The Systemic Link Between Oral Health and Cardiovascular Disease: Contemporary Evidence, Mechanisms, and Risk Factor Implications
Abstract
1. Introduction
2. Literature Review
- ➢
- Search Strategy
- ➢
- Inclusion and Exclusion Criteria
- ✓
- Peer-reviewed articles published in English between January 2000 and May 2025;
- ✓
- Studies involving adult human subjects;
- ✓
- Original research (observational or interventional), systematic reviews, or meta-analyses examining links between oral and cardiovascular health.
- ✓
- Case reports, editorials, and conference abstracts;
- ✓
- Studies focusing solely on pediatric populations;
- ✓
- Papers not directly addressing the oral–cardiovascular connection.
- ➢
- Study Selection and Quality Appraisal
- ➢
- Data Synthesis
2.1. Systemic Inflammation (Elevated CRP, IL-6, TNF-α)
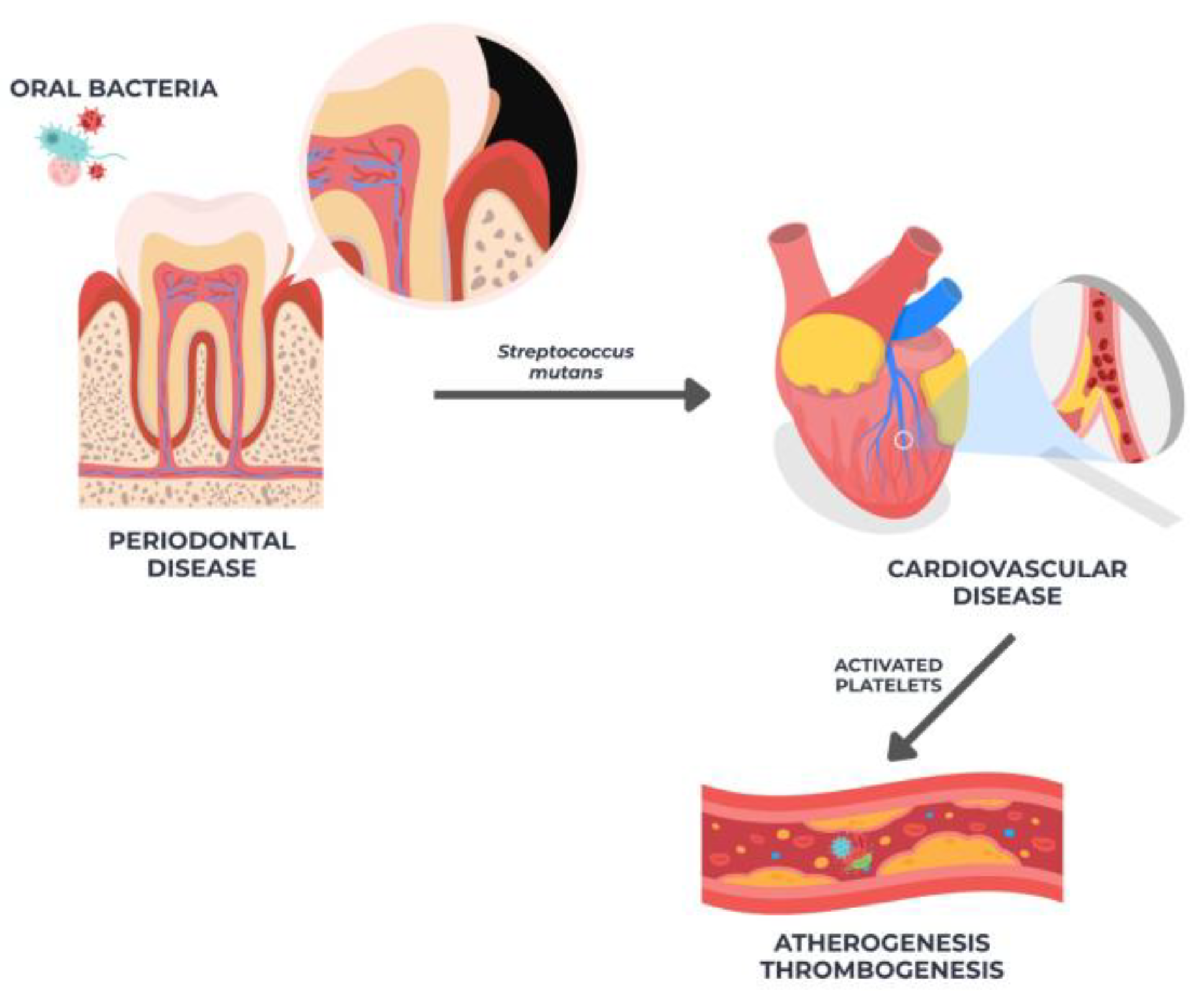
2.2. Oral Microbiota in Atheromatous Tissue
2.3. Xerostomia Associated with Antihypertensives
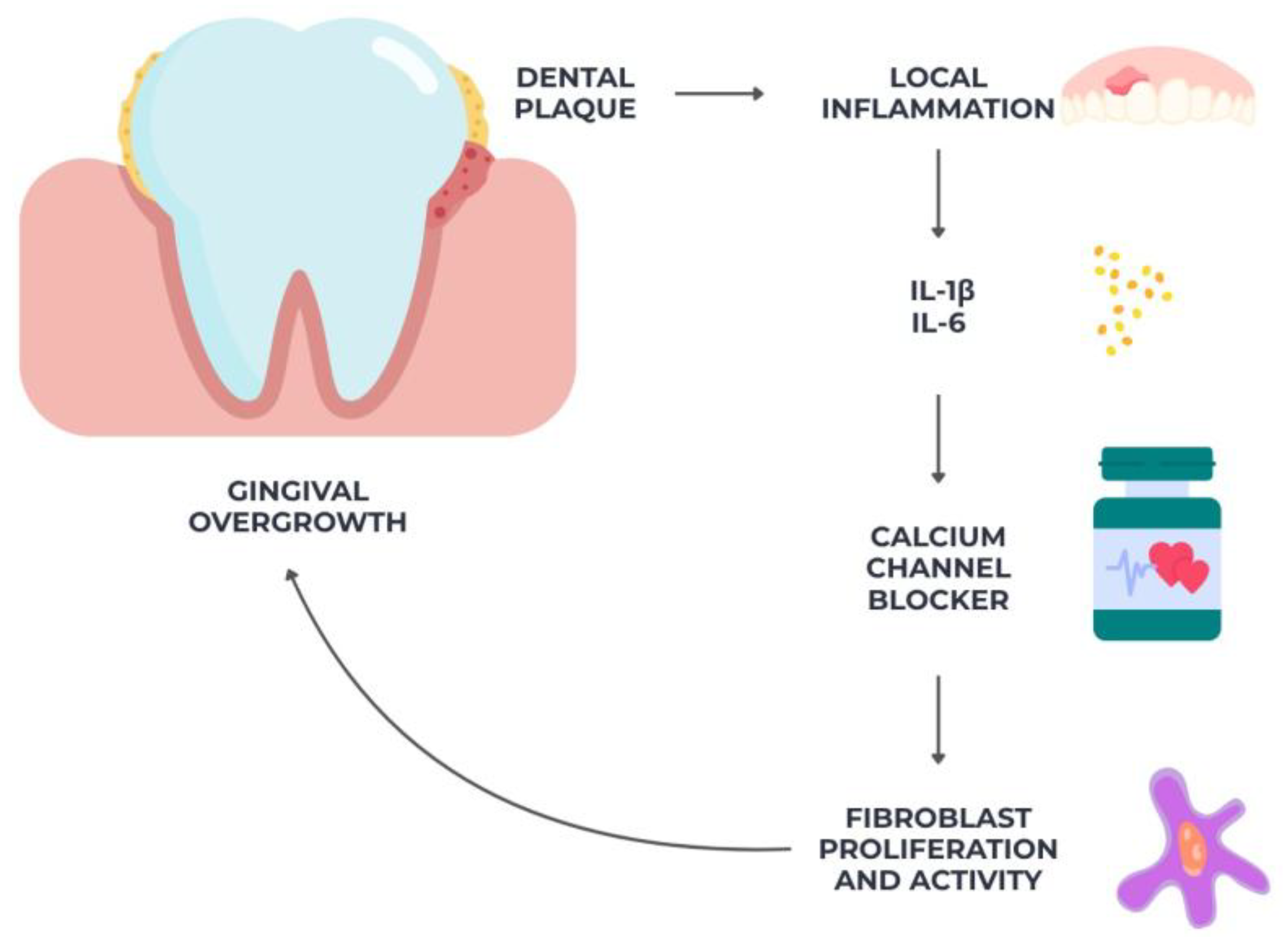
2.4. Gingival Overgrowth from Calcium Channel Blockers
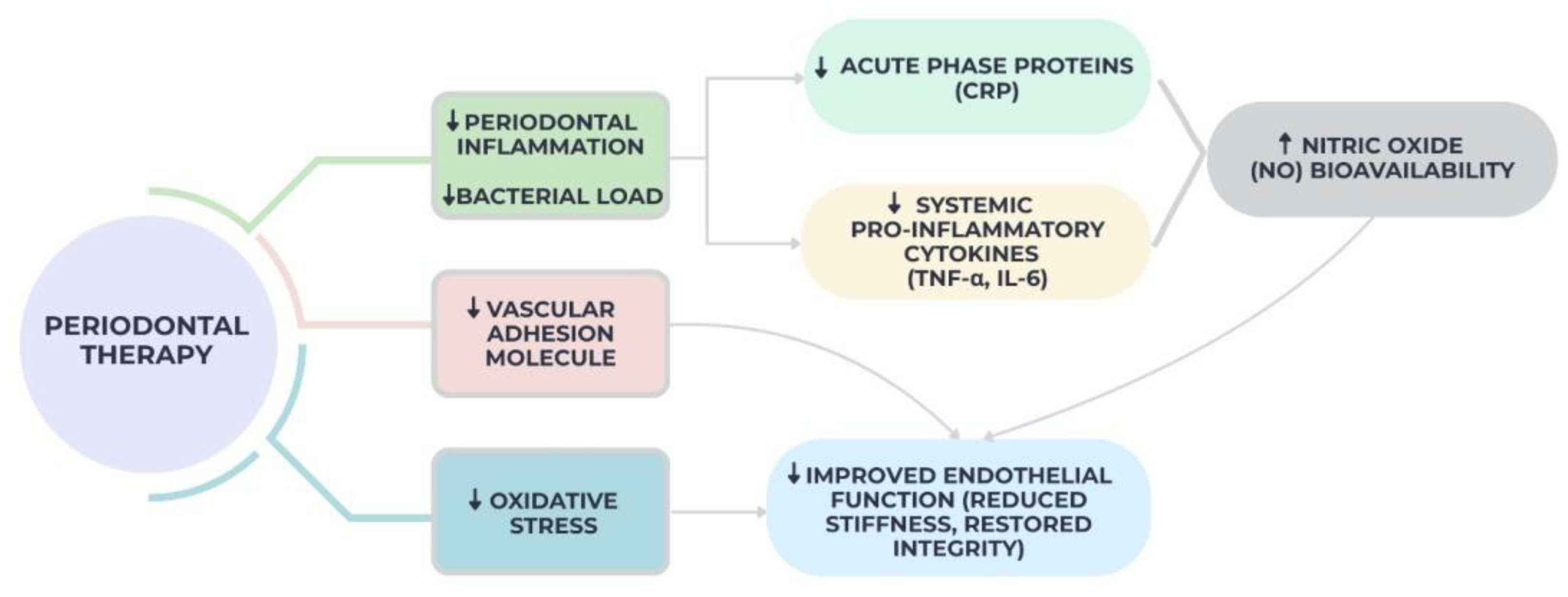
2.5. Improved Endothelial Function After Periodontal Therapy
2.6. Oral Bleeding and Delayed Healing in Anticoagulated Patients
2.7. Glossodynia and Burning Mouth in CVD Patients
3. Recommendations for Practice and Research
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Disease |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-alpha |
| (IL)-1 | Interleukin-1 |
| PCR | Polymerase Chain Reaction |
| ACE | Angiotensin-Converting Enzyme |
| UWS | Unstimulated Whole Saliva |
| TGF-β1 | Growth Factor-Beta 1 |
| IL-1β | Interleukin-1 Beta |
| NO | nitric oxide |
| FMD | Flow-Mediated Dilation |
| BMS | Burning Mouth Syndrome |
| AI | Artificial Intelligence |
| VCAM-1 | Cell Adhesion Molecule-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| SCFAs | Short-Chain Fatty Acids |
| Toll-like receptors | TLR2 and TLR4 |
References
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Orlandi, M.; Gunsolley, J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Clin. Periodontol. 2013, 40, S85–S105. [Google Scholar] [CrossRef] [PubMed]
- Dhadse, P.; Gattani, D.; Mishra, R. The link between periodontal disease and cardiovascular disease: How far we have come in last two decades? J. Indian Soc. Periodontol. 2010, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, M.; Demmer, R.; Papapanou, P. “Gum bug, leave my heart alone!”—Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010, 89, 879–902. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: Periodontitis and atherosclerotic cardiovascular disease. J. Periodontol. 2009, 80, 1021–1032. [Google Scholar] [CrossRef]
- Di Spirito, F. Oral and Systemic Health in the Elderly. Appl. Sci. 2022, 12, 11718. [Google Scholar] [CrossRef]
- Tatarciuc, D.; Curca, F.R.; Virvescu, D.I.; Butnaru, O.M.; Goriuc, A.; Bida, S.; Luchian, I.; Surlari, Z.; Scurtu, M.; Ursu, R.G.; et al. Alzheimer’s Disease and Oral Health from Clinical Challenges to Interdisciplinary Care: A Narrative Review. J. Clin. Med. 2025, 14, 6696. [Google Scholar] [CrossRef]
- Surlari, Z.; Virvescu, D.I.; Baciu, E.-R.; Vasluianu, R.-I.; Budală, D.G. The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma? Appl. Sci. 2021, 11, 12100. [Google Scholar] [CrossRef]
- Müller, F.; Shimazaki, Y.; Kahabuka, F.; Schimmel, M. Oral Health for an Ageing Population: The Importance of a Natural Dentition in Older Adults. Int. Dent. J. 2017, 67, 7–13. [Google Scholar] [CrossRef]
- Tibeica, S.C.; Virvescu, D.I.; Lupu, I.C.; Budala, D.G.; Luchian, I.; Tibeica, A.; Surlari, Z.; Carausu, E.M. Patients’ Satisfaction Regarding Oral Healthcare Services in the North-East Region of Romania: A Preliminary Questionnaire Survey. Healthcare 2024, 12, 1195. [Google Scholar] [CrossRef]
- Beukers, N.G.; van der Heijden, G.J.; van Wijk, A.J.; Loos, B.G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J. Epidemiol. Community Health 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.A.C.; Bafadhel, M.; Russell, R.E.K.; Sheikh, A.; Brindle, P.; Channon, K.M. Development and validation of a new algorithm for improved cardiovascular risk prediction. Nat. Med. 2024, 30, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Navarro, B.; Egido Moreno, S.; Omaña Cepeda, C.; Estrugo Devesa, A.; Jane Salas, E.; Lopez Lopez, J. Relationship between Oral Lichen Planus and Cardiovascular Disease of Atherosclerotic Origin: Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4630. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Wang, X.; Lam, T.H.; Kim, H.C.; Ho, S.; Ninomiya, T.; Woodward, M. Clustering of risk factors and the risk of incident cardiovascular disease in Asian and Caucasian populations: Results from the Asia Pacific Cohort Studies Collaboration. BMJ Open 2018, 8, e019335. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef]
- Rhee, M.; Lee, J.; Lee, E.Y.; Yoon, K.H.; Lee, S.H. Lipid Variability Induces Endothelial Dysfunction by Increasing Inflammation and Oxidative Stress. Endocrinol. Metab. 2024, 39, 511–520. [Google Scholar] [CrossRef]
- Badrov, M.; Miskovic, M.; Glavina, A.; Tadin, A. Oral–Systemic Health Awareness Among Physicians and Dentists in Croatian Primary Healthcare: A Cross-Sectional Study. Epidemiologia 2025, 6, 43. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.-H. Common Medical and Dental Problems of Older Adults: A Narrative Review. Geriatrics 2021, 6, 76. [Google Scholar] [CrossRef]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are Local Drug Delivery Systems a Challenge in Clinical Periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Visco, V.; Loria, F.; Squillante, A.; Iannarella, C.; Guerriero, A.; Cirillo, A.; Barbato, M.G.; Ferrigno, O.; Augusto, A.; et al. Cardiovascular Nursing in Rehabilitative Cardiology: A Review. J. Cardiovasc. Dev. Dis. 2025, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Bakhsh, A. Association between Endodontic Infection, Its Treatment and Systemic Health: A Narrative Review. Medicina 2022, 58, 931. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Hanafiah, A. Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. Int. J. Mol. Sci. 2025, 26, 4264. [Google Scholar] [CrossRef]
- Kramer, C.D.; Simas, A.M.; He, X.; Ingalls, R.R.; Weinberg, E.O.; Genco, C.A. Distinct roles for dietary lipids and Porphyromonas gingivalis infection on atherosclerosis progression and the gut microbiota. Anaerobe 2017, 45, 19–30. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Velsko, I.M.; Rivera-Kweh, M.F.; Zheng, D.; Lucas, A.R.; Kesavalu, L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoE null Mice. PLoS ONE 2015, 10, e0143291. [Google Scholar] [CrossRef]
- Butnaru, O.; Bida, F.C.; Tatarciuc, D.; Rotundu, G.; Sîrghe, A.; Lionte, C.; Luchian, I.; Haba, D. Research on the Bidirectional Relationship between Periodontal Disease and High Blood Pressure. Med.-Surg. J. 2024, 128, 613–623. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral Sci. 2022, 30, 20210349. [Google Scholar] [CrossRef]
- Fratila, D.N.; Virvescu, D.I.; Luchian, I.; Hancianu, M.; Baciu, E.R.; Butnaru, O.; Budala, D.G. Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels 2024, 10, 661. [Google Scholar] [CrossRef]
- Denis, F.; Clement, C. Oral Health: A Major Global Public Health Concern. J. Clin. Med. 2025, 14, 4101. [Google Scholar] [CrossRef]
- Ben Yahya, I. Global oral health initiative: World Health Organization strategic action plan. J. Dent. Educ. 2024, 88, 699–702. [Google Scholar] [CrossRef]
- Benzian, H.; Kavanaugh, D.; Naidoo, S.; Mathur, M.R. Oral disease must be central in policies to improve global health. BMJ 2025, 389, r1070. [Google Scholar] [CrossRef]
- Kuppa, A.; Tripathi, H.; Al-Darraji, A.; Tarhuni, W.M.; Abdel-Latif, A. C-Reactive Protein Levels and Risk of Cardiovascular Diseases: A Two-Sample Bidirectional Mendelian Randomization Study. Int. J. Mol. Sci. 2023, 24, 9129. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, S.; Deskins, D.L.; Young, P.P. Membrane TNF-alpha-activated programmed necrosis is mediated by Ceramide-induced reactive oxygen species. J. Mol. Signal. 2013, 8, 12. [Google Scholar] [CrossRef]
- Sheriff, A. Special Issue “C-Reactive Protein and Cardiovascular Disease: Clinical Aspects”. J. Clin. Med. 2022, 11, 3610. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease—Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Shen, C.; Chen, Y.; Zhao, X.; Wei, P.; Sun, J.; Ji, Y.; Chen, X.; Yang, S. Association of high sensitive C-reactive protein with coronary heart disease: A Mendelian randomization study. BMC Med. Genet. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, T.E.R.F. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Rifai, N.; Blake, G.J.; Gaziano, J.M.; Ridker, P.M. C-reactive protein and the risk of developing hypertension. JAMA 2003, 290, 2945–2951. [Google Scholar] [CrossRef]
- Badimon, L.; Pena, E.; Arderiu, G.; Padro, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Hage, F.G. C-reactive protein and hypertension. J. Hum. Hypertens. 2014, 28, 410–415. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D.; et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009, 302, 37–48. [Google Scholar] [CrossRef]
- Bida, F.C.; Agop-Forna, D.; Bolat, M.; Tudorici, T.; Bârlean, M.C.; Budala, D.G.; Taraboanta-Gamen, A.C.; Virvescu, D.I. Quality of life and dental health in the elderly patients: A clinical investigation. Rom. J. Oral Rehab. 2025, 1, 811–817. [Google Scholar] [CrossRef]
- Li, X.; Peng, S.; Guan, B.; Chen, S.; Zhou, G.; Wei, Y.; Gong, C.; Xu, J.; Lu, X.; Zhang, X.; et al. Genetically Determined Inflammatory Biomarkers and the Risk of Heart Failure: A Mendelian Randomization Study. Front. Cardiovasc. Med. 2021, 8, 734400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kaplan, R.C.; Burk, R.D.; Qi, Q. The Oral Microbiota, Microbial Metabolites, and Immuno-Inflammatory Mechanisms in Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 12337. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Papaioannou, W.; Sakkas, L.I.; Kousvelari, E. The Autoimmunity-Oral Microbiome Connection. Oral Dis. 2017, 23, 828–839. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The Oral Microbiome in the Pathophysiology of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 386. [Google Scholar] [CrossRef]
- Lucchese, A. Streptococcus Mutans Antigen I/II and Autoimmunity in Cardiovascular Diseases. Autoimmun. Rev. 2017, 16, 456–460. [Google Scholar] [CrossRef]
- Kaschwich, M.; Behrendt, C.; Heydecke, G.; Bayer, A.; Debus, E.S.; Seedorf, U.; Aarabi, G. The Association of Periodontitis and Peripheral Arterial Occlusive Disease-A Systematic Review. Int. J. Mol. Sci. 2019, 20, 2936. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The Oral Microbiota and Cardiometabolic Health: A Comprehensive Review and Emerging Insights. Front. Immunol. 2022, 13, 1010368. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Martínez-Acitores, L.; Hernández Ruiz de Azcárate, F.; Casañas, E.; Serrano, J.; Hernández, G.; López-Pintor, R.M. Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs. Int. J. Environ. Res. Public Health 2020, 17, 2478. [Google Scholar] [CrossRef] [PubMed]
- Budală, D.G.; Baciu, E.R.; Virvescu, D.I.; Armencia, A.; Scutariu, M.M.; Surlari, Z.; Balcoș, C. Quality of Life of Complete Denture Wearers—A Comparative Study between Conventional Dentures and Acrylic Dentures with Vitamin B12 Incorporated. Medicina 2021, 57, 820. [Google Scholar] [CrossRef] [PubMed]
- Carramolino-Cuéllar, E.; Lauritano, D.; Silvestre, F.J.; Carinci, F.; Lucchese, A.; Silvestre-Rangil, J. Salivary flow and xerostomia in patients with type 2 diabetes. J. Oral Pathol. Med. 2018, 47, 526–530. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Nederfors, T.; Nauntofte, B.; Twetman, S. Effects of furosemide and bendroflumethiazide on saliva flow rate and composition. Arch. Oral Biol. 2004, 49, 507–513. [Google Scholar] [CrossRef]
- Ivanovski, K.; Pesevka, S.; Ristoska, S.; Dirjanska, K.; Mindova, S.; Pandilova, M.; Georgieva, S.; Stefanovska, E.; Filipce, V.; Apostolska, S.; et al. The impact of antihypertensive medications on quantitative and qualitative characteristics of saliva. J. Pharm. Biol. Chem. Sci. 2015, 6, 1356–1364. [Google Scholar]
- Speroni, S.; Giuffrè, M.; Tura, T.; Al Jawaheri, Q.A.S.; Antonelli, L.; Coccoluto, L.; Bortune, G.; Sarnelli, F.; Abati, S. Calcium Antagonist-Induced Gingival Overgrowth: A Case Report and Literature Review. Diagnostics 2025, 15, 320. [Google Scholar] [CrossRef]
- Droździk, A.; Droździk, M. Drug-induced gingival overgrowth—Molecular aspects of drug actions. Int. J. Mol. Sci. 2023, 24, 5448. [Google Scholar] [CrossRef]
- Reddy, S.C.; Midha, N.; Chhabra, V.; Kumar, D.; Bohra, G.K. Amlodipine Induced Gum Hypertrophy: A Rare Case Report. Curr. Drug Saf. 2022, 17, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Ray-Chaudhuri, A. Calcium channel blocker induced gingival enlargement following implant placement in a fibula free flap reconstruction of the mandible: A case report. Int. J. Implant. Dent. 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, G.A.G.; Edsor, E.; Sajesh, S.; Neha, K.; Gangadhar, R. Calcium Channel Blockers- Induced Iatrogenic Gingival Hyperplasia: Case Series. J. Pharm. Bioallied Sci. 2023, 15 (Suppl. S1), S821–S824. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M.; Herman, W. Calcium channel blocker induced gingival overgrowth. J. Clin. Hypertens. 2002, 4, 310–311. [Google Scholar] [CrossRef]
- Fang, L.; Tan, B.C. Clinical presentation and management of drug-induced gingival overgrowth: A case series. World J. Clin. Cases 2021, 9, 9926–9934. [Google Scholar] [CrossRef]
- Lauritano, D.; Lucchese, A.; Di Stasio, D.; Della Vella, F.; Cura, F.; Palmieri, A.; Carinci, F. Molecular Aspects of Drug-Induced Gingival Overgrowth: An In Vitro Study on Amlodipine and Gingival Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2047. [Google Scholar] [CrossRef]
- Angjelova, A.; Jovanova, E.; Polizzi, A.; Laganà, L.; Santonocito, S.; Ragusa, R.; Isola, G. Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. J. Clin. Med. 2024, 13, 3781. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-De-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef] [PubMed]
- Budala, D.G.; Luchian, I.; Virvescu, D.I.; Tudorici, T.; Constantin, V.; Surlari, Z.; Butnaru, O.; Bosinceanu, D.N.; Bida, C.; Hancianu, M. Salivary Biomarkers as a Predictive Factor in Anxiety, Depression, and Stress. Curr. Issues Mol. Biol. 2025, 47, 488. [Google Scholar] [CrossRef] [PubMed]
- Lazureanu, P.C.; Popescu, F.G.; Stef, L.; Focsa, M.; Vaida, M.A.; Mihaila, R. The Influence of Periodontal Disease on Oral Health Quality of Life in Patients with Cardiovascular Disease: A Cross-Sectional Observational Single-Center Study. Medicina 2022, 58, 584. [Google Scholar] [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Lee, J.-I.; Seo, H.; Cho, Y.-C.; Son, J.-H.; Sung, I.-Y. Analysis of Postoperative Bleeding After Oral Surgery in Patients Receiving Anticoagulants: A Retrospective Study. Medicina 2025, 61, 425. [Google Scholar] [CrossRef]
- Lababidi, E.; Breik, O.; Savage, J.; Engelbrecht, H.; Kumar, R.; Crossley, C.W. Assessing an oral surgery specific protocol for patients on direct oral anticoagulants: A retrospective controlled cohort study. Int. J. Oral Maxillofac. Surg. 2018, 47, 940–946. [Google Scholar] [CrossRef]
- Yeh, C.H.; Hogg, K.; Weitz, J.I. Overview of the new oral anticoagulants: Opportunities and challenges. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1056–1065. [Google Scholar] [CrossRef]
- Bajkin, B.V.; Vujkov, S.B.; Milekic, B.R.; Vuckovic, B.A. Risk factors for bleeding after oral surgery in patients who continued using oral anticoagulant therapy. J. Am. Dent. Assoc. 2015, 146, 375–381. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Rusche, B.; Clemm, R.; Neukam, F.W.; Buchbender, M. Management of anticoagulated patients in dentoalveolar surgery: A clinical comparative study. Clin. Oral Investig. 2020, 24, 2653–2662. [Google Scholar] [CrossRef]
- Buchbender, M.; Rößler, F.; Kesting, M.R.; Frohwitter, G.; Adler, W.; Rau, A. Management of anticoagulated patients in dentoalveolar surgery: A retrospective study comparing bridging with heparin versus unpaused vitamin K antagonist medication. BMC Oral Health 2021, 21, 96. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Pollio, A.; Fortuna, G.; Leuci, S.; Ruoppo, E.; Adamo, D.; Zarrelli, C. Unexplained somatic comorbidities in patients with burning mouth syndrome: A controlled clinical study. J. Orofac. Pain 2011, 25, 131–140. [Google Scholar] [PubMed]
- Albuquerque, R.J.C.; de Leeuw, R.; Carlson, C.R.; Okeson, J.P.; Miller, C.S.; Andersen, A.H. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain 2006, 122, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Calabria, E.; Cuocolo, R.; Ugga, L.; Buono, G.; Marenzi, G.; Gasparro, R.; Pecoraro, G.; Aria, M.; D’Aniello, L.; et al. Burning Fog: Cognitive Impairment in Burning Mouth Syndrome. Front. Aging Neurosci. 2021, 13, 727417. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Pecoraro, G.; Fortuna, G.; Amato, M.; Marenzi, G.; Aria, M.; Mignogna, M.D. Assessment of oral health-related quality of life, measured by OHIP-14 and GOHAI, and psychological profiling in burning mouth syndrome: A case-control clinical study. J. Oral Rehabil. 2020, 47, 42–52. [Google Scholar] [CrossRef]
- Canfora, F.; Calabria, E.; Pecoraro, G.; Aniello, L.D.; Aria, M.; Marenzi, G.; Sammartino, P.; Mignogna, M.D.; Adamo, D. The use of self-report questionnaires in an analysis of the multidimensional aspects of pain and a correlation with the psychological profile and quality of life in patients with burning mouth syndrome: A case-control study. J. Oral Rehabil. 2022, 49, 890–914. [Google Scholar] [CrossRef]
- Muñoz Aguilera, E.; Leira, Y.; Miró Catalina, Q.; Orlandi, M.; Czesnikiewicz-Guzik, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J. Intern. Med. 2021, 289, 532–546. [Google Scholar] [CrossRef]
- Chen, D.Y.; Lin, C.H.; Chen, Y.M.; Chen, H.H. Risk of atrial fibrillation or flutter associated with periodontitis: A nationwide, population-based, cohort study. PLoS ONE 2016, 11, e0165601. [Google Scholar] [CrossRef]
- Gualtero, D.F.; Lafaurie, G.I.; Buitrago, D.M.; Castillo, Y.; Vargas-Sanchez, P.K.; Castillo, D.M. Oral microbiome mediated inflammation, a potential inductor of vascular diseases: A comprehensive review. Front. Cardiovasc. Med. 2023, 10, 1250263. [Google Scholar] [CrossRef]
- Messina, B.M.; Grippaudo, C.; Polizzi, A.; Blasi, A.; Isola, G. The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Appl. Sci. 2025, 15, 6847. [Google Scholar] [CrossRef]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Martellacci, L.; Quaranta, G.; Patini, R.; Isola, G.; Gallenzi, P.; Masucci, L. A Literature Review of Metagenomics and Culturomics of the Peri-implant Microbiome: Current Evidence and Future Perspectives. Materials 2019, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Sasaki, S.; Yoshizumi, M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: A comparison of dippers and non-dippers. J. Am. Coll. Cardiol. 2002, 40, 2039–2043. [Google Scholar] [CrossRef]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D. Burning mouth syndrome: A review of the literature and case series. J. Oral Pathol. Med. 2014, 43, 163–170. [Google Scholar]
- Glavina, A.; Trlaja, A.; Martinović, D.; Tadin, A.; Lugović-Mihić, L. Stratification of Patients with Burning Mouth Syndrome in the Croatian Population: A Single-Center Cross-Sectional Study. NeuroSci 2025, 6, 33. [Google Scholar] [CrossRef]
- Rakic, M.; Calciolari, E.; Grant, M.M.; Radovanovic, S.; Bostanci, N.; Preshaw, P.M. Host Markers of Periodontal Diseases: Meta-Analysis of Diagnostic Accuracy Studies. J. Clin. Periodontol. 2025, 52, 155–181. [Google Scholar] [CrossRef]
- Reddahi, S.; Bouziane, A.; Rida, S.; Tligui, H.; Ennibi, O. Salivary biomarkers in periodontitis patients: A pilot study. Int. J. Dent. 2022, 2022, 3664516. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. Eur. Heart J. 2025, 29, ehaf190. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Haller, P.M.; Beer, B.N.; Tonkin, A.M.; Blankenberg, S.; Neumann, J.T. Role of Cardiac Biomarkers in Epidemiology and Risk Outcomes. Clin. Chem. 2021, 67, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Haider, Z.M.; Hussain, J.; Malik, F.H.; Talib, I.; Abdullah, S. Comprehensive Analysis of Cardiovascular Diseases: Symptoms, Diagnosis, and AI Innovations. Bioengineering 2024, 11, 1239. [Google Scholar] [CrossRef]
| Outcome | No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Overall Quality |
|---|---|---|---|---|---|---|---|
| Systemic inflammation (elevated CRP, IL-6, TNF-α) [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] | 18 | RCTs+ Cohort Studies | Low | Moderate | No | Moderate | Moderate |
| Oral microbiota In atheromatous tissue [52,53,54,55,56,57,58,59,60,61,62] | 11 | Case–Control Studies | Low | Low | No | Low | High |
| Xerostomia associated with antihypertensives [28,63,64,65,66,67,68,69,70] | 9 | Cross-sectional Studies | Moderate | Moderate | No | Low | Moderate |
| Gingival overgrowth from calcium channel blockers [71,72,73,74,75,76,77,78] | 8 | Observational studies | Moderate | Low | No | Moderate | High |
| Improved endothelial function after periodontal therapy [79,80,81,82,83,84,85] | 7 | Clinical Trials | Low | Low | Yes | Moderate | Moderate |
| Oral bleeding and delayed healing in anticoagulated patients [86,87,88,89,90,91] | 6 | Observational + case reports | Moderate | Moderate | Yes | Moderate | Moderate |
| Glossodynia and burning mouth in CVD patients [92,93,94,95,96] | 5 | Cross-sectional Studies | Moderate | High | Yes | High | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bida, F.C.; Curca, F.R.; Lupusoru, R.-V.; Virvescu, D.I.; Scurtu, M.; Rotundu, G.; Butnaru, O.M.; Tudorici, T.; Luchian, I.; Budala, D.G. The Systemic Link Between Oral Health and Cardiovascular Disease: Contemporary Evidence, Mechanisms, and Risk Factor Implications. Diseases 2025, 13, 354. https://doi.org/10.3390/diseases13110354
Bida FC, Curca FR, Lupusoru R-V, Virvescu DI, Scurtu M, Rotundu G, Butnaru OM, Tudorici T, Luchian I, Budala DG. The Systemic Link Between Oral Health and Cardiovascular Disease: Contemporary Evidence, Mechanisms, and Risk Factor Implications. Diseases. 2025; 13(11):354. https://doi.org/10.3390/diseases13110354
Chicago/Turabian StyleBida, Florinel Cosmin, Florin Razvan Curca, Raoul-Vasile Lupusoru, Dragos Ioan Virvescu, Mihaela Scurtu, Gabriel Rotundu, Oana Maria Butnaru, Teona Tudorici, Ionut Luchian, and Dana Gabriela Budala. 2025. "The Systemic Link Between Oral Health and Cardiovascular Disease: Contemporary Evidence, Mechanisms, and Risk Factor Implications" Diseases 13, no. 11: 354. https://doi.org/10.3390/diseases13110354
APA StyleBida, F. C., Curca, F. R., Lupusoru, R.-V., Virvescu, D. I., Scurtu, M., Rotundu, G., Butnaru, O. M., Tudorici, T., Luchian, I., & Budala, D. G. (2025). The Systemic Link Between Oral Health and Cardiovascular Disease: Contemporary Evidence, Mechanisms, and Risk Factor Implications. Diseases, 13(11), 354. https://doi.org/10.3390/diseases13110354










