Abstract
In light of the elevated incidence and consequential prognostic implications associated with colorectal cancer, a comprehensive investigation into the impact exerted by inflammatory status on patient management becomes imperative. A retrospective study spanning 7 years was conducted, involving the retrospective collection of data on colorectal cancer patients undergoing surgical intervention. We evaluated six inflammation ratios derived from complete peripheral blood counts. A thorough analysis of these markers’ prognostic capacity was conducted, revealing that patients who died postoperatively displayed significantly higher preoperative Aggregate Index of Systemic Inflammation—AISI (p = 0.014) and Systemic Inflammation Response Index—SII (p = 0.0197) levels compared to those with successful discharge. Noteworthy variations in neutrophil-to-lymphocyte ratio (p = 0.0103), platelet-to-lymphocyte ratio (p = 0.0041), AISI (p < 0.001), and SII (p = 0.0045) were observed in patients necessitating postoperative Intensive Care Unit (ICU) monitoring. Furthermore, patients with complications, such as an intestinal fistula, exhibited significantly elevated AISI (p = 0.0489). Inflammatory biomarkers stand out as valuable prognostic tools for colorectal cancer patients, offering potential assistance in predicting their prognosis.
1. Introduction
Colorectal cancer (CRC) poses a significant health challenge, ranking as the third most common cancer globally according to Globocan 2020, with more than 1.9 million new cases of colorectal cancer and more than 930,000 deaths due to colorectal cancer estimated to have occurred worldwide in 2020 [1]. Over two decades (from 1999 to 2019), Romania has witnessed a concerning increase in CRC incidence and mortality rates, reaching 17.74/100,000 inhabitants [2]. Despite considerable advancements in CRC treatment, long-term survival remains suboptimal, with approximately 60% of patients experiencing a 5-year survival rate post-curative resection [3].
Surgical intervention remains essential in CRC treatment, with modern technologies markedly improving the outcomes and overall survival. However, precise preoperative tumor staging remains a challenge, emphasizing the crucial role of identifying prognostic biomarkers in optimizing patient management [4].
The investigation of biological markers has become pivotal in understanding systemic inflammation and its role in cancer progression. Various ratios, including neutrophil-to-lymphocyte (NLR), platelet-to-lymphocyte (PLR), and monocyte-to-lymphocyte (MLR), are extensively studied in colorectal cancer (CRC) to offer insights into patients’ inflammatory status and predict disease progression [2,3]. Notably, studies present that an elevated NLR (> 5) has been linked to a decrease in overall survival [5], while increased PLR values (>140) have been correlated with higher postoperative morbidity rate [6]. Hemoglobin, NLR, and platelet count serve as additional biomarkers, offering insights into the pre-surgery tumor status. Furthermore, emphasizing the significance of inflammatory indices such as AISI (aggregate index of systemic inflammation), SIRI (systemic inflammation response index), and SII (Systemic Immune–Inflammation Index) have the potential to predict outcomes in CRC [7,8].
Understanding the intricate interplay between systemic inflammation and CRC progression is essential for refining prognostic assessments and therapeutic strategies. The identified biomarkers offer valuable information, underscoring the necessity for personalized approaches based on the inflammatory status of CRC patients.
Our study aimed to evaluate the preoperative inflammation status of patients with colorectal cancer, identify associations between this status and several parameters, and evaluate, if any, have a possible impact on postoperative prognosis.
2. Materials and Methods
We conducted our study according to the Declaration of Helsinki and received approval from the “Pius Brinzeu” Clinical Emergency Hospital Ethical Commission prior to collecting data (approval no. 434/29 January 2024).
2.1. Design and Settings
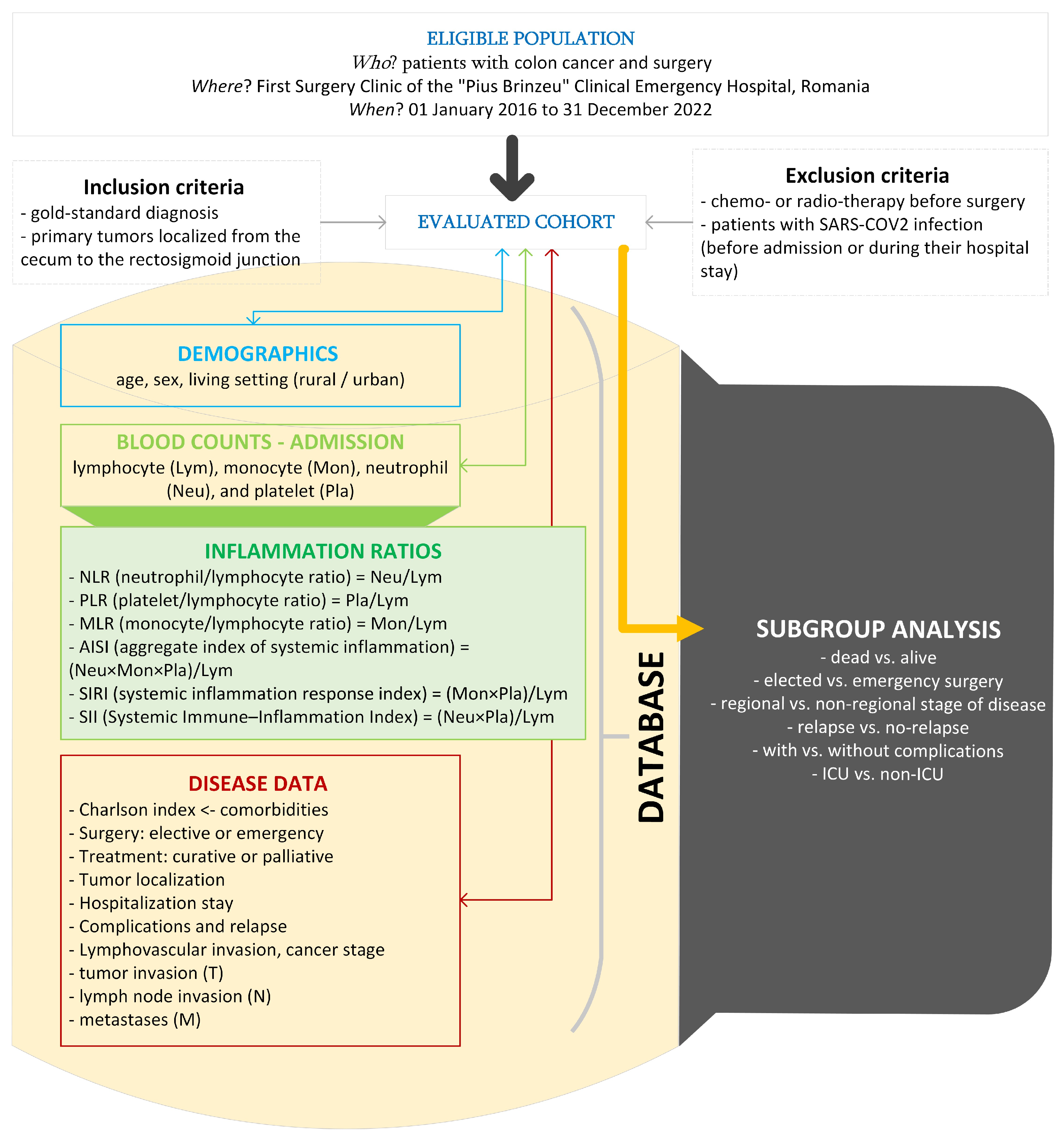
To reach our aim, we conducted an observational, analytical study with a retrospective collection of data from medical charts. Patients undergoing surgical intervention for primary colorectal cancer treatment at the First Surgery Clinic of the “Pius Brinzeu” Clinical Emergency Hospital, Romania, represented the eligible population. The time frame of our study covers 7 years, from 1 January 2016 to 31 December 2022.
Figure 1 presents the flow of the applied methods. The histopathological examination was the gold-standard diagnosis in our study. Medical charts of patients were the source of raw data.
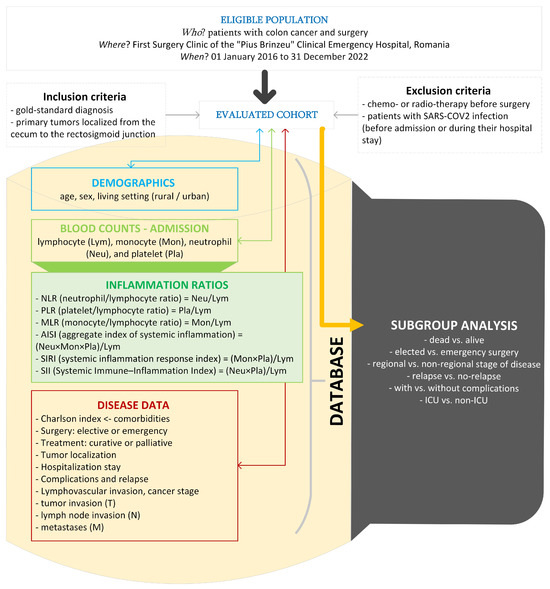
Figure 1.
The setting, raw, and derived data in our study (drawn with Microsoft Visio, v. 16.0 2019, Microsoft Corporation, Redmond, WA, USA).
We excluded patients with chemotherapy or radiotherapy before the surgery because their effects on systemic inflammation (either increased or decreased) were observed for months after treatment, and Zou et al. mentioned that adjuvant chemotherapy appeared to be more effective in patients with CRC with high NLR or PLR [9,10,11]. Similarly, SARS-CoV-2 infection is associated with a systemic inflammatory response, besides the inflammation associated with the presence of colorectal cancer [12]; in other words, patients with a history of this infection prior to the intervention or patients who developed an infection during the hospital stay were excluded.
We included patients with primary tumors localized from the cecum to the rectosigmoid junction. Four categories were used for tumor location: right colon (tumors at the cecum, ascending colon, hepatic flexure), transverse colon, and left colon (tumors at the splenic flexure, descending colon, sigmoid colon, and rectosigmoid junction).
2.2. Data Analysis
We used centrality and dispersion indicators to summarize the characteristics of the sample. We reported the mean and standard deviation for normally distributed data (Shapiro–Wilk test). For non-normally distributed quantitative variables, we used the median and interquartile range [Q1 to Q3]. We report qualitative variables as numbers and percentages. We applied the Mann–Whitney test to compare two independent groups and the Kruskal–Wallis test to compare two groups for quantitative data that violated, in at least one sub-group, the normal distribution. We applied the Chi-squared test or Fisher’s exact test to compare groups in the case of attribute data.
We used Statistica (v.13.5, TIBCO Software Inc., Palo Alto, CA, USA) to conduct our exploratory statistical analysis. We obtained the graphical representations with Jasp (v. 0.18.3.0, available at: https://jasp-stats.org/ (accessed on 10 January 2024)). We used a significance level of 5% and considered the p-values smaller than 0.05 as statistically significant.
3. Results
We evaluated 282 patients aged from 26 to 97 years with a balance among sex. As expected, due to restricted access to clinical care, the number of cases was lower during the COVID-19 pandemic (39 in 2016, 43 in 2017, 65 in 2018, 41 in 2019, 31 in 2020, 29 in 2021, and 34 in 2022).
3.1. Deceased vs. Alive
The patients who died during hospitalization were older, had a CHARLSON score higher than 3, most frequently had the surgery in emergency, had postoperative complications, and needed intensive care in the ICU (Table 1).

Table 1.
Cohort features and comparison between deceased and alive patients.
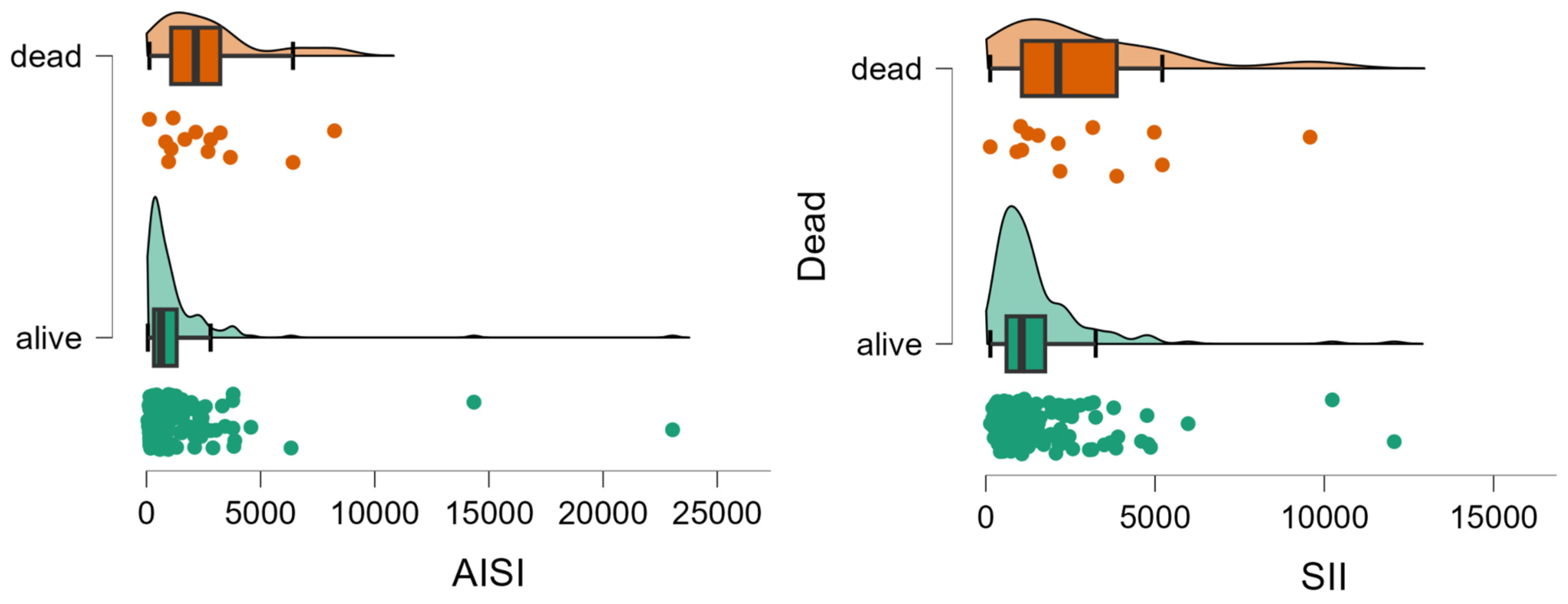
Patients who died showed significantly less lymphocytes and neutrophils, and higher values of AISI and SII than those who survived (Table 2, Figure 2).

Table 2.
Evaluated biomarkers and associated ratios by groups.
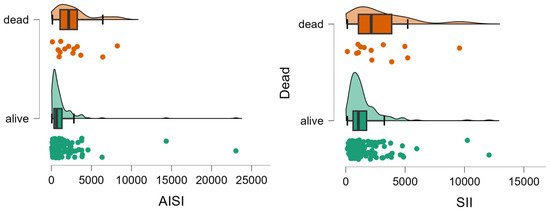
Figure 2.
Distribution of AISI and SII values by group. The circles represent the raw data, the box shows the median (middle line), first and third quartiles (box), respectively, and minimum and maximum values (whiskers).
3.2. Elected vs. Emergency Surgery
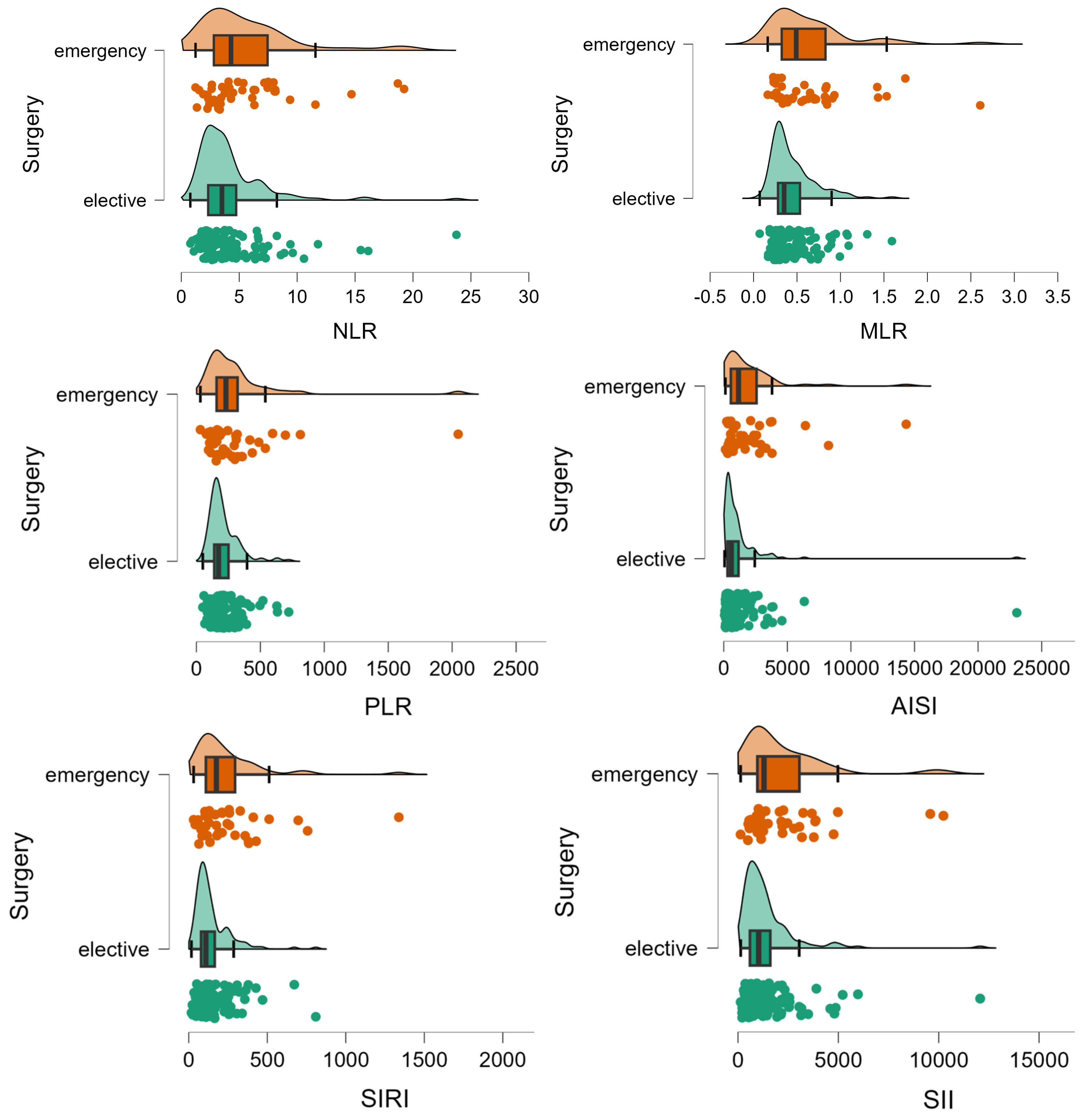
Patients who had an unplanned surgery were similar to those who had a planned surgery in terms of age (p-value = 0.1008), sex (p-value = 0.2468), living in rural areas (p-value = 0.2310), hospitalization stay (p-value = 0.0540), presence of relapse (p-value = 0.4353), post-surgery complications (p-value = 0.2278), and CHARLSON score (p = 0.3185). A statistically significant smaller percentage of patients with unplanned surgery had a curative intervention (104 (77%) vs. 128 (87.1%), p-value = 0.0275), significantly higher frequency of lymphovascular invasion (70 (56.9%) vs. 58 (42%); p-value= 0.0164), with a lower percentage of patients in stage I (4 (3.3%) vs. 19 (13.7%)) and a higher percentage of patients in stage IV (24 (19.5%) vs. 18 (12.9%)) (χ2 = 11.2, p-value = 0.0105). Patients with emergency surgery showed a different inflammation profile compared to those with planned surgery (Table 3, Figure 3).

Table 3.
Inflammation profile of patients with or without an elected surgery.
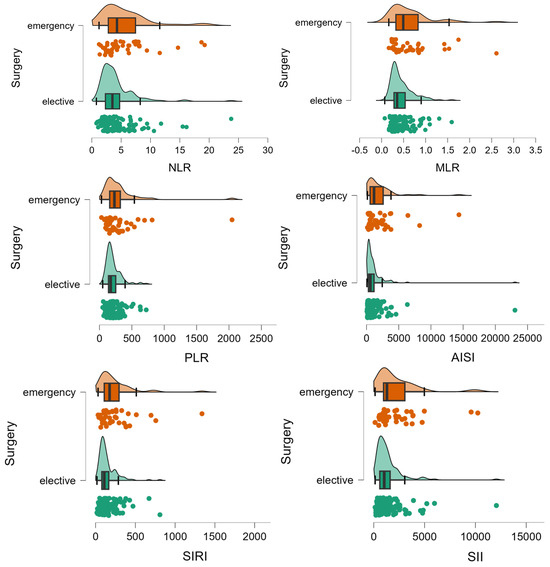
Figure 3.
Distribution of inflammation ratios on patients with (elective) and without elective surgery (emergency). The circles represent the raw data, the box showed the median (middle line), first and third quartiles (box), respectively minimum and maximum values (whiskers).
3.3. Stage of the Disease
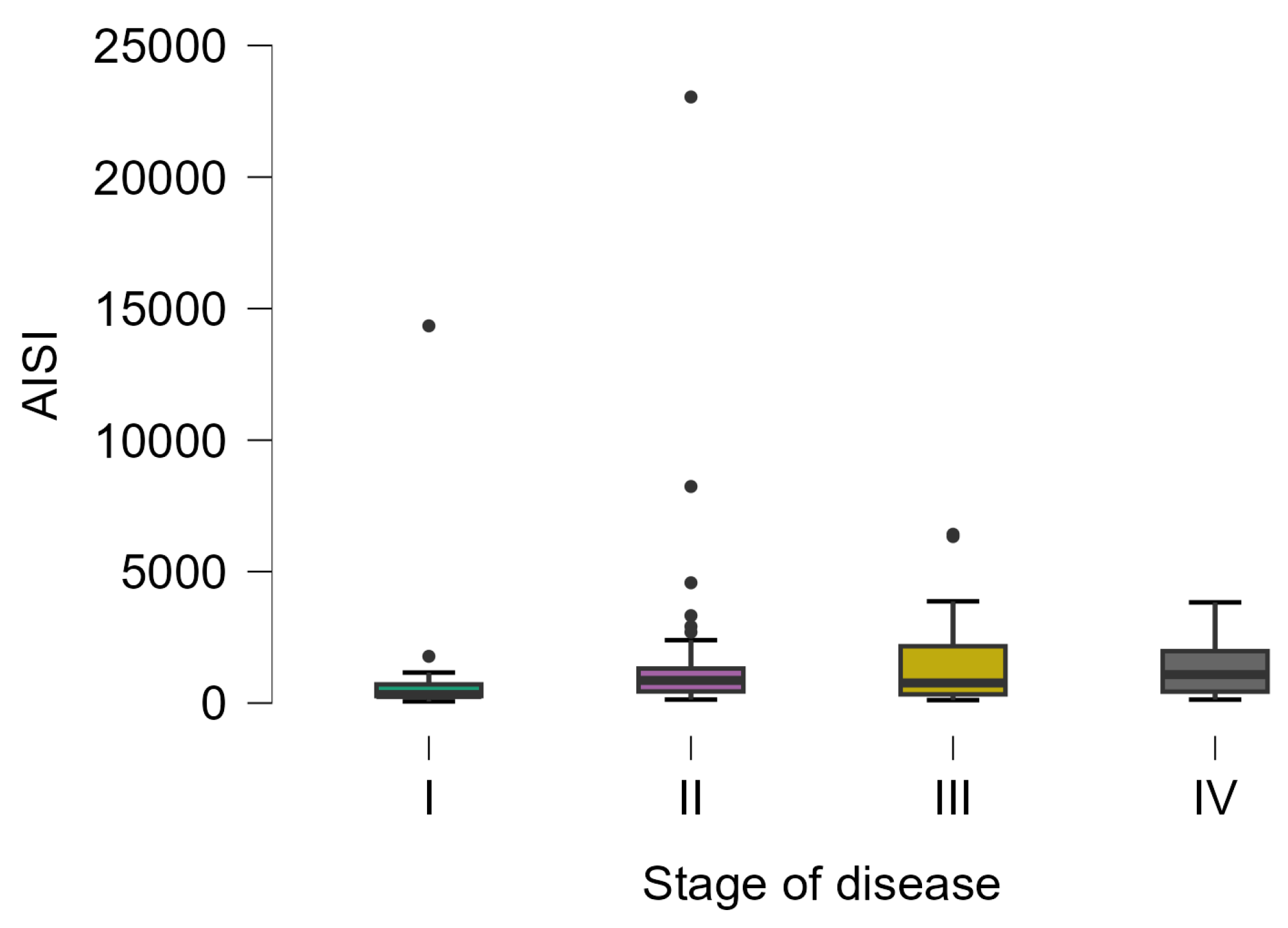
The AISI varied from 62 to 23,044, with statistically significant differences in patients with different stages of the disease (Kruskal–Wallis test; p-value = 0.0313). The lowest values were observed in patients with stage I (323 [266.5 to 710.5], 20) and the highest values in patients with stage IV (1082 [433.5 to 1975], 23), with a statistically significant difference in posthoc analysis (p-value = 0.0328) (Figure 4).
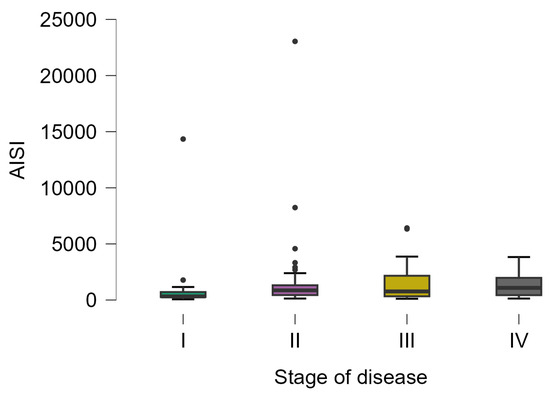
Figure 4.
Distribution of AISI on patients with different disease stages. The circles represent the raw data, and the box shows the median (middle line), first and third quartiles (box), respectively, and minimum and maximum values (whiskers).
Patients with a regional stage of disease (serosa invasion, pT = 4) are similar to those without serosa penetration in terms of demographic characteristics. The number of neutrophils (6540 [5290 to 8570], 53 vs. 5360 [4000 to 7340], 99; Mann–Whitney test: 2.69 (0.0073)) and platelets (6540 [5290 to 8570], 53 vs. 5360 [4000 to 7340], 99; Mann–Whitney test: 2.98 (0.0029)) showed statistically significant different values compared to those without a regional stage of disease. Four of the evaluated inflammation ratios demonstrated higher values for patients with serosa penetration than those without (Table 4).

Table 4.
Inflammation ratios by regional stage of disease.
3.4. Relapse, Early Complications and Post-Surgery Intensive Care Admission
Twenty-seven patients (9.6%) in our cohort were with relapse. Patients with relapse were younger (60 [56.5 to 67.5], 27 vs. 69 [60.5 to 75], 255; p-value = 0.0034) and had more hospitalization stays (16 [11.5 to 23], 27 vs. 13 [10 to 17], 255; p-value = 0.0058). The evaluated ratios were similar between those with and without relapse (p-values > 0.15).
Seventeen (6.0%) patients had a fistula and exhibited three times higher value of AISI than those without a fistula (2373 [971 to 3675], 9 vs. 743 [332 to 1344.5], 156; p-value = 0.0489).
Post-surgery, thirty-five patients were admitted to the ICU (12.4%). Patients with post-surgery ICU were older (72 years [64 to 77.5], n = 35 vs. 67 years [59 to 74], n = 247; p-value = 0.0247), have statistically significant NLR (6.3 [3.4 to 8.3], n = 13 vs. 3.5 [2.3 to 5.2], n = 152; p-value = 0.0103), PLR (250 [174.5 to 330.5], n = 35 vs. 177 [134 to 261.5], n = 247; p-value = 0.0041), AISI (2817 [1169 to 4575], n = 13 vs. 646.5 [323.5 to 1313.3], n = 152; p-value = 0.0001), and SII (2207 [1065 to 3846], n = 13 vs. 1061.5 [611.5 to 1711.3], n = 152; p-value = 0.0045).
4. Discussion
The evaluated inflammation ratios showed different patterns on specific outcomes. Specifically, AISI and SII showed higher values in deceased patients, NLR, PLR, AISI, and SII exhibited elevated values, and MLR and SIRI showed lower values in patients who underwent emergency surgery. Furthermore, AISI showed elevated values in patients with a higher stage of disease, and NLR, PLR, AISI, and SII proved statistically significantly higher in patients with a regional stage compared to those without a regional stage.
The timeframe of our study includes the COVID-19 pandemic, during which surgical interventions decreased by over 50% in the first year compared to the pre-pandemic period, in line with global trends [13,14,15]. To mitigate potential interference with results due to the impact of SARS-CoV-2 infection on the inflammatory system [16,17,18], patients with a history of SARS-CoV-2 infection or those who acquired the infection during hospitalization were excluded from the study.
Our cohort had an average age of 68 years, predominantly male, consistent with the literature findings on colorectal cancer incidence. Siegel et al. reported an incidence of 40.7 per 100,000 individuals for colorectal cancer, with a higher frequency in males [19]. The decline in colorectal cancer mortality since 1980 can be attributed to improved screening methods, such as endoscopic detection of colonic polyps and minimally invasive surgical interventions [20]. Incidence peaks in the fourth to sixth decades of life, with age-specific rates increasing with each subsequent decade [21]. In our study, deceased patients were statistically significantly older, underwent emergency surgeries at a higher proportion, and had a higher CHARLSON index over 3 (Table 1). Associated pathologies, acid-base, electrolyte imbalances, and complications requiring emergency surgical intervention contributed to a poorer prognosis [22]. Deceased patients also required intensive care monitoring at a significantly higher proportion (p < 0.0001) and experienced an intestinal fistula as a postoperative complication (p < 0.0001, Table 1). The incidence of stage IV disease was higher in deceased individuals compared to survivors (25.7% vs. 13.4%, Table 1). Our findings align with the scientific literature emphasizing the impact of negative prognostic factors for colorectal cancer patients undergoing surgical intervention [23,24]. The inflammatory status has been long recognized as a pivotal factor in the progression of cancer in patients [25]. The emergence of cancer is often linked with persistent inflammatory conditions, with infections contributing to over 15% of malignancies [26]. The findings of our study reveal substantial disparities among patients with specific outcomes. Notably, the count of lymphocytes exhibits a significant contrast, showing a decline in non-survivors compared to survivors (Table 2). Hence, the hematologic equilibrium and response of patients exert a significant influence on postoperative outcomes and overall prognosis [27]. Similarly, the count of neutrophils displays a marked elevation among deceased individuals relative to survivors (Table 2), while the numerical increase in NLR among deceased patients compared to survivors showed only a tendency to statistical significance (0.01 < p-value < 0.10).
Several studies have underscored the utility of NLR as a prognostic indicator in CRC patients prior to surgery [28,29], although a universally accepted threshold remains undetermined. Despite the fact that MLR has been associated with less favorable prognoses [30,31], our study shows similar values in patients who died than in those who survived (Table 2). The mean NLR value for deceased patients stood at 4.47 in our study, a result in line with scientific data with a threshold of 3.3 [32]. Additionally, Ding et al. showed that an elevated preoperative NLR (>4) emerges as an independent predictor of poorer survival in CRC patients [33]. Other studies have yielded similar outcomes, emphasizing the significance of this metric. For instance, Shibutani et al. demonstrated that a preoperative NLR > 2.5 significantly forecasts poorer cancer-specific survival in CRC patients [34]. Within this study, a notable increase in AISI is also evident among patients with a fistula, underscoring once again the link between imbalances in the inflammatory response and adverse prognostic factors [23,24].
As expected, the examination of the relationship between biomarkers and the regional stage of colorectal cancer unveils notable disparities. NLR demonstrates a significant surge in patients with regional stages compared to those with non-regional stages (Table 4). Similarly, PLR shows a substantial increase in patients with regional stages, and AISI and SII exhibit analogous trends, with significant elevations in patients with regional stages (Table 4). AISI showed a high variability (from 62 to 23,044), with marked distinctions between patients with varying disease stages (Kruskal–Wallis test; p-value = 0.0313). The lowest values were observed in patients with stage I, while the highest values were recorded in patients with stage IV (p-value = 0.0328), the reaction of the organism increasing with the stage of the disease.
Patients with the regional stage of the disease (lymphovascular invasion, pT = 4) are similar to those without serosa penetration in terms of demographic characteristics but showed significantly higher numbers of neutrophils (p = 0.0073) and platelets (p = 0.0029). Four of the evaluated inflammation markers showed higher values in patients with serosa penetration. Similarly, an extensive study highlighting significant associations between NLR, derived neutrophil-to-lymphocyte ratio (dNLR), and T stage in colorectal cancer has been reported in the scientific literature, suggesting their potential clinical relevance [22]. Additionally, MLR shows an inverse relationship with the T stage. Hu et al. [35] reported that SII is also associated with poor histological differentiation, larger tumor sizes, and advanced T, N, and M stages, validating the hypothesis that increased inflammatory response could promote tumor proliferation, progression, and metastasis [36]. The combined evaluation of NLR and SII reflects the clinical utility of these individual ratios [37]. While NLR proves valuable in identifying patients with positive lymph nodes [38], MLR does not reach the reliability demonstrated by NLR or dNLR [39]. Including PLR in TNM staging could enhance prognostic capacity [40].
It is pertinent to highlight that a derived neutrophil-to-lymphocyte ratio (dNLR) equal to or greater than 3.125 effectively doubles the risk of mortality, while each year of advancing age escalates the risk by 4%. Notably, both neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) maintain significant associations with the localized stage of the tumor, irrespective of gender and age. An initial NLR exceeding 3.105 prior to treatment amplifies the likelihood of an advanced T stage, whereas each increment in the MLR ratio diminishes this probability. Moreover, an NLR surpassing 4.255 significantly triples the likelihood of metastasis [22]. Several studies corroborate the potential of inflammatory markers in prognostic prediction and risk assessment in colorectal cancer [41,42,43,44,45]. Results regarding the type of surgical intervention—elective or emergency—reveal significant insights. The number of lymphocytes, although not statistically significant (Table 3), shows a marginal decrease in patients with emergency interventions compared to those with scheduled interventions. On the other hand, the number of monocytes is significantly lower in patients with emergency interventions compared to those with elective interventions (Table 3), showing the different baseline biomarkers between these two groups. This study has highlighted significant differences in inflammatory markers between patients undergoing emergency and elective surgeries for colorectal cancer. NLR shows a significant increase in emergency surgeries, while the monocyte-to-lymphocyte ratio indicates a substantial rise in emergency surgery patients (Table 3). Patients undergoing emergency surgeries exhibit a significantly increased PLR, AISI, and SII (Table 3). These findings highlight the baseline response of the body to the presence of CRC that could explain the burn and complexity of the disease at presentation. Additionally, since patients admitted to postoperative intensive care had a significantly higher mortality rate, it is noteworthy that they presented significantly higher values for NLR (p = 0.0103), PLR (p = 0.0041), AISI (p = 0.0001), and SII (p = 0.0045).
Thus, following the presentation of our study results and their link with the relevant literature, it can be concluded that a significant variation in inflammatory status parameters can be employed in the prognostic analysis of these patients. Stotz et al. [45] underscore the preoperative predictive value of MLR and its correlation with shortened long-term survival. Huang et al. [46] present, in a meta-analysis, the predictive superiority of PLR, demonstrating its association with reduced survival and increased recurrence. However, SII takes center stage as the most effective predictor of long-term survival outcomes, surpassing NLR and PLR. Its comprehensive reflection of inflammatory and immune responses positions SII in a key role in prognostic assessments for colorectal cancer, offering valuable perspectives on potential implications [7,8,47,48]. These findings collectively emphasize the intricate connection between inflammatory markers and outcomes in the context of colorectal cancer patients, providing crucial insights into prognostic implications. Furthermore, the correlation between age, postoperative intensive care unit admission, and elevated levels of NLR, PLR, AISI, and SII underscores the complex impact of these markers, adding depth to the understanding of their clinical relevance in the postoperative setting [8,22,23,24,48,49].
Our findings highlight significant patterns in inflammatory markers and the regional stage of colorectal cancer, providing a more detailed understanding of potential clinical implications. Through a careful analysis of the data obtained in this study, promising perspectives for clinical management and prognosis of patients with colorectal cancer are outlined.
Study Limitations
The conclusions drawn from our study demand meticulous consideration, as they are accompanied by several limitations that require careful acknowledgment. First, the retrospective nature of our data collection precluded our ability to adequately control for potential confounding variables. This limitation may have resulted in the exaggeration or underestimation of the associations identified, particularly considering the omission of influential factors such as smoking, inflammatory conditions, medication usage, genetic predispositions, diabetes, and obesity. Second, it is essential to acknowledge the potential for misclassification of outcomes, notably the staging of tumors (T stage and M stage). Variations in imaging techniques and interpretations by different healthcare professionals may have contributed to a misclassification, potentially impacting the perceived clinical utility of the investigated ratios. To address these limitations and enhance the robustness of future research, a prospective approach with standardized protocols for outcome assessment is warranted. Furthermore, comprehensive consideration of potential confounding variables, including those omitted in our study, is imperative. Additionally, investigating the dynamic changes in ratios before and after interventions could yield valuable insights into their clinical relevance. However, the significant contributions of this study to understanding the role of inflammation in colorectal cancer underscore the importance of continued research to identify and validate relevant inflammatory markers with both prognostic and therapeutic potential.
5. Conclusions
Our results revealed distinct trends of evaluated inflammation ratios linked to various outcomes. Notably, AISI and SII demonstrated elevated levels among deceased patients, while NLR, PLR, AISI, and SII exhibited heightened values among patients undergoing emergency surgery. Moreover, AISI levels were found to be elevated in patients with advanced disease stages, and statistically significant increases in NLR, PLR, AISI, and SII were observed in patients with regional disease stages compared to those without.
Moving forward, adopting a prospective approach with standardized protocols and accounting for comprehensive confounding variables are imperative to strengthen future research endeavors. Despite the limitations, our study underscores the importance of further exploration to validate inflammatory markers’ prognostic and therapeutic potential in colorectal cancer management.
Author Contributions
Conceptualization, C.V.I.F. and C.M.; methodology, C.V.I.F. and S.D.B.; software, C.V.I.F.; validation, C.M., S.D.B. and C.V.I.F.; formal analysis, S.D.B. and C.V.I.F.; investigation, S.D.B. and C.V.I.F.; resources, C.V.I.F. and S.O.; data curation, C.M. and S.D.B.; writing—original draft preparation, C.V.I.F. and S.D.B.; writing—review and editing, S.O. and C.M.; visualization C.M. and S.O.; supervision, S.O.; project administration, C.V.I.F. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the “Victor Babeș” University of Medicine and Pharmacy, Timisoara, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of PIUS BRINZEU” EMERGENCY COUNTY CLINICAL HOSPITAL IN TIMISOARA, ROMANIA (434/29 January 2024).
Informed Consent Statement
As this study was conducted retrospectively and did not involve any additional interventions or the collection of private information, patient consent was waived.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Rapacchietta, L. Differences in colorectal cancer surveillance epidemiology and screening in the WHO European Region. Oncol. Lett. 2019, 17, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.G.; Wallace, R.; McKee, R.F.; Anderson, J.H.; Going, J.J.; Edwards, J.; Horgan, P.G. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2012, 14, 1493–1499. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Xia, L.J.; Li, W.; Zhai, J.C.; Yan, C.W.; Chen, J.B.; Yang, H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Ohtani, Y.; Sakamoto, I.; Hosoda, A.; Ihara, A.; Naitoh, T. Systemic Immune-Inflammation Index Predicts Tumor Recurrence after Radical Resection for Colorectal Cancer. Tohoku J. Exp. Med. 2023, 261, 229–238. [Google Scholar] [CrossRef]
- Dong, M.; Shi, Y.; Yang, J.; Zhou, Q.; Lian, Y.; Wang, D.; Ma, T.; Zhang, Y.; Mi, Y.; Gu, X.; et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: A meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937425. [Google Scholar] [CrossRef]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Liu, H.L.; Ning, N.; Li, S.Y.; Du, X.H.; Li, R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol. Lett. 2016, 11, 2241–2248. [Google Scholar] [CrossRef]
- Salazar-Onfray, F.; López, M.N.; Mendoza-Naranjo, A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007, 18, 171–182. [Google Scholar] [CrossRef]
- Porosnicu, T.M.; Sirbu, I.O.; Oancea, C.; Sandesc, D.; Bratosin, F.; Rosca, O.; Jipa, D.; Boeriu, E.; Bandi, S.S.S.; Pricop, M. The Impact of Therapeutic Plasma Exchange on Inflammatory Markers and Acute Phase Reactants in Patients with Severe SARS-CoV-2 Infection. Medicina 2023, 59, 867. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Pi, F.; Tao, J.; Wei, Z. Impact of the COVID-19 pandemic on surgical outcomes in patients undergoing colorectal cancer surgery: A retrospective study and meta-analysis of data from 11,082 participants. Front. Public Health 2022, 10, 907571. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, G.; Guerra, F.; Messinese, S.; Santelli, F.; Salvischiani, L.; Esposito, S.; Ferraro, L.; Esposito, A.; De Pastena, M.; Rega, D.; et al. The COVID-AGICT Collaborative group. The COVID-AGICT study: COVID-19 and advanced gastro-intestinal cancer surgical treatment. A multicentric Italian study on the SARS-CoV-2 pandemic impact on gastro-intestinal cancers surgical treatment during the 2020. Analysis of perioperative and short-term oncological outcomes. Surg. Oncol. 2023, 47, 101907. [Google Scholar] [CrossRef] [PubMed]
- Uyan, M.; Özdemir, A.; Kalcan, S.; Tomas, K.; Demiral, G.; Pergel, A.; Tarım, İ.A. Effects of COVID-19 pandemic on colorectal cancer surgery. Sao Paulo Med. J. 2022, 140, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Fericean, R.M.; Rosca, O.; Citu, C.; Manolescu, D.; Bloanca, V.; Toma, A.O.; Boeriu, E.; Dumitru, C.; Ravulapalli, M.; Barbos, V.; et al. COVID-19 Clinical Features and Outcomes in Elderly Patients during Six Pandemic Waves. J. Clin. Med. 2022, 11, 6803. [Google Scholar] [CrossRef]
- Antonucci, F.; Di Carlo, D.; Falcone, M. Evaluation of a potential prognostic parameter for the inflammatory status in COVID-19 patients: The inflammatory protein ratio. Electrophoresis 2022, 43, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, M.L.; Citu, I.M.; Citu, C.; Chiriac, V.D.; Gorun, F.; Levai, M.C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Gurumurthy, S.; et al. Evaluation of FIB-4, NFS, APRI and Liver Function Tests as Predictors for SARS-CoV-2 Infection in the Elderly Population: A Matched Case-Control Analysis. J. Clin. Med. 2022, 11, 5149. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970-2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide variations in colorectal cancer. CA A Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National Trends in Colorectal Cancer Incidence among Older and Younger Adults in Canada. JAMA 2019, 2, e198090. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, A.; Ciocan, R.A.; Al Hajjar, N.; Gherman, C.D.; Bolboacă, S.D. Abilities of Pre-Treatment Inflammation Ratios as Classification or Prediction Models for Patients with Colorectal Cancer. Diagnostics 2021, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Tonini, V.; Zanni, M. Impact of anastomotic leakage on long-term prognosis after colorectal cancer surgery. World J. Gastrointest. Surg. 2023, 15, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Feng, B.; Lu, A.G.; Wang, M.L.; Hu, W.G.; Li, J.W.; Mao, Z.H.; Zheng, M.H. Laparoscopic low anterior resection for rectal carcinoma: Complications and management in 132 consecutive patients. World J. Gastroenterol. 2010, 16, 4605–4610. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; Salmond, J.M.; Horgan, P.G.; Oien, K.A.; McMillan, D.C. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann. Surg. 2009, 249, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000, 248, 171–183. [Google Scholar] [CrossRef]
- Leitch, E.F.; Chakrabarti, M.; Crozier, J.E.; McKee, R.F.; Anderson, J.H.; Horgan, P.G.; McMillan, D.C. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br. J. Cancer 2007, 97, 1266–1270. [Google Scholar] [CrossRef]
- Kishi, Y.; Kopetz, S.; Chun, Y.S.; Palavecino, M.; Abdalla, E.K.; Vauthey, J.N. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann. Surg. Oncol. 2009, 16, 614–622. [Google Scholar] [CrossRef]
- Halazun, K.J.; Aldoori, A.; Malik, H.Z.; Al-Mukhtar, A.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2008, 34, 55–60. [Google Scholar] [CrossRef]
- Absenger, G.; Szkandera, J.; Stotz, M.; Postlmayr, U.; Pichler, M.; Ress, A.L.; Schaberl-Moser, R.; Loibner, H.; Samonigg, H.; Gerger, A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013, 33, 4591–4594. [Google Scholar]
- Absenger, G.; Szkandera, J.; Pichler, M.; Stotz, M.; Arminger, F.; Weissmueller, M.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br. J. Cancer 2013, 109, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.M.; von Eyben, R.; Pai, J.S.; Anderson, E.M.; Welton, M.L.; Shelton, A.A.; Kin, C.; Koong, A.C.; Chang, D.T. The Prognostic Significance of Pretreatment Hematologic Parameters in Patients Undergoing Resection for Colorectal Cancer. Am. J. Clin. Oncol. 2017, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.R.; An, X.; Zhang, R.X.; Fang, Y.J.; Li, L.R.; Chen, G.; Wu, X.J.; Lu, Z.H.; Lin, J.Z.; Kong, L.H.; et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int. J. Color. Dis. 2010, 25, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Noda, E.; Ohtani, H.; Nishiguchi, Y.; Hirakawa, K. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013, 33, 3291–3294. [Google Scholar]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nature reviews. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Yu, W.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int. J. Cancer 2016, 139, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Turri, G.; Barresi, V.; Valdegamberi, A.; Gecchele, G.; Conti, C.; Ammendola, S.; Guglielmi, A.; Scarpa, A.; Pedrazzani, C. Clinical Significance of Preoperative Inflammatory Markers in Prediction of Prognosis in Node-Negative Colon Cancer: Correlation between Neutrophil-to-Lymphocyte Ratio and Poorly Differentiated Clusters. Biomedicines 2021, 9, 94. [Google Scholar] [CrossRef]
- Özgehan, G.; Kahramanca, Ş.; Kaya, İ.O.; Bilgen, K.; Bostanci, H.; Güzel, H.; Küçükpinar, T.; Kargici, H. Neutrophil-lymphocyte ratio as a predictive factor for tumor staging in colorectal cancer. Turk. J. Med. Sci. 2014, 44, 365–368. [Google Scholar] [CrossRef]
- Basile, D.; Garattini, S.K.; Corvaja, C.; Montico, M.; Cortiula, F.; Pelizzari, G.; Gerratana, L.; Audisio, M.; Lisanti, C.; Fanotto, V.; et al. The MIMIC Study: Prognostic Role and Cutoff Definition of Monocyte-to-Lymphocyte Ratio and Lactate Dehydrogenase Levels in Metastatic Colorectal Cancer. Oncologist 2020, 25, 661–668. [Google Scholar] [CrossRef]
- Josse, J.M.; Cleghorn, M.C.; Ramji, K.M.; Jiang, H.; Elnahas, A.; Jackson, T.D.; Okrainec, A.; Quereshy, F.A. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2016, 18, O236–O242. [Google Scholar] [CrossRef]
- Chen, X.Q.; Xue, C.R.; Hou, P.; Lin, B.Q.; Zhang, J.R. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J. Gastroenterol. 2019, 25, 4970–4984. [Google Scholar] [CrossRef]
- Palin, R.P.; Devine, A.T.; Hicks, G.; Burke, D. Association of pretreatment neutrophil-lymphocyte ratio and outcome in emergency colorectal cancer care. Ann. R. Coll. Surg. Engl. 2018, 100, 308–315. [Google Scholar] [CrossRef]
- Farolfi, A.; Petrone, M.; Scarpi, E.; Gallà, V.; Greco, F.; Casanova, C.; Longo, L.; Cormio, G.; Orditura, M.; Bologna, A.; et al. Inflammatory Indexes as Prognostic and Predictive Factors in Ovarian Cancer Treated with Chemotherapy Alone or Together with Bevacizumab. A Multicenter, Retrospective Analysis by the MITO Group (MITO 24). Target. Oncol. 2018, 13, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Stotz, M.; Pichler, M.; Absenger, G.; Szkandera, J.; Arminger, F.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 2014, 110, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Chen, W.J.; Zhang, X.; Wu, C.C.; Zhang, C.Y.; Sun, S.S.; Wu, J. An Elevated Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Colorectal Cancer: A Meta-Analysis. Dis. Markers 2017, 2017, 1053125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.M.; Xu, J.B.; Peng, J.J.; Chen, C.Q.; He, Y.L.; Cai, S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.K.; Chen, P.; Hu, W.M.; Sun, P.; He, W.Z.; Jiang, C.; Kong, P.F.; Liu, S.S.; Chen, H.T.; Yang, Y.Z.; et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J. Transl. Med. 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.; Dranoff, G. Dual roles for immunity in gastrointestinal cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).