN1, MEN2, MEN4, and hyperparathyroidism-jaw tumor syndrome [57,58,59]. While MEN1, MEN4, and hyperparathyroidism-jaw tumor syndrome involve inactivating pathogenic variants of tumor suppressor genes MEN1, CDKN1B, and CDC73, respectively, MEN2 is caused by activating mutations of RET proto-oncogene [60,61,62,63]. These syndromes often present a variety of symptom clusters and multi-layered complications; hence, their management represents a complex process [64,65,66]. Differentiating between the familial causes of PHPT is one of the most important steps for an adequate multimodal management and active search/screening for other associated endocrine and non-endocrine manifestations of the syndromes, as well as for initiating a genetic screening among the family members [66].
While PHPT is a cardinal feature of hyperparathyroidism-jaw tumor syndrome [67] and it is the main manifestation of MEN1, affecting over 90% of patients [68], the prevalence of PHPT in MEN2 is lower, with a penetrance around 30% (e.g., recent data reported a prevalence of up to 31.3% [45], as identified across our analysis). Another key difference between these syndromes is the number of parathyroid glands involved; typically, familial syndromes have multi-glandular involvement [69,70]. However, our analysis highlighted unexpected high rates of single-gland disease, as mentioned by some studies [36,37,38,47]. Despite a low frequency for a multi-gland involvement, PHPT post-surgery recurrence was increased, up to 23% [38].
In MEN2 subjects, PHPT is rarely the first manifested endocrine disorder [47]. Recognizing MEN2 in these patients is extremely important, so that MTC screening may be early performed for a better overall prognosis [71]. Figueiredo et al. [36] highlighted key differences between distinct hereditary PHPT forms and found that the lowest prevalence was among familial PHPT (of 22.9%) compared with 41.7% in patients with hyperparathyroidism-jaw tumor syndrome, or 35.4% in MEN1 [36]. The importance of PHPT diagnosis in MEN2 patients should not be downplayed by the relatively less severe presentation compared with other syndromic manifestations such as the presence of a potentially severe thyroid malignancy, especially if PHPT co-occurs with MTC, making preoperative diagnosis of both conditions crucial for the surgical approach [72,73]. Notably, a case–control analysis of the serum calcium levels in PHPT amid MEN2 confirmation (in order to highlight the impact of acute hypercalcemia and a potentially more severe presentation) was not provided by all the studies we could identify [35,38,39,40,41,42,43] or the results only included data with a relatively low statistical power [37]. On the other hand, as mentioned, Figueiredo et al. [36] showed that serum total calcium and PTH were statistically significantly lower in MEN2 than MEN1, of 10.6 10.6 ± 1.1 versus 11.7 ± 1.2 mg/dL (p = 0.03) and 108 versus 196.9 pg/mL (p = 0.01), respectively, but, also, than found in hyperparathyroidism-jaw tumor syndrome, of 12.9 ± 1.8 mg/dL (p < 0.001) and 383.5 pg/mL (p = 0.01) [36], respectively.
Primary Hyperparathyroidism in the Setting of MEN2
MEN2 leads to the development of neoplasia in the thyroid, adrenals, and parathyroid glands. The first clinical manifestation, with the highest penetrance, occurring in virtually all patients with MEN2, is MTC [74,75,76]. The strong link between RET and MEN2 is further highlighted by the prevalence of germline RET pathogenic variants in MTC patients in the general population, which exceeds 16% [77]. Considering that most patients with MEN2 are typically diagnosed with MTC during childhood, many undergo prophylactic thyroidectomy at an early age [78,79,80]. In order to achieve the best cure rate, RET genetic screening and early diagnosis, as well as identifying the pathogenic variant risk category for better surgical planning, are essential [80].
According to ATA guidelines, the risk of MTC is stratified by the RET pathogenic variant in distinct categories, the highest being in codon M918T, while codon C634 and codon A883F also involve a high risk of MTC [78,79,80]. Recent data analyzed characteristics of PHPT based on this classification. As mentioned, two studies reported a higher prevalence of PHPT in high-risk pathogenic variants [40,41]. However, the age at PTx was similar across these pathogenic variant groups [40].
A new classification of RET variants associated with MEN2 has been recently proposed by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology and includes the introduction of two new categories, likely benign and likely pathogenic, as well as the re-classification of the significance in certain RET variants [81].
In association with MTC and PHPT, PC affects 30% of the MEN2 subjects; its penetrance is connected to the MTC aggressiveness [82]. PC usually manifests during the third decade of life, and due to the life-threatening complications, early detection across the biochemical screening is advised [83,84]. Identifying PC prior to other surgical interventions is crucial, as hidden/unrecognized PC may receive inappropriate alpha-adrenergic receptor blockade, with severe perioperative consequences, including cardiac arrest and fatal outcome [85]. Surgical treatment requires preoperative blood pressure control and the prevention of intraoperative hypertensive crises, and the presence of PHPT-related hypercalcemia should be carefully taken into consideration as well [86]. When synchronously occurring with MTC or PHPT, PC needs immediate attention and adrenalectomy should be performed before thyroidectomy or PTx [87]. In some cases, simultaneous (single-time) adrenalectomy and thyroidectomy have successfully been performed, and it requires an experienced surgical team [88].
PHPT, a less frequent manifestation of MEN2, associates, however, a much higher prevalence than seen in the general population which, although rising, is less than 1% [89,90]. Typically, sporadic PHPT affects menopausal women, while hereditary PHPT manifests at much younger ages with similar prevalence in men and women [2,91]. In MEN2, however, while the prevalence in men and women is similar, PHPT usually manifests later in life compared to other familial syndromes, in the third and fourth decades of life, as also reflected by our sample-based analysis [35,36,38,40,47,48].
Typical symptoms of PHPT include nephrolithiasis, low-trauma/spontaneous fractures and osteoporosis, cardio-metabolic complications, non-specific symptoms such as digestive symptoms, and fatigue, as well as various clinical features that are induced by acute hypercalcemia [92,93,94]. Yet, nowadays, due to calcium screening protocols, the most often presentation is asymptomatic or mildly symptomatic PHPT [95,96,97]. Another disease form is normocalcemic PHPT, often diagnosed during supplementary investigations of subjects with kidney stones, low bone mineral density, or incidental fractures, an entity characterized by normal levels of albumin-adjusted serum calcium and ionized calcium and high levels of PTH in the absence of secondary causes of high PTH [96,98,99,100,101]. Recent data (across our methods of search) did not particularly report or explore this disease form in MEN2-PHPT, and future studies are needed. As mentioned, classical symptoms of PHPT such as nephrolithiasis and osteoporosis were described in some studies [38,47]. Additionally, about 6% of patients with PHPT may develop pancreatitis [46], a manifestation that might be the sole indication of PHPT in some cases [102,103]. Moreover, non-classical features in PHPT (e.g., depression, anxiety, cardiovascular manifestations, or glucose metabolism changes [104,105]) were not distinctly analyzed in this MEN2-PHPT-focused analysis.
Of note, PHPT increases the fragility fracture risk due to cortical bone loss, especially of the distal radius, as well as altered bone microarchitecture, as reflected by low trabecular bone score [106,107,108]. Therefore, the presence of osteoporosis represents a surgical indication in PHPT [109]. Holm et al. [38] identified that 12.5% of the PHPT subjects had osteoporosis [38]; however, we could not identify any more specific data across the other studies regarding the bone status.
4.2. Surgery Candidates
Surgical indications in MEN2-related PHPT are similar to those applied to PHPT in the general population (e.g., the identification of skeletal or renal complications, serum total calcium levels higher than 1 mg/dL above the upper limit or patient age below 50 years, etc.) [109]. Surgical planning is highly individualized and has several particularities. For instance, PTx often needs to be performed on an already explored neck, making the surgery more difficult and exposing the patient to a higher rate of complications [110,111]. Typically, the first manifestation of MEN2 is MTC, a severe disease with a high mortality and morbidity that requires total thyroidectomy with prophylactic or curative lateral neck dissection [112,113,114]. Due to the high penetrance of MTC, especially in high-risk variants, prophylactic thyroidectomy is practiced in RET pathogenic variants carriers at very early ages [115,116]. While prophylactic thyroidectomy in children may be performed without lymph node dissection in certain stages/mutations, MTC surgery may need large neck dissection with multiple postoperative complications [117,118,119]. Hence, a future lateral neck exploration is more difficult due to fibrosis, etc. [120,121].
Notably, preoperative imaging scans represent another crucial step for surgical planning, especially considering the multi-glandular disease in PHPT (with asynchronous presentation) [47,122]. Moreover, the preoperative assessment should include the evaluation of previous forearm grafts from prior PTxs, and of possible complications from prior surgery(s) such as laryngeal nerve palsy, etc. [122].
Prophylactic PTx in MEN2 patients who undergo thyroidectomy for MTC was explored in the past, but it is not currently supported due to the fact that PHPT is actually diagnosed later in life (during adulthood) and PHPT penetrance is only 30% [123]. Older data showed that, while some authors are in support of total PTx and auto-transplantation since it seems a safe approach [124,125], nowadays, if parathyroid glands are macroscopically normal, we consider that they should not be removed, noting the low rate of recurrence and of surgical complications [126,127,128,129,130]. Currently, a less aggressive and minimally invasive approach is preferred in order to reduce the post-operatory rate of complications, including hypocalcemia and hypoparathyroidism [127,128,129,130]. The use of intra-operatory PTH assays helps the rate of complete gland/tumor removal and avoids unnecessary redo surgery [126,127,128,129,130].
If total PTx with auto-transplantation is performed, regular follow-up is still needed, due to PHPT recurrence or multi-gland involvement, including in ectopic parathyroid [129,130]. As mentioned, we identified a single study that explored intraoperative auto-fluorescence [42], a novel technique used for identifying parathyroid glands based on the natural fluorescence emitted by the parathyroid tissue during exposure to near-infrared light (which is especially useful in bilateral neck exploration) [131,132,133,134]. The clinical and therapeutic implications in MEN2 patients, however, remain an emerging topic that needs further studies. No additional data with respect to using intraoperative indocyanine green angiography for glands localization in MEN2 we could identify, neither in using cryopreservation, but these seem promising alternatives for selected cases amid a tailored multimodal management in MEN2-related PHPT.
4.3. Case Report-Focused Analysis
Exploring the fascinating domain of PTH, from PTH-producing tumors amid genetic conditions to the practical use of PTH analogues in daily endocrine practice [135], might pinpoint aspects with a lower level of statistical evidence. Hence, across our search, we identified a collateral result in terms of five novel reports [136,137,138,139,140] in MEN2-PHPT in subjects who underwent genetic testing for RET pathogenic variants. Among them, four cases included adult patients (three males and one female) with ages between 26 and 68 years, diagnosed with PHPT in the setting of MEN2 [136,137,138,139]. All patients were surgically treated for PHPT: selective PTx was performed in one patient [136], while another underwent total PTx with re-implantation in the forearm [139]. Thoracoscopy was necessary in the case of a 26-year-old male for an ectopic parathyroid mass, after having undergone a prior PTx with post-surgery persistent PHPT. Following the resection of the ectopic mass, the patient developed hypoparathyroidism [137]. Synchronous MTC occurred in 3/5 subjects requiring thyroidectomy [136.137,139]. Two patients associated PC [136,137], while another had a pancreatic paraganglioma [138]. Family history was positive only in 1/5 subjects [139]. However, while the other three did not have a family history of endocrine neoplasia [136,137,138], another had three relatives positive for the same RET pathogenic variant [136].
The complexity of hereditary forms of PHPT was reflected by a 28-year-old male who developed asymptomatic PHPT, multifocal MTC, and bilateral PC in the setting of two pathogenic variants of the RET gene (Cys630Tyr) and of the MEN1 gene (p.Ala176Leufs*10) [139]. One case in particular highlighted the phenotypic variability and interconnection between MEN2 and MEN3 manifestations. In this case of a 7-year-old girl, reported by Giani et al. [140], the p.Asp631_Leu633delinsGlu de novo RET variant manifested suggestive features for MEN2 (e.g., PHPT) and distinct aspects of MEN3 (including marfanoid habitus and mucosal neuromas). In addition, the patient had MTC and a history of plexiform neurofibroma and ganglioneuromatosis [140] (Table 8).

Table 8.
Case reports of PHPT in MEN2 patients (the display starts with the most recent publication date) [136,137,138,139,140].
4.4. Limits and Further Expansion
This sample-focused analysis was introduced across a non-systematic review in order to not restrain the original studies data to similar statistical parameters, a type of analysis which is less likely feasible at this point in the field of MEN2-PHPT. As mentioned across this narrative review, our search did not detect distinct results in terms of PHPT-connected clinical elements such as osteoporosis or fracture prevalence, etc. Further longitudinal studies are necessary to assess the long-term outcome in patients with MEN2-PHPT. Moreover, we raise the issue of epidemiologic data with concern to new PHPT subtypes such as normocalcemic or asymptomatic in MEN2 individuals. As specified, novel surgical procedures, including minimally invasive approaches, might minimize the impact of the surgery in these patients and provide a better outcome (Figure 2).
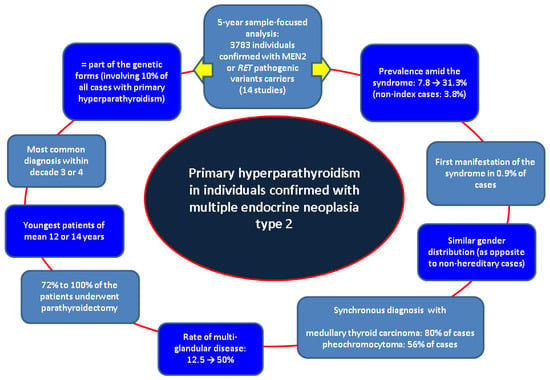
Figure 2.
Flowchart of the main findings across our methods [35,36,37,38,39,40,41,42,43,44,45,46,47,48].
5. Conclusions
PHPT in MEN2 patients may occasionally present as the first manifestation of the syndrome. In contrast to other familial forms of PHPT, it usually manifests later in life during the third and fourth decades and frequently has single-gland involvement. In spite of a relatively low prevalence compared to the other manifestations of MEN2, PHPT should not be overlooked considering the chance of recurrence and the high frequency in high-risk mutations. Surgical planning needs to be tailored to every case and takes into consideration previous neck surgery, possible preexisting complications, and the transformation of transplanted parathyroid tissue. Apart from early diagnosis and surgical treatment, life-long follow-up represents the key to the management of PHPT in MEN2 individuals.
Author Contributions
Conceptualization, A.-M.G., C.N., A.-F.F. and M.C.; methodology, A.-M.G., C.N., A.-F.F. and M.C.; software, A.-M.G., C.N., A.-F.F. and M.C.; validation, A.-M.G., C.N., A.-F.F. and M.C.; formal analysis, A.-M.G., C.N., A.-F.F. and M.C.; investigation, A.-M.G., C.N., A.-F.F. and M.C.; resources, A.-M.G., C.N., A.-F.F. and M.C.; data curation, A.-M.G., C.N., A.-F.F. and M.C.; writing—original draft preparation, A.-M.G.; writing—review and editing, M.C.; visualization, A.-M.G., C.N., A.-F.F. and M.C.; supervision, A.-M.G., C.N., A.-F.F. and M.C.; project administration, A.-M.G. and M.C.; funding acquisition, A.-M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Carol Davila” University of Medicine and Pharmacy, PhD research “Primary hyperparathyroidism: cardio-metabolic, osseous and surgical aspects”—2023.
Acknowledgments
This is part of the PhD research belonging to the PhD Doctoral School of “Carola Davila” University of Medicine and Pharmacy, entitled “Primary hyperparathyroidism: cardio-metabolic, osseous and surgical aspects”—28374/2 October 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ATA | American Thyroid Association |
| CI | confidence interval |
| HR | hazard ratio |
| IQR | interquartile range |
| MEN | multiple endocrine neoplasia |
| MTC | medullary thyroid carcinoma |
| PC | pheochromocytoma |
| PHPT | primary hyperparathyroidism |
| PTx | parathyroidectomy |
| PTH | parathormone |
| SD | standard deviation |
References
- Palermo, A.; Tabacco, G.; Makras, P.; Zavatta, G.; Trimboli, P.; Castellano, E.; Yavropoulou, M.P.; Naciu, A.M.; Anastasilakis, A.D. Primary hyperparathyroidism: From guidelines to outpatient clinic. Rev. Endocr. Metab. Disord. 2024, 25, 875–896. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Arnold, A.; Belaya, Z.; Brandi, M.L.; Clarke, B.L.; Hannan, F.M.; Hofbauer, L.C.; Insogna, K.L.; Lacroix, A.; Liberman, U.; et al. Epidemiology, Pathophysiology, and Genetics of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gillis, A.; Lindeman, B.; Chen, H.; Fazendin, J. Normocalcemic primary hyperparathyroidism: From pathophysiology to clinical management. Am. J. Surg. 2024, 235, 115812. [Google Scholar] [CrossRef] [PubMed]
- Cetani, F.; Dinoi, E.; Pierotti, L.; Pardi, E. Familial states of primary hyperparathyroidism: An update. J. Endocrinol. Investig. 2024, 47, 2157–2176. [Google Scholar] [CrossRef]
- English, K.A.; Lines, K.E.; Thakker, R.V. Genetics of hereditary forms of primary hyperparathyroidism. Hormones 2024, 23, 3–14. [Google Scholar] [CrossRef]
- Manea, M.M.; Dragos, D.; Ghenu, M.I.; Enache, I.I.; Stoican, I.C.; Ciulavu, C.; Vasiliu, O.; Sirbu, C.A.; Tuta, S. The Neurocardiogenic Impact of Ischemic Stroke: Intricacies of Cardiac Enzymes and the Vegetative System. Rom. J. Mil. Med. 2025, 128, 36–42. [Google Scholar] [CrossRef]
- Mitrica, M.; Vasiliu, O.; Plesa, A.; Sirbu, O.M. Multinodular and vacuolating neuronal tumor. Rom. J. Mil. Med. 2025, 128, 10–16. [Google Scholar] [CrossRef]
- Lauricella, E.; Chaoul, N.; D’Angelo, G.; Giglio, A.; Cafiero, C.; Porta, C.; Palmirotta, R. Neuroendocrine Tumors: Germline Genetics and Hereditary Syndromes. Curr. Treat. Options Oncol. 2025, 26, 55–71. [Google Scholar] [CrossRef]
- Balachandra, S.; Fazendin, J.; Chen, H. Complex Primary Hyperparathyroidism: Hereditary and Recurrent Disease. Surg. Clin. N. Am. 2024, 104, 811–823. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Grossrubatscher, E.M.; Hasballa, I.; Tarsitano, M.G.; Centello, R.; Isidori, A.M.; Colao, A.; Pellegata, N.S.; et al. Multiple endocrine neoplasia type 4 (MEN4): A thorough update on the latest and least known men syndrome. Endocrine 2023, 82, 480–490. [Google Scholar] [CrossRef]
- Ababneh, E.; Nosé, V. Para This, Fibromin That: The Role of CDC73 in Parathyroid Tumors and Familial Tumor Syndromes. Surg. Pathol. Clin. 2023, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mitrică, M.; Lorusso, L.; Badea, A.A.; Sîrbu, C.A.; Pleșa, A.; Stănescu, A.A.; Pleșa, F.C.; Sîrbu, O.M.; Munteanu, A.E. The Hidden Heart: Exploring Cardiac Damage Post-Stroke: A Narrative Review. Medicina 2024, 60, 1699. [Google Scholar] [CrossRef]
- Walker, T.J.; Mulligan, L.M. Cellular mechanisms of RET receptor dysfunction in multiple endocrine neoplasia 2. Endocr. Relat. Cancer 2024, 32, e240187. [Google Scholar] [CrossRef]
- McDonnell, J.E.; Gild, M.L.; Clifton-Bligh, R.J.; Robinson, B.G. Multiple endocrine neoplasia: An update. Intern. Med. J. 2019, 49, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Huai, J.X.; Wang, F.; Zhang, W.H.; Lou, Y.; Wang, G.X.; Huang, L.J.; Sun, J.; Zhou, X.Q. Unveiling new chapters in medullary thyroid carcinoma therapy: Advances in molecular genetics and targeted treatment strategies. Front. Endocrinol. 2024, 15, 1484815. [Google Scholar] [CrossRef]
- Poturnajova, M.; Altanerova, V.; Kostalova, L.; Breza, J.; Altaner, C. Novel germline mutation in the transmembrane region of RET gene close to Cys634Ser mutation associated with MEN 2A syndrome. J. Mol. Med. 2005, 83, 287–295. [Google Scholar] [CrossRef]
- Greenberg, L.A. Multiple Endocrine Neoplasia Type 1, Type 2A, and Type 2B. Prim. Care 2024, 51, 483–494. [Google Scholar] [CrossRef]
- Romei, C.; Ciampi, R.; Casella, F.; Tacito, A.; Torregrossa, L.; Ugolini, C.; Basolo, F.; Materazzi, G.; Vitti, P.; Elisei, R. RET mutation heterogeneity in primary advanced medullary thyroid cancers and their metastases. Oncotarget 2018, 9, 9875–9884. [Google Scholar] [CrossRef] [PubMed]
- Giacché, M.; Tacchetti, M.C.; Agabiti-Rosei, C.; Torlone, F.; Bandera, F.; Izzi, C.; Agabiti-Rosei, E. Pheochromocytoma-Paraganglioma Syndrome: A Multiform Disease with Different Genotype and Phenotype Features. Biomedicines 2024, 12, 2385. [Google Scholar] [CrossRef]
- Nica, S.; Sionel, R.; Maciuca, R.; Csutak, O.; Ciobica, M.L.; Nica, M.I.; Chelu, I.; Radu, I.; Toma, M. Gender-Dependent Associations Between Digit Ratio and Genetic Polymorphisms, BMI, and Reproductive Factors. Rom. J. Mil. Med. 2025, 128, 78–86. [Google Scholar] [CrossRef]
- Erickson, L.A.; Mete, O.; Juhlin, C.C.; Perren, A.; Gill, A.J. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr. Pathol. 2022, 33, 64–89. [Google Scholar] [CrossRef]
- Alevizaki, M.; Saltiki, K. Primary Hyperparathyroidism in MEN2 Syndromes. Recent Results Cancer Res. 2015, 204, 179–186. [Google Scholar] [CrossRef]
- Vasiliu, O.; Panea, C.A.; Mangalagiu, A.G.; Petrescu, B.M.; Cândea, C.A.; Manea, M.M.; Ciobanu, A.M.; Sîrbu, C.A.; Mitrică, M. Case Management of Delirium in Patients with Major Neurocognitive Disorders. Rom. J. Mil. Med. 2025, 128, 67–77. [Google Scholar] [CrossRef]
- Tonon, C.R.; Silva, T.A.A.L.; Pereira, F.W.L.; Queiroz, D.A.R.; Junior, E.L.F.; Martins, D.; Azevedo, P.S.; Okoshi, M.P.; Zornoff, L.A.M.; de Paiva, S.A.R.; et al. A Review of Current Clinical Concepts in the Pathophysiology, Etiology, Diagnosis, and Management of Hypercalcemia. Med. Sci. Monit. 2022, 28, e935821. [Google Scholar] [CrossRef]
- Cormier, C.; Koumakis, E. Bone and primary hyperparathyroidism. Jt. Bone Spine 2022, 89, 105129. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobica, L.M.; Negru, M.M.; Jurcut, C.; Otlocan, L.; Coca, A. Bone mineral density and vitamin D levels in patients with rheumatoid arthritis. Osteoporos. Int. 2017, 28, S435–S436. [Google Scholar]
- Stanciu, S.; Enciu, C.; Raduta, I.; Stoicescu, D.; Anghel, A.; Anghel, D.; Olan, B.; Ciobica, L. The role of contrast-enhanced ultrasound in risk assessment of carotid atheroma. Rom. J. Mil. Med. 2016, 119, 9–11. [Google Scholar]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef]
- Cristina, E.V.; Alberto, F. Management of familial hyperparathyroidism syndromes: MEN1, MEN2, MEN4, HPT-Jaw tumour, Familial isolated hyperparathyroidism, FHH, and neonatal severe hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 861–875. [Google Scholar] [CrossRef]
- Aggarwal, P.; Gunasekaran, V.; Sood, A.; Mittal, B.R. Localization in primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 39, 101967. [Google Scholar] [CrossRef]
- Iacobone, M.; Citton, M.; Viel, G.; Schiavone, D.; Torresan, F. Surgical approaches in hereditary endocrine tumors. Updates Surg. 2017, 69, 181–191. [Google Scholar] [CrossRef]
- Frye, C.C.; Brown, T.C.; Olson, J.A., Jr. Evaluation and Surgical Management of Multiple Endocrine Neoplasias. Surg. Clin. N. Am. 2024, 104, 909–928. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Haissaguerre, M.; Tresallet, C.; Kuczma, P.; Marciniak, C.; Cardot-Bauters, C. Chapter 6: Syndromic primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101695. [Google Scholar] [CrossRef]
- Romanet, P.; Coppin, L.; Molin, A.; Santucci, N.; Le Bras, M.; Odou, M.F. Chapter 5: The role of genetics in primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101694. [Google Scholar] [CrossRef]
- Binter, T.; Baumgartner-Parzer, S.; Schernthaner-Reiter, M.H.; Arikan, M.; Hargitai, L.; Niederle, M.B.; Niederle, B.; Scheuba, C.; Riss, P. Does Genotype-Specific Phenotype in Patients with Multiple Endocrine Neoplasia Type 2 Occur as Current Guidelines Predict? Cancers 2024, 16, 494. [Google Scholar] [CrossRef]
- Figueiredo, A.A.; Saramago, A.; Cavaco, B.M.; Simões-Pereira, J.; Leite, V. Familial parathyroid tumours-comparison of clinical profiles between syndromes. J. Endocrinol. Investig. 2023, 46, 1799–1806. [Google Scholar] [CrossRef]
- Gasior, J.; Kelz, R.R.; Karakousis, G.C.; Fraker, D.L.; Wachtel, H. Primary Hyperparathyroidism in Young Adult Patients. Ann. Surg. Oncol. 2023, 30, 4156–4164. [Google Scholar] [CrossRef]
- Holm, M.; Vestergaard, P.; Poulsen, M.M.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Bay, M.; Rolighed, L.; Londero, S.; Pedersen, H.B.; Hahn, C.H.; et al. Primary Hyperparathyroidism in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Population-Based Retrospective Study. Cancers 2023, 15, 2125. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Brandenburg, T.; Führer-Sakel, D.; Dralle, H. Clinical presentation of MEN 2A in index vs. non-index patients. Endocrine 2023, 82, 450–455. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Brandenburg, T.; Führer-Sakel, D.; Weber, F.; Dralle, H. The Changing Face of Multiple Endocrine Neoplasia 2A: From Symptom-Based to Preventative Medicine. J. Clin. Endocrinol. Metab. 2023, 108, e734–e742. [Google Scholar] [CrossRef]
- Rosenblum, R.C.; Hirsch, D.; Grozinsky-Glasberg, S.; Benbassat, C.; Yoel, U.; Ishay, A.; Zolotov, S.; Bachar, G.; Banne, E.; Levy, S.; et al. Clinical characteristics of a large familial cohort with Medullary thyroid cancer and germline Cys618Arg RET mutation in an Israeli multicenter study. Front. Endocrinol. 2023, 14, 1268193. [Google Scholar] [CrossRef]
- Berber, E.; Akbulut, S.; Avci, S.; Isiktas, G. Comparison of Parathyroid Autofluorescence Signals in Different Types of Hyperparathyroidism. World J. Surg. 2022, 46, 807–812. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Medullary thyroid cancer and pheochromocytoma in MEN2A: Are there parent of origin effects on disease expression? Fam. Cancer 2022, 21, 473–478. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Sex differences in MEN 2A penetrance and expression according to parental inheritance. Eur. J. Endocrinol. 2022, 186, 469–476. [Google Scholar] [CrossRef]
- Milicevic, S.; Krajc, M.; Blatnik, A.; Peric, B. Medullary Thyroid Carcinoma and Associated Endocrinopathies in Slovenia from 1995 to 2021. Life 2022, 12, 1091. [Google Scholar] [CrossRef]
- Diwaker, C.; Sarathi, V.; Jaiswal, S.K.; Shah, R.; Deshmukh, A.; Thomas, A.E.; Prakash, G.; Malhotra, G.; Patil, V.; Lila, A.; et al. Hereditary medullary thyroid carcinoma syndromes: Experience from western India. Fam. Cancer 2021, 20, 241–251. [Google Scholar] [CrossRef]
- Larsen, L.V.; Mirebeau-Prunier, D.; Imai, T.; Alvarez-Escola, C.; Hasse-Lazar, K.; Censi, S.; Castroneves, L.A.; Sakurai, A.; Kihara, M.; Horiuchi, K.; et al. Primary hyperparathyroidism as first manifestation in multiple endocrine neoplasia type 2A: An international multicenter study. Endocr. Connect. 2020, 9, 489–497. [Google Scholar] [CrossRef]
- Machens, A.; Elwerr, M.; Lorenz, K.; Weber, F.; Dralle, H. 100-Year evolution of precision medicine and surgery for multiple endocrine neoplasia type 2A. Endocrine 2020, 68, 368–376. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic autoimmune thyroiditis and obesity. Arch. Balk. Med. Union. 2018, 53, 64–69. [Google Scholar]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union 2016, 51, 476–480. [Google Scholar]
- Marini, F.; Giusti, F.; Brandi, M.L. Congenital primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 101982. [Google Scholar] [CrossRef] [PubMed]
- Răcătăianu, N.; Leach, N.; Bondor, C.I.; Mârza, S.; Moga, D.; Valea, A.; Ghervan, C. Thyroid disorders in obese patients. Does insulin resistance make a difference? Arch. Endocrinol. Metabol. 2017, 61, 575–583. [Google Scholar] [CrossRef]
- Das, L.; Dutta, P. Association of primary hyperparathyroidism with pituitary adenoma and management issues. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 101978. [Google Scholar] [CrossRef]
- Valea, A.; Ghervan, C.; Morar, A.; Pop, D.D.; Carsote, M.; Albu, S.E.; Georgescu, C.E.; Chiorean, A. Hashimoto’s thyroiditis and breast cancer: Coincidence or correlation? Arch. Balk. Med. Union 2016, 51, 129–132. [Google Scholar]
- Newey, P.J.; Hannan, F.M.; Wilson, A.; Thakker, R.V. Genetics of monogenic disorders of calcium and bone metabolism. Clin. Endocrinol. 2022, 97, 483–501. [Google Scholar] [CrossRef]
- De Sousa, S.M.C.; Carroll, R.W.; Henderson, A.; Burgess, J.; Clifton-Bligh, R.J. A contemporary clinical approach to genetic testing for heritable hyperparathyroidism syndromes. Endocrine 2022, 75, 23–32. [Google Scholar] [CrossRef]
- Mazarico-Altisent, I.; Capel, I.; Baena, N.; Bella-Cueto, M.R.; Barcons, S.; Guirao, X.; Pareja, R.; Muntean, A.; Arsentales, V.; Caixàs, A.; et al. Genetic testing for familial hyperparathyroidism: Clinical-genetic profile in a Mediterranean cohort. Front. Endocrinol. 2023, 14, 1244361. [Google Scholar] [CrossRef]
- Watanabe, A.; Wiseman, S.M. Multiple endocrine neoplasia type 4 & primary hyperparathyroidism: What the surgeon needs to know. Am. J. Surg. 2022, 224, 1017–1018. [Google Scholar] [CrossRef]
- Majer, A.D.; Hua, X.; Katona, B.W. Menin in Cancer. Genes 2024, 15, 1231. [Google Scholar] [CrossRef]
- Razavipour, S.F.; Harikumar, K.B.; Slingerland, J.M. p27 as a Transcriptional Regulator: New Roles in Development and Cancer. Cancer Res. 2020, 80, 3451–3458. [Google Scholar] [CrossRef]
- Simonds, W.F.; Li, Y.; Jha, S. Genotype-Phenotype Correlations in the Hyperparathyroidism-Jaw Tumor Syndrome. J. Clin. Endocrinol. Metab. 2025, 110, dgae909. [Google Scholar] [CrossRef]
- Gopinath, S.; Ramaiyan, V. Molecular diagnostic approaches in detecting rearranged during transfection oncogene mutations in multiple endocrine neoplasia type 2. World J. Clin. Cases 2024, 12, 6436–6440. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Carsote, M.; Cocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [CrossRef]
- Popa, F.L.; Diaconu, C.; Canciu, A.; Ciortea, V.M.; Iliescu, M.G.; Stanciu, M. Medical management and rehabilitation in posttraumatic common peroneal nerve palsy. Balneo PRM Res. J. 2022, 13, 496. [Google Scholar] [CrossRef]
- Jha, S.; Simonds, W.F. Molecular and Clinical Spectrum of Primary Hyperparathyroidism. Endocr. Rev. 2023, 44, 779–818. [Google Scholar] [CrossRef] [PubMed]
- Tora, R.; Welch, J.; Sun, J.; Agarwal, S.K.; Bell, D.A.; Merino, M.; Weinstein, L.S.; Simonds, W.F.; Jha, S. Phenotypic Profiling and Molecular Mechanisms in Hyperparathyroidism-jaw Tumor Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 3165–3177. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Cadiot, G.; Calender, A.; Goudet, P.; Chanson, P. Clinical aspects of multiple endocrine neoplasia type 1. Nat. Rev. Endocrinol. 2021, 17, 207–224. [Google Scholar] [CrossRef]
- Frank-Raue, K.; Rybicki, L.A.; Erlic, Z.; Schweizer, H.; Winter, A.; Milos, I.; Toledo, S.P.; Toledo, R.A.; Tavares, M.R.; Alevizaki, M.; et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum. Mutat. 2011, 32, 51–58. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, Y.H.; Hong, N.; Won, D.; Rhee, Y. Germline Mutations Related to Primary Hyperparathyroidism Identified by Next-Generation Sequencing. Front. Endocrinol. 2022, 13, 853171. [Google Scholar] [CrossRef]
- Febrero, B.; Rodríguez, J.M.; Ríos, A.; Segura, P.; Pérez-Sánchez, B.; Torregrosa, N.; Hernández, A.M.; Parrilla, P. Prophylactic thyroidectomy in multiple endocrine neoplasia 2 (MEN2) patients with the C634Y mutation: A long-term follow-up in a large single-center cohort. Eur. J. Surg. Oncol. 2019, 45, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Haciyanli, S.G.; Karaisli, S.; Suataman, B.; Karahan, F.; Haciyanli, M. Primary Hyperparathyroidism with Thyroid Cancer: Clinicopathologic Features. Sisli Etfal Hastan. Tip Bul. 2022, 56, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Simonds, W.F. Clinical and Molecular Genetics of Primary Hyperparathyroidism. Horm. Metab. Res. 2020, 52, 578–587. [Google Scholar] [CrossRef]
- Saravana-Bawan, B.; Pasternak, J.D. Multiple endocrine neoplasia 2: An overview. Ther. Adv. Chronic Dis. 2022, 13, 20406223221079246. [Google Scholar] [CrossRef]
- Sahakian, N.; Castinetti, F.; Romanet, P.; Reznik, Y.; Brue, T. Updates on the genetics of multiple endocrine neoplasia. Ann. Endocrinol. 2024, 85, 127–135. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Effraimidis, G.; Rossing, M.; Rasmussen, Å.K.; Hoejberg, L.; Bastholt, L.; Godballe, C.; Oturai, P.; Feldt-Rasmussen, U. Multiple endocrine neoplasia type 2: A review. Semin. Cancer Biol. 2022, 79, 163–179. [Google Scholar] [CrossRef]
- Xu, B.; Viswanathan, K.; Ahadi, M.S.; Ahmadi, S.; Alzumaili, B.; Bani, M.A.; Baudin, E.; Behrman, D.B.; Capelletti, M.; Chau, N.G.; et al. Association of the Genomic Profile of Medullary Thyroid Carcinoma with Tumor Characteristics and Clinical Outcomes in an International Multicenter Study. Thyroid 2024, 34, 167–176. [Google Scholar] [CrossRef]
- Hensley, S.G.; Hu, M.I.; Bassett, R.L.; Ying, A.K.; Zafereo, M.E.; Perrier, N.D.; Busaidy, N.L.; Hyde, S.M.; Grubbs, E.G.; Waguespack, S.G. Pediatric Medullary Thyroid Carcinoma: Clinical Presentations and Long-Term Outcomes in 144 Patients Over 6 Decades. J. Clin. Endocrinol. Metab. 2024, 109, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, Y.; Hwang, S.; Yoon, J.H.; Kim, G.H.; Yoo, H.W.; Choi, J.H. Impact of Early Diagnostic and Therapeutic Interventions and Clinical Course in Children and Adolescents with Multiple Endocrine Neoplasia Types 1 and 2. Exp. Clin. Endocrinol. Diabetes 2024, 132, 39–46. [Google Scholar] [CrossRef]
- Raue, F.; Frank-Raue, K. Update on Multiple Endocrine Neoplasia Type 2: Focus on Medullary Thyroid Carcinoma. J. Endocr. Soc. 2018, 2, 933–943. [Google Scholar] [CrossRef]
- Margraf, R.L.; Alexander, R.Z.; Fulmer, M.L.; Miller, C.E.; Coupal, E.; Mao, R. Multiple endocrine neoplasia type 2 (MEN2) and RET specific modifications of the ACMG/AMP variant classification guidelines and impact on the MEN2 RET database. Hum. Mutat. 2022, 43, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Mucha, L.; Leidig-Bruckner, G.; Frank-Raue, K.; Bruckner, T.; Kroiss, M.; Raue, F.; German study group for rare thyroid cancer. Phaeochromocytoma in multiple endocrine neoplasia type 2: RET codon-specific penetrance and changes in management during the last four decades. Clin. Endocrinol. 2017, 87, 320–326. [Google Scholar] [CrossRef]
- Thosani, S.; Ayala-Ramirez, M.; Palmer, L.; Hu, M.I.; Rich, T.; Gagel, R.F.; Cote, G.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J. Clin. Endocrinol. Metab. 2013, 98, E1813–E1819. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Dralle, H. Peak incidence of pheochromocytoma and primary hyperparathyroidism in multiple endocrine neoplasia 2: Need for age-adjusted biochemical screening. J. Clin. Endocrinol. Metab. 2013, 98, E336–E345. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Qiu, Y.; Zhang, X.; Qian, Y.; Xu, J. Unexpected pheochromocytoma leading to cardiac arrest during the perioperative period: A case report and literature review. BMC Anesthesiol. 2024, 24, 463. [Google Scholar] [CrossRef]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr.; Endocrine Society. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef]
- Ungureanu, S.; Şipitco, N.; Alexa, Z.; Gonţa, V.; Bujac, M.; Parnov, M.; Romanenco, R. MEN 2A syndrome-Multiple endocrine neoplasia with autosomal dominant transmission. Int. J. Surg. Case Rep. 2020, 73, 141–145. [Google Scholar] [CrossRef]
- Łokaj, M.; Szewc, M.; Forma, A.; Szymańska, M.; Lubikowski, J.; Prowans, P.; Sitarz, R. Simultaneous unilateral laparoscopic adrenalectomy for pheochromocytoma and thyroidectomy in MEN 2A and MEN 2B syndrome. Endokrynol. Pol. 2022, 73, 383–384. [Google Scholar] [CrossRef]
- Alevizaki, M. Management of hyperparathyroidism (PHP) in MEN2 syndromes in Europe. Thyroid Res. 2013, 6 (Suppl. S1), S10. [Google Scholar] [CrossRef]
- Soto-Pedre, E.; Newey, P.J.; Leese, G.P. Stable Incidence and Increasing Prevalence of Primary Hyperparathyroidism in a Population-based Study in Scotland. J. Clin. Endocrinol. Metab. 2023, 108, e1117–e1124. [Google Scholar] [CrossRef]
- Newey, P.J. Hereditary Primary Hyperparathyroidism. Endocrinol. Metab. Clin. N. Am. 2021, 50, 663–681. [Google Scholar] [CrossRef]
- Popa, F.L.; Stanciu, M.; Banciu, A.; Berteanu, M. Association between low bone mineral density, metabolic syndrome and sex steroids deficiency in men. Acta Endocrinol.-Buchar. 2016, 12, 418–422. [Google Scholar] [CrossRef]
- Răcătăianu, N.; Leach, N.V.; Bolboacă, S.D.; Soran, M.L.; Opriş, O.; Dronca, E.; Valea, A.; Ghervan, C. Interplay between metabolic and thyroid parameters in obese pubertal children. Does visceral adipose tissue make the first move? Acta Clin. Belg. 2021, 76, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.; Bera, L.G.; Popescu, M.; Grosu, F.; Popa, L.F. Hashimoto’s thyroiditis associated with thyroid adenoma with Hurthle cells-case report. Rom. J. Morphol. Embryol. 2017, 58, 241–248. [Google Scholar]
- Magalhães, P.K.; Antonini, S.R.; de Paula, F.J.; de Freitas, L.C.; Maciel, L.M. Primary hyperparathyroidism as the first clinical manifestation of multiple endocrine neoplasia type 2A in a 5-year-old child. Thyroid 2011, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.J.; Buła, G.; Żądło, D.; Gawrychowska, A.; Gawrychowski, J. Primary hyperparathyroidism. Endokrynol. Pol. 2020, 71, 260–270. [Google Scholar] [CrossRef]
- Clarke, B.L. Asymptomatic Primary Hyperparathyroidism. Front. Horm. Res. 2019, 51, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Cetani, F. Normocalcemic primary hyperparathyroidism. Arq. Endocrinol. Metabol. 2022, 66, 666–677. [Google Scholar] [CrossRef]
- Muñoz de Nova, J.L.; Sampedro-Nuñez, M.; Huguet-Moreno, I.; Marazuela Azpiroz, M. A practical approach to normocalcemic primary hyperparathyroidism. Endocrine 2021, 74, 235–244. [Google Scholar] [CrossRef]
- Popa, F.L.; Stanciu, M.; Bighea, A.; Berteanu, M.; Totoianu, I.G.; Rotaru, M. Decreased serum levels of sex steroids associated with osteoporosis in a group of Romanian male patients. Rev. Romana Med. Lab. 2016, 24, 75–82. [Google Scholar] [CrossRef]
- Meriam, H.; Kaaroud, H.; Karray, R.; Ben Hamida, F.; Bouzid, K.; Abderrahim, E. Recurrent Urolithiasis Revealing Primary Hyperparathyroidism in a Nephrology Department. Case Rep. Nephrol. 2024, 2024, 1265364. [Google Scholar] [CrossRef]
- Misgar, R.A.; Bhat, M.H.; Rather, T.A.; Masoodi, S.R.; Wani, A.I.; Bashir, M.I.; Wani, M.A.; Malik, A.A. Primary hyperparathyroidism and pancreatitis. J. Endocrinol. Investig. 2020, 43, 1493–1498. [Google Scholar] [CrossRef]
- Desmedt, V.; Desmedt, S.; D’heygere, E.; Vereecke, G.; Van Moerkercke, W. Hypercalcemia induced pancreatitis as a rare presentation of primary hyperparathyroidism. Acta Gastroenterol. Belg. 2021, 84, 367–370. [Google Scholar] [CrossRef]
- Dandurand, K.; Ali, D.S.; Khan, A.A. Primary Hyperparathyroidism: A Narrative Review of Diagnosis and Medical Management. J. Clin. Med. 2021, 10, 1604. [Google Scholar] [CrossRef]
- El-Hajj Fuleihan, G.; Chakhtoura, M.; Cipriani, C.; Eastell, R.; Karonova, T.; Liu, J.M.; Minisola, S.; Mithal, A.; Moreira, C.A.; Peacock, M.; et al. Classical and Non-classical Manifestations of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2330–2350. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Liu, E.; Vandenput, L.; Lorentzon, M.; McCloskey, E.V.; Bouillon, R.; Abrahamsen, B.; Rejnmark, L.; Johansson, H.; et al. Primary hyperparathyroidism and fracture probability. Osteoporos. Int. 2023, 34, 489–499. [Google Scholar] [CrossRef]
- Iwanowska, M.; Kochman, M.; Szatko, A.; Zgliczyński, W.; Glinicki, P. Bone Disease in Primary Hyperparathyroidism-Changes Occurring in Bone Metabolism and New Potential Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 11639. [Google Scholar] [CrossRef]
- Popa, F.L.; Boicean, L.C.; Iliescu, M.G.; Stanciu, M. The importance of association between sex steroids deficiency, reduction of bone mineral density and falling risk in men with implications in medical rehabilitation. Balneo PRM Res. J. 2021, 12, 318–322. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Silverberg, S.J.; Bandeira, F.; Cetani, F.; Chandran, M.; Cusano, N.E.; Ebeling, P.R.; Formenti, A.M.; Frost, M.; Gosnell, J.; et al. Management of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2391–2403. [Google Scholar] [CrossRef]
- Cetani, F.; Saponaro, F.; Borsari, S.; Marcocci, C. Familial and Hereditary Forms of Primary Hyperparathyroidism. Front. Horm. Res. 2019, 51, 40–51. [Google Scholar] [CrossRef]
- Giusti, F.; Cavalli, L.; Cavalli, T.; Brandi, M.L. Hereditary hyperparathyroidism syndromes. J. Clin. Densitom. 2013, 16, 69–74. [Google Scholar] [CrossRef]
- Jayasinghe, R.; Basnayake, O.; Jayarajah, U.; Seneviratne, S. Management of medullary carcinoma of the thyroid: A review. J. Int. Med. Res. 2022, 50, 3000605221110698. [Google Scholar] [CrossRef]
- Laganà, M.; Cremaschi, V.; Alberti, A.; Vodopivec Kuri, D.M.; Cosentini, D.; Berruti, A. The Evolving Treatment Landscape of Medullary Thyroid Cancer. Curr. Treat. Options Oncol. 2023, 24, 1815–1832. [Google Scholar] [CrossRef]
- Shaghaghi, A.; Salari, A.; Jalaeefar, A.; Shirkhoda, M. Management of lymph nodes in medullary thyroid carcinoma: A review. Ann. Med. Surg. 2022, 81, 104538. [Google Scholar] [CrossRef]
- Torresan, F.; Censi, S.; Pennelli, G.; Galuppini, F.; Mian, C.; Iacobone, M. Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients. Cancers 2022, 14, 6226. [Google Scholar] [CrossRef]
- Machens, A.; Elwerr, M.; Lorenz, K.; Weber, F.; Dralle, H. Long-term outcome of prophylactic thyroidectomy in children carrying RET germline mutations. Br. J. Surg. 2018, 105, e150–e157. [Google Scholar] [CrossRef]
- Garcés Visier, C.; Espinoza Vega, M.; Guillén Redondo, P.; Ollero Fresno, J.C.; Souto Romero, H.; Luis Huertas, A.; Espinosa Góngora, R.; Rico Espiñeira, C.; Bautista, F.J.; Alonso Calderón, J.L. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A in children: A single centre experience. J. Pediatr. Endocrinol. Metab. 2019, 32, 889–893. [Google Scholar] [CrossRef]
- Rocke, D.J.; Mulder, H.; Cyr, D.; Kahmke, R.; Lee, W.T.; Puscas, L.; Schulz, K.; Witsell, D.L. The effect of lateral neck dissection on complication rate for total thyroidectomy. Am. J. Otolaryngol. 2020, 41, 102421. [Google Scholar] [CrossRef]
- Campbell, J.C.; Lee, H.J.; Cannon, T.Y.; Kahmke, R.R.; Lee, W.T.; Puscas, L.; Rocke, D.J. Lateral neck dissection surgeon volume and complications in head and neck endocrine malignancy. Gland. Surg. 2023, 12, 917–927. [Google Scholar] [CrossRef]
- Deng, J.; Wulff-Burchfield, E.M.; Murphy, B.A. Late Soft Tissue Complications of Head and Neck Cancer Therapy: Lymphedema and Fibrosis. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz005. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Waśniewska-Okupniak, E.; Banaszewski, J.; Pabiszczak, M.; Piorunek, T. Evaluation of outcomes after reoperative neck dissection due to thyroid cancer. Contemp. Oncol. 2014, 18, 268–272. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Grubbs, E.G. Surgical Management of Multiple Endocrine Neoplasia 1 and Multiple Endocrine Neoplasia 2. Surg. Clin. N. Am. 2019, 99, 693–709. [Google Scholar] [CrossRef]
- Kavazis, C.; Romanidis, K.; Pitiakoudis, M.; Kesisoglou, I.; Laskou, S.; Sapalidis, K. The role of prophylactic parathyroidectomy during thyroidectomy for MTC in patients with MEN2A syndrome. Folia Med. 2023, 65, 720–727. [Google Scholar] [CrossRef]
- Yoshida, S.; Imai, T.; Kikumori, T.; Wada, M.; Sawaki, M.; Takada, H.; Yamada, T.; Sato, S.; Sassa, M.; Uchida, H.; et al. Long term parathyroid function following total parathyroidectomy with auto-transplantation in adult patients with MEN2A. Endocr. J. 2009, 56, 545–551. [Google Scholar] [CrossRef]
- Moley, J.F.; Skinner, M.; Gillanders, W.E.; Lairmore, T.C.; Rowland, K.J.; Traugott, A.L.; Jin, L.X.; Wells, S.A., Jr. Management of the Parathyroid Glands During Preventive Thyroidectomy in Patients with Multiple Endocrine Neoplasia Type 2. Ann. Surg. 2015, 262, 641–646. [Google Scholar] [CrossRef]
- Tonelli, F.; Giudici, F.; Marcucci, T.; Cavalli, T.; Spini, S.; Gheri, R.G.; Brandi, M.L. Surgery in MEN 2A Patients Older Than 5 Years with Micro-MTC: Outcome at Long-Term Follow-Up. Otolaryngol. Head. Neck Surg. 2016, 155, 787–789. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.; Sun, W.Y. Recurrent hyperparathyroidism due to proliferation of autotransplanted parathyroid tissue in a multiple endocrine neoplasia type 2A patient. Ann. Surg. Treat. Res. 2016, 91, 145–148. [Google Scholar] [CrossRef]
- Twigt, B.A.; Scholten, A.; Valk, G.D.; Rinkes, I.H.; Vriens, M.R. Differences between sporadic and MEN related primary hyperparathyroidism; clinical expression, preoperative workup, operative strategy and follow-up. Orphanet J. Rare Dis. 2013, 8, 50. [Google Scholar] [CrossRef]
- Scholten, A.; Schreinemakers, J.M.; Pieterman, C.R.; Valk, G.D.; Vriens, M.R.; Borel Rinkes, I.H. Evolution of surgical treatment of primary hyperparathyroidism in patients with multiple endocrine neoplasia type 2A. Endocr. Pract. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Perrier, N.; Lang, B.H.; Farias, L.C.B.; Poch, L.L.; Sywak, M.; Almquist, M.; Vriens, M.R.; Yeh, M.W.; Shariq, O.; Duh, Q.Y.; et al. Surgical Aspects of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2373–2390. [Google Scholar] [CrossRef]
- Pace-Asciak, P.; Russell, J.; Solorzano, C.; Berber, E.; Singer, M.; Shaha, A.R.; Khafif, A.; Angelos, P.; Nixon, I.; Tufano, R.P. The utility of parathyroid autofluorescence as an adjunct in thyroid and parathyroid surgery 2023. Head Neck 2023, 45, 3157–3167. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Thomas, G.; Patel, A.; Fan, R.; Ye, F.; Willmon, P.A.; Solórzano, C.C. Does the Use of Probe-based Near-infrared Autofluorescence Parathyroid Detection Benefit Parathyroidectomy?: A Randomized Single-center Clinical Trial. Ann. Surg. 2023, 278, 549–558. [Google Scholar] [CrossRef]
- Frey, S.; Bannani, S.; Caillard, C.; Le Bras, M.; Drui, D.; Ansquer, C.; Guillot, P.; Le Thuaut, A.; Mirallié, E. Parathyroid near-infrared autofluorescence use for parathyroidectomy in mild primary hyperparathyroidism: Results from a randomized monocentric trial. Surgery 2025, 177, 108878. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.; Jung, J.; Kim, S.; Kim, S.W.; Hwang, S.H. Near-infrared autofluorescence-based parathyroid glands identification in the thyroidectomy or parathyroidectomy: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2022, 407, 491–499. [Google Scholar] [CrossRef]
- Pipernea, R.; Popa, F.L.; Ciortea, V.M.; Irsay, L.; Ungur, R.A.; Pintea, A.L.; Iliescu, M.G.; Cipăian, R.C.; Stanciu, M. The role of rehabilitation and anabolic treatment in severe osteoporosis associated with significant vitamin D deficiency–case report. Balneo PRM Res. J. 2023, 14, 539. [Google Scholar] [CrossRef]
- La Greca, A.; Dawes, D.; Albuja-Cruz, M.; Raeburn, C.; Axell, L.; Ku, L.; Klein, C.; Marshall, C.; Fishbein, L. MEN2 phenotype in a family with germline heterozygous rare RET K666N variant. Endocrinol. Diabetes Metab. Case Rep. 2024, 2024, 24-0009. [Google Scholar] [CrossRef]
- Jones, J.; Mann, G.N.; Chaudhuri, A.; Ramos-Santillan, V. Diagnostic and Surgical Challenges Associated With Sporadic Multiple Endocrine Neoplasia 2A Presenting as Non-syndromic Primary Hyperparathyroidism. Am. Surg. 2024, 90, 1753–1755. [Google Scholar] [CrossRef]
- Kim, M.; Aploks, K.; Vargas-Pinto, S.; Dong, X. RET T244I Germline Variant Mutation in a Patient with Pancreatic Paraganglioma and Primary Hyperparathyroidism. Int. J. Endocrinol. Metab. 2022, 20, e121056. [Google Scholar] [CrossRef]
- Brown, S.J.; Riconda, D.L.; Zheng, F.; Jackson, G.L.; Suo, L.; Robbins, R.J. Features of Multiple Endocrine Neoplasia Type 1 and 2A in a Patient with Both RET and MEN1 Germline Mutations. J. Endocr. Soc. 2020, 4, bvaa020. [Google Scholar] [CrossRef]
- Giani, C.; Ramone, T.; Romei, C.; Ciampi, R.; Tacito, A.; Valerio, L.; Agate, L.; Ugolini, C.; Marinò, M.; Basolo, F.; et al. A New MEN2 Syndrome with Clinical Features of Both MEN2A and MEN2B Associated with a New RET Germline Deletion. Case Rep. Endocrinol. 2020, 2020, 4147097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
