Blood Urea/Creatinine Ratio and Mortality in Ambulatory Patients with Heart Failure with Reduced Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
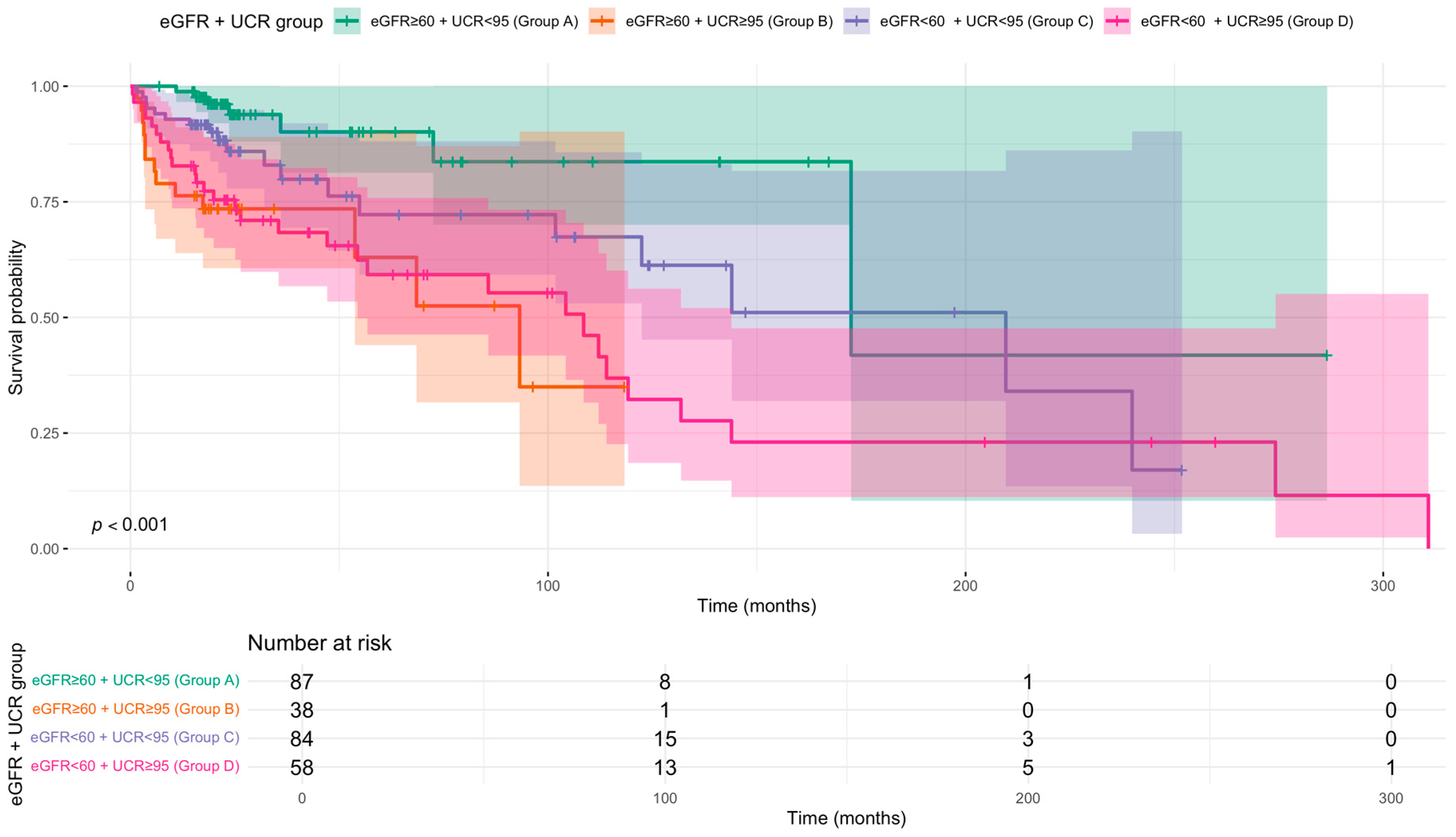
3.2. Prognostic Value of UCR Independent of Renal Function
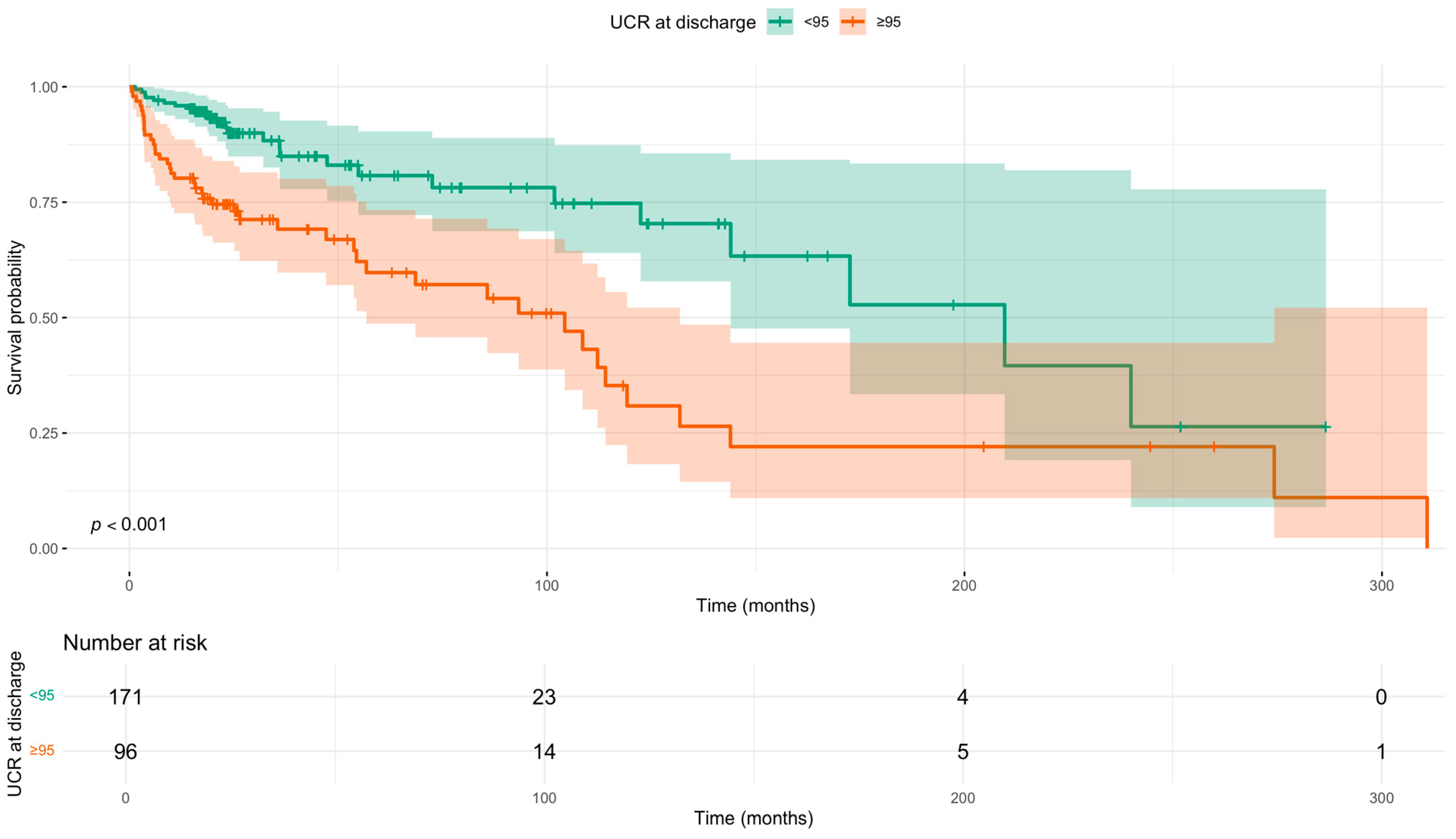
3.3. Prognostic Value of UCR at Discharge
3.4. UCR as a Continuous Predictor and Model Validation
3.5. Association of UCR with Cardiovascular-Specific Mortality
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEi | angiotensin-converting enzyme inhibitor |
| ARB | angiotensin receptor blocker |
| ARNI | angiotensin receptor-neprilysin inhibitor |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| eGFR | estimated glomerular filtration rate |
| HR | Hazard Ratio |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| MRA | mineralocorticoid receptor antagonist |
| NYHA | New York Heart Association |
| SGLT2i | sodium-glucose cotransporter-2 inhibitor |
| UCR | urea/creatinine ratio |
Appendix A
| Cox Regression: Sensitivity Analysis | |||
|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | 95% CI | p-Value |
| Age (years) | 1.00 | 0.98, 1.02 | >0.9 |
| Male | 0.80 | 0.46, 1.38 | 0.4 |
| Cerebrovascular Disease | 3.21 | 1.75, 5.90 | <0.001 |
| MRA | 0.32 | 0.18, 0.56 | <0.001 |
| SGLT2i | 0.48 | 0.28, 0.82 | 0.007 |
| ACEi/ARB/ARNI | 0.74 | 0.42, 1.29 | 0.3 |
| UCR at Discharge (per 1-unit increase) | 1.01 | 1.00, 1.02 | 0.038 |
| Moderate or Greater Valvular Disease | 0.90 | 0.53, 1.53 | 0.7 |
| Left Ventricular Ejection Fraction (%) | 1.00 | 0.97, 1.03 | >0.9 |
| Haemoglobin (g/L) | 0.99 | 0.98, 1.01 | 0.3 |
| Furosemide Equivalent Dose (mg) | 1.00 | 1.00, 1.01 | 0.3 |
| Concordance = 0.77|Likelihood ratio test p < 0.001|n = 258, events = 67 | |||
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. The Heart Failure Association Atlas: Heart Failure Epidemiology and Management Statistics 2019. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of Patients with Chronic Heart Failure in the Community: A Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef]
- Darvish, M.; Shakoor, A.; Feyz, L.; Schaap, J.; van Mieghem, N.M.; de Boer, R.A.; Brugts, J.J.; van der Boon, R.M.A. Heart Failure: Assessment of the Global Economic Burden. Eur. Heart J. 2025, 46, 3069–3078. [Google Scholar] [CrossRef]
- Lang, C.C.; Struthers, A.D. Targeting the Renin–Angiotensin–Aldosterone System in Heart Failure. Nat. Rev. Cardiol. 2013, 10, 125–134. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of Kidney Function throughout the Heart Failure Trajectory—A Position Statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Schrier Robert, W. Blood Urea Nitrogen: A Marker for Adverse Effects of Loop Diuretics? J. Am. Coll. Cardiol. 2011, 58, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; von Haehling, S.; Anker, S.D.; Hillege, H.L.; Struck, J.; Hartmann, O.; Bergmann, A.; Squire, I.; van Veldhuisen, D.J.; Dickstein, K.; et al. C-Terminal Provasopressin (Copeptin) Is a Strong Prognostic Marker in Patients with Heart Failure after an Acute Myocardial Infarction: Results from the OPTIMAAL Study. Eur. Heart J. 2009, 30, 1187–1194. [Google Scholar] [CrossRef]
- Sands, J.M.; Blount, M.A.; Klein, J.D. Regulation of Renal Urea Transport by Vasopressin. Trans. Am. Clin. Climatol. Assoc. 2011, 122, 82. [Google Scholar]
- Schrier, R.W. Blood Urea Nitrogen and Serum Creatinine: Not Married in Heart Failure. Circ. Heart Fail. 2008, 1, 2–5. [Google Scholar]
- Brisco, M.A.; Coca, S.G.; Chen, J.; Owens, A.T.; McCauley, B.D.; Kimmel, S.E.; Testani, J.M. Blood Urea Nitrogen/Creatinine Ratio Identifies a High-Risk but Potentially Reversible Form of Renal Dysfunction in Patients With Decompensated Heart Failure. Circ. Heart Fail. 2013, 6, 233–239. [Google Scholar] [CrossRef]
- Filippatos, G.; Rossi, J.; Lloyd-Jones, D.M.; Stough, W.G.; Ouyang, J.; Shin, D.D.; O’Connor, C.; Adams, K.F.; Orlandi, C.; Gheorghiade, M. Prognostic Value of Blood Urea Nitrogen in Patients Hospitalized With Worsening Heart Failure: Insights From the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) Study. J. Card. Fail. 2007, 13, 360–364. [Google Scholar] [CrossRef]
- Mickey, R.M.; Greenland, S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Coca, S.G.; Shannon, R.P.; Kimmel, S.E.; Cappola, T.P. Influence of Renal Dysfunction Phenotype on Mortality in the Setting of Cardiac Dysfunction: Analysis of Three Randomized Controlled Trials. Eur. J. Heart Fail. 2011, 13, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, P.; Butt, J.H.; Kondo, T.; Campo, G.; Desai, A.S.; Jhund, P.S.; Køber, L.; Lefkowitz, M.P.; Rouleau, J.L.; Solomon, S.D.; et al. Independent Prognostic Importance of Blood Urea Nitrogen to Creatinine Ratio in Heart Failure. Eur. J. Heart Fail. 2024, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Liang, W.; Tan, W.; Dong, B.; Wu, Y.; Liu, C.; Xue, R. Prognostic Significance of Blood Urea Nitrogen/Creatinine Ratio in Chronic HFpEF. Eur. J. Clin. Investig. 2022, 52, e13761. [Google Scholar] [CrossRef]
- Kazory, A. Emergence of Blood Urea Nitrogen as a Biomarker of Neurohormonal Activation in Heart Failure. Am. J. Cardiol. 2010, 106, 694–700. [Google Scholar] [CrossRef]
- Parrinello, G.; Torres, D.; Testani, J.M.; Almasio, P.L.; Bellanca, M.; Pizzo, G.; Cuttitta, F.; Pinto, A.; Butler, J.; Paterna, S. Blood Urea Nitrogen to Creatinine Ratio Is Associated with Congestion and Mortality in Heart Failure Patients with Renal Dysfunction. Intern. Emerg. Med. 2015, 10, 965–972. [Google Scholar] [CrossRef]
- Bashi, N.; Karunanithi, M.; Fatehi, F.; Ding, H.; Walters, D. Remote Monitoring of Patients with Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e18. [Google Scholar]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of Telemedical Interventional Management in Patients with Heart Failure (TIM-HF2): A Randomised, Controlled, Parallel-Group, Unmasked Trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating Heart Failure Biomarkers beyond Natriuretic Peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef]
- Noveanu, M.; Breidthardt, T.; Potocki, M.; Reichlin, T.; Twerenbold, R.; Uthoff, H.; Socrates, T.; Arenja, N.; Reiter, M.; Meissner, J.; et al. Direct Comparison of Serial B-Type Natriuretic Peptide and NT-proBNP Levels for Prediction of Short- and Long-Term Outcome in Acute Decompensated Heart Failure. Crit. Care 2011, 15, R1. [Google Scholar] [CrossRef]
- Luo, H.; Chen, X. Prognostic Value of the Serum Creatinine/Albumin Ratio for 28-Day Mortality in Heart Failure: A Retrospective Cohort Study. Front. Cardiovasc. Med. 2025, 12, 1586327. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Adler, E.; Campagnari, C.; Yagil, A. A Machine Learning Risk Score Predicts Mortality across the Spectrum of Left Ventricular Ejection Fraction. Eur. J. Heart Fail. 2021, 23, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.; Avila, D.X.; Candia, A.M.; Scaramussa, V.D.; Villacorta, H. Role of Urea to Creatinine Ratio in the Prediction of Outcomes in Chronic Heart Failure. Eur. Heart J. 2024, 45, ehae666.980. [Google Scholar] [CrossRef]
| Baseline Characteristics by Mortality | |||
|---|---|---|---|
| Variable | Alive 1 | Dead 1 | p-Value 2 |
| Sex | 0.6 | ||
| Female | 90 (34%) | 29 (38%) | |
| Male | 171 (66%) | 47 (62%) | |
| Age (years) | 71 (15) | 79 (11) | <0.001 |
| Cardiovascular Death | 40 (53%) | ||
| Cancer Death | 7 (9%) | ||
| Non-Cardiovascular or Cancer Death | 29 (38%) | ||
| BMI (kg/m2) | 28 (7) | 26 (7) | 0.010 |
| Atrial Fibrillation | 112 (43%) | 29 (39%) | 0.5 |
| Ischemic Heart Disease | 108 (42%) | 35 (47%) | 0.5 |
| Cerebrovascular Disease | 29 (11%) | 19 (25%) | 0.004 |
| Chronic Obstructive Pulmonary Disease | 44 (17%) | 17 (23%) | 0.3 |
| Asthma | 35 (13%) | 4 (5.4%) | 0.065 |
| Smoker (current or former) | 78 (30%) | 30 (40%) | 0.12 |
| Left Ventricular Ejection Fraction (%) 3 | 34 (9) | 31 (8) | 0.062 |
| Left Ventricular Ejection Fraction Group 3 | 0.009 | ||
| ≥60% | 3 (1.1%) | 0 (0%) | |
| 50–59% | 14 (5.4%) | 0 (0%) | |
| 40–49% | 45 (17%) | 15 (20%) | |
| 30–39% | 118 (45%) | 24 (32%) | |
| <30% | 81 (31%) | 37 (49%) | |
| Aortic Regurgitation 4 | <0.001 | ||
| None | 199 (77%) | 42 (57%) | |
| Mild | 43 (17%) | 29 (39%) | |
| Moderate | 17 (6.6%) | 3 (4.1%) | |
| Moderate–Severe | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Tricuspid Regurgitation 4 | 0.13 | ||
| None | 105 (41%) | 28 (38%) | |
| Mild | 120 (47%) | 31 (42%) | |
| Moderate | 25 (9.8%) | 10 (14%) | |
| Moderate–Severe | 1 (0.4%) | 3 (4.1%) | |
| Severe | 5 (2.0%) | 1 (1.4%) | |
| Pulmonary Regurgitation 4 | 0.065 | ||
| None | 145 (69%) | 31 (54%) | |
| Mild | 61 (29%) | 26 (46%) | |
| Moderate | 4 (1.9%) | 0 (0%) | |
| Moderate–Severe | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Mitral Stenosis 4 | 0.011 | ||
| None | 260 (100%) | 73 (96%) | |
| Mild | 0 (0%) | 3 (3.9%) | |
| Moderate | 0 (0%) | 0 (0%) | |
| Moderate–Severe | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Aortic Stenosis 4 | 0.2 | ||
| None | 233 (90%) | 62 (83%) | |
| Mild | 18 (6.9%) | 7 (9.3%) | |
| Moderate | 6 (2.3%) | 4 (5.3%) | |
| Moderate–Severe | 1 (0.4%) | 0 (0%) | |
| Severe | 2 (0.8%) | 2 (2.7%) | |
| Tricuspid Stenosis 4 | >0.9 | ||
| None | 252 (100%) | 74 (100%) | |
| Mild | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | |
| Moderate–Severe | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Pulmonary Stenosis 4 | >0.9 | ||
| None | 229 (100%) | 60 (100%) | |
| Mild | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | |
| Moderate–Severe | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Systolic BP (mmHg) | 121 (18) | 115 (18) | 0.032 |
| Haemoglobin (g/L) | 140 (18) | 126 (25) | <0.001 |
| Urea (diagnosis) (mmol/L) | 7.8 (3.6) | 9.9 (7.1) | 0.049 |
| Creatinine (diagnosis) (µmol/L) | 93 (31) | 112 (66) | 0.019 |
| eGFR (diagnosis) (mL/min/1.73 m2) | 0.005 | ||
| <15 | 0 (0%) | 1 (1.3%) | |
| 15–29 | 6 (2.3%) | 7 (9.3%) | |
| 30–44 | 26 (10%) | 12 (16%) | |
| 45–59 | 44 (17%) | 14 (19%) | |
| ≥60 | 183 (71%) | 41 (55%) | |
| UCR (diagnosis) | 84 (28) | 87 (31) | 0.3 |
| Potassium (diagnosis) (mmol/L) | 4.31 (0.52) | 4.20 (0.49) | 0.2 |
| Sodium (diagnosis) (mmol/L) | 138.8 (8.4) | 138.1 (4.3) | 0.057 |
| Urea (discharge) (mmol/L) | 9 (7) | 19 (31) | <0.001 |
| Creatinine (discharge) (µmol/L) | 110 (42) | 164 (130) | <0.001 |
| UCR (discharge) | 83 (26) | 106 (37) | <0.001 |
| eGFR (discharge) (mL/min/1.73 m2) | <0.001 | ||
| <15 | 0 (0%) | 6 (7.9%) | |
| 15–29 | 17 (6.5%) | 13 (17%) | |
| 30–44 | 47 (18%) | 20 (26%) | |
| 45–59 | 61 (23%) | 16 (21%) | |
| ≥60 | 135 (52%) | 21 (28%) | |
| Sodium (discharge) (mmol/L) | 139.3 (2.8) | 138.0 (7.6) | 0.067 |
| Potassium (discharge) (mmol/L) | 4.53 (0.45) | 4.47 (0.77) | 0.11 |
| NYHA Functional Classification | <0.001 | ||
| 1 | 56 (22%) | 4 (5.8%) | |
| 2 | 157 (62%) | 29 (42%) | |
| 3 | 34 (13%) | 29 (42%) | |
| 4 | 5 (2.0%) | 7 (10%) | |
| GDMT agents | <0.001 | ||
| 1 agent | 6 (2.3%) | 18 (24%) | |
| 2 agents | 53 (21%) | 22 (29%) | |
| 3 agents | 84 (33%) | 26 (34%) | |
| 4 agents | 114 (44%) | 10 (13%) | |
| Beta-blocker | 221 (85%) | 64 (84%) | >0.9 |
| MRA | 182 (70%) | 29 (38%) | <0.001 |
| SGLT2i | 192 (74%) | 39 (51%) | <0.001 |
| ACEi/ARB/ARNI | 225 (86%) | 48 (63%) | <0.001 |
| Deprivation Decile (Scottish Index of Multiple Deprivation) | 10.5 (5.5) | 9.8 (5.2) | 0.3 |
| Diuretic (Furosemide (mg)) | 30 (32) | 57 (50) | <0.001 |
| Multivariable Cox Regression Analysis for All-Cause Mortality by eGFR and UCR Group | |||
|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | p-Value |
| Age (years) | 1.00 | 0.97, 1.02 | 0.7 |
| Male | 0.76 | 0.45, 1.26 | 0.3 |
| Cerebrovascular Disease | 2.68 | 1.49, 4.83 | 0.001 |
| ACEi/ARB/ARNI | 0.77 | 0.45, 1.33 | 0.4 |
| SGLT2i | 0.49 | 0.29, 0.83 | 0.007 |
| MRA | 0.27 | 0.15, 0.46 | <0.001 |
| eGFR + UCR group | |||
| eGFR ≥ 60 + UCR < 95 (Group A) | – | – | |
| eGFR ≥ 60 + UCR ≥ 95 (Group B) | 4.03 | 1.50, 10.9 | 0.006 |
| eGFR < 60 + UCR < 95 (Group C) | 2.11 | 0.84, 5.30 | 0.112 |
| eGFR < 60 + UCR ≥ 95 (Group D) | 2.85 | 1.16, 7.01 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, A.S.; Hussain, M.S.; Roshan, M.H.K.; Pigazzani, F.; Choy, A.-M.; Khan, F.; Mordi, I.R.; Lang, C.C. Blood Urea/Creatinine Ratio and Mortality in Ambulatory Patients with Heart Failure with Reduced Ejection Fraction. Diseases 2025, 13, 362. https://doi.org/10.3390/diseases13110362
Oswald AS, Hussain MS, Roshan MHK, Pigazzani F, Choy A-M, Khan F, Mordi IR, Lang CC. Blood Urea/Creatinine Ratio and Mortality in Ambulatory Patients with Heart Failure with Reduced Ejection Fraction. Diseases. 2025; 13(11):362. https://doi.org/10.3390/diseases13110362
Chicago/Turabian StyleOswald, Andrew S., Muhammad S. Hussain, Mohsin H. K. Roshan, Filippo Pigazzani, Anna-Maria Choy, Faisel Khan, Ify R. Mordi, and Chim C. Lang. 2025. "Blood Urea/Creatinine Ratio and Mortality in Ambulatory Patients with Heart Failure with Reduced Ejection Fraction" Diseases 13, no. 11: 362. https://doi.org/10.3390/diseases13110362
APA StyleOswald, A. S., Hussain, M. S., Roshan, M. H. K., Pigazzani, F., Choy, A.-M., Khan, F., Mordi, I. R., & Lang, C. C. (2025). Blood Urea/Creatinine Ratio and Mortality in Ambulatory Patients with Heart Failure with Reduced Ejection Fraction. Diseases, 13(11), 362. https://doi.org/10.3390/diseases13110362







