Clinical Research into Central Nervous System Inflammatory Demyelinating Diseases Related to COVID-19 Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report
2.2. Literature Review
2.3. Statistical Analysis
3. Results
3.1. Case Report
3.2. Literature Review
3.3. Protein Sequence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegen, H.; Reindl, M. Recent developments in MOG-IgG associated neurological disorders. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420945135. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Aktas, O.; Ayzenberg, I.; Bellmann-Strobl, J.; Berthele, A.; Giglhuber, K.; Häußler, V.; Havla, J.; Hellwig, K.; Hümmert, M.W.; et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD)—Revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J. Neurol. 2023, 270, 3341–3368. [Google Scholar] [CrossRef]
- Papp, V.; Magyari, M.; Aktas, O.; Berger, T.; Broadley, S.A.; Cabre, P.; Jacob, A.; Kira, J.I.; Leite, M.I.; Marignier, R.; et al. Worldwide Incidence and Prevalence of Neuromyelitis Optica: A Systematic Review. Neurology 2021, 96, 59–77. [Google Scholar] [CrossRef] [PubMed]
- de Mol, C.L.; Wong, Y.; van Pelt, E.D.; Wokke, B.; Siepman, T.; Neuteboom, R.F.; Hamann, D.; Hintzen, R.Q. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult. Scler. 2020, 26, 806–814. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Hamilton-Shield, A.; Woodhall, M.; Messina, S.; Mariano, R.; Waters, P.; Ramdas, S.; Leite, M.I.; Palace, J. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire, UK. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Wynford-Thomas, R.; Jacob, A.; Tomassini, V. Neurological update: MOG antibody disease. J. Neurol. 2019, 266, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Mohammad, S.; Tantsis, E.; Nguyen, T.K.; Merheb, V.; Fung, V.S.C.; White, O.B.; Broadley, S.; Lechner-Scott, J.; Vucic, S.; et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry 2018, 89, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jurynczyk, M.; Messina, S.; Woodhall, M.R.; Raza, N.; Everett, R.; Roca-Fernandez, A.; Tackley, G.; Hamid, S.; Sheard, A.; Reynolds, G.; et al. Clinical presentation and prognosis in MOG-antibody disease: A UK study. Brain 2017, 140, 3128–3138. [Google Scholar] [CrossRef]
- López-Chiriboga, A.S.; Majed, M.; Fryer, J.; Dubey, D.; McKeon, A.; Flanagan, E.P.; Jitprapaikulsan, J.; Kothapalli, N.; Tillema, J.M.; Chen, J.; et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol. 2018, 75, 1355–1363. [Google Scholar] [CrossRef]
- Cobo-Calvo, A.; Ruiz, A.; Maillart, E.; Audoin, B.; Zephir, H.; Bourre, B.; Ciron, J.; Collongues, N.; Brassat, D.; Cotton, F.; et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology 2018, 90, e1858–e1869. [Google Scholar] [CrossRef]
- Pohl, D.; Alper, G.; Van Haren, K.; Kornberg, A.J.; Lucchinetti, C.F.; Tenembaum, S.; Belman, A.L. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology 2016, 87, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Nishiyama, S.; Manzano, G.S.; Lydston, M.; Levy, M. COVID-19 and the risk of CNS demyelinating diseases: A systematic review. Front. Neurol. 2022, 13, 970383. [Google Scholar] [CrossRef] [PubMed]
- Bennetto, L.; Scolding, N. Inflammatory/post-infectious encephalomyelitis. J. Neurol. Neurosurg. Psychiatry 2004, 75 (Suppl. S1), i22–i28. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Lewis, E.; Goddard, K.; Fireman, B.; Bakshi, N.; DeStefano, F.; Gee, J.; Tseng, H.F.; Naleway, A.L.; Klein, N.P. Acute Demyelinating Events Following Vaccines: A Case-Centered Analysis. Clin. Infect. Dis. 2016, 63, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, F.; Xu, Y.; Chu, X.; Zhang, J. Vaccines and the risk of acute disseminated encephalomyelitis. Vaccine 2018, 36, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J.; Mba-Jonas, A.; Dimova, R.B.; Alimchandani, M.; Zinderman, C.E.; Nair, N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February–July 2021. JAMA 2021, 326, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients—United States, April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 651–656. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef]
- Su, S.C.; Lyu, R.K.; Chang, C.W.; Tseng, W.J. The First Guillain-Barr? Syndrome after SARS-CoV-2 Vaccination in Taiwan. Acta Neurol. Taiwan 2022, 31, 46–51. [Google Scholar]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Romano, A.; Bozzao, A.; Salvetti, M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult. Scler. 2021, 28, 13524585211040222. [Google Scholar] [CrossRef] [PubMed]
- Permezel, F.; Borojevic, B.; Lau, S.; de Boer, H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci. Med. Pathol. 2021, 18, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pagenkopf, C.; Südmeyer, M. A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J. Neuroimmunol. 2021, 358, 577606. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.Y.; Yusof Khan, A.H.K.; Mohd Yaakob, M.N.; Abdul Rashid, A.M.; Loh, W.C.; Baharin, J.; Ibrahim, A.; Ismail, M.R.; Inche Mat, L.N.; Wan Sulaiman, W.A.; et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: A case report. BMC Neurol. 2021, 21, 395. [Google Scholar] [CrossRef]
- Notghi, A.A.; Atley, J.; Silva, M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin. Med. 2021, 21, e535–e538. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Tsai, M.J.; Chen, Y.H.; Hsu, C.F. Acute Transverse Myelitis after COVID-19 Vaccination. Medicina 2021, 57, 1010. [Google Scholar] [CrossRef]
- Vegezzi, E.; Ravaglia, S.; Buongarzone, G.; Bini, P.; Diamanti, L.; Gastaldi, M.; Prunetti, P.; Rognone, E.; Marchioni, E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: Casual or causal association? J. Neuroimmunol. 2021, 359, 577686. [Google Scholar] [CrossRef]
- Singh Malhotra, H.; Gupta, P.; Prabhu, V.; Garg, R.K.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. QJM 2021, 114, 591–593. [Google Scholar] [CrossRef]
- Tahir, N.; Koorapati, G.; Prasad, S.; Jeelani, H.M.; Sherchan, R.; Shrestha, J.; Shayuk, M. SARS-CoV-2 Vaccination-Induced Transverse Myelitis. Cureus 2021, 13, e16624. [Google Scholar] [CrossRef] [PubMed]
- Badrawi, N.; Kumar, N.; Albastaki, U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: Case report & MRI findings. Radiol. Case Rep. 2021, 16, 3864–3867. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Ogaki, K.; Nakamura, R.; Kado, E.; Nakajima, S.; Kurita, N.; Watanabe, M.; Yamashiro, K.; Hattori, N.; Urabe, T. An 88-year-old woman with acute disseminated encephalomyelitis following messenger ribonucleic acid-based COVID-19 vaccination. eNeurologicalSci 2021, 25, 100381. [Google Scholar] [CrossRef]
- Vogrig, A.; Janes, F.; Gigli, G.L.; Curcio, F.; Negro, I.D.; D’Agostini, S.; Fabris, M.; Valente, M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021, 208, 106839. [Google Scholar] [CrossRef]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2021, 269, 1093–1106. [Google Scholar] [CrossRef]
- Alshararni, A. Acute Transverse Myelitis Associated with COVID-19 vaccine: A Case Report. Int. J. Res. Pharm. Sci. 2021, 12, 5. [Google Scholar] [CrossRef]
- McLean, P.; Trefts, L. Transverse myelitis 48 hours after the administration of an mRNA COVID-19 vaccine. Neuroimmunol. Rep. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Kania, K.; Ambrosius, W.; Tokarz Kupczyk, E.; Kozubski, W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann. Clin. Transl. Neurol. 2021, 8, 2000–2003. [Google Scholar] [CrossRef]
- Fujikawa, P.; Shah, F.A.; Braford, M.; Patel, K.; Madey, J. Neuromyelitis Optica in a Healthy Female after Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine. Cureus 2021, 13, e17961. [Google Scholar] [CrossRef]
- Gao, J.J.; Tseng, H.P.; Lin, C.L.; Shiu, J.S.; Lee, M.H.; Liu, C.H. Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines 2021, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Shrestha, A.K.; Colantonio, M.A.; Liberio, R.N.; Sriwastava, S. Acute transverse myelitis following SARS-CoV-2 vaccination: A case report and review of literature. J. Neurol. 2022, 269, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, W.; Nance, C.S. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. SSRN 2021, 7, 3841558. [Google Scholar] [CrossRef]
- Ozgen Kenangil, G.; Ari, B.C.; Guler, C.; Demir, M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol. Belg. 2021, 121, 1089–1091. [Google Scholar] [CrossRef]
- Erdem, N.; Demirci, S.; Özel, T.; Mamadova, K.; Karaali, K.; Çelik, H.T.; Uslu, F.I.; Özkaynak, S.S. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy. Sz. 2021, 74, 273–276. [Google Scholar] [CrossRef]
- Cao, L.; Ren, L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. Acta Neurol. Belg. 2021, 122, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, M.; Yazdi, N.; Rohani, M.; Emamikhah, M. Cervical longitudinally extensive myelitis after vaccination with inactivated virus-based COVID-19 vaccine. Radiol. Case Rep. 2022, 17, 303–305. [Google Scholar] [CrossRef]
- Chen, S.; Fan, X.R.; He, S.; Zhang, J.W.; Li, S.J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. 2021, 42, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, D.G.; Cañete, L.A.Q.; Dos Santos, G.A.C.; de Oliveira, R.V.; Brandão, C.O.; da Cruz, L.C.H., Jr. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: Cause or coincidence? Clin. Imaging 2021, 80, 348–352. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ohyama, A.; Kubota, T.; Ikeda, K.; Kaneko, K.; Takai, Y.; Warita, H.; Takahashi, T.; Misu, T.; Aoki, M. MOG Antibody-Associated Disorders Following SARS-CoV-2 Vaccination: A Case Report and Literature Review. Front. Neurol. 2022, 13, 845755. [Google Scholar] [CrossRef]
- Dams, L.; Kraemer, M.; Becker, J. MOG-antibody-associated longitudinal extensive myelitis after ChAdOx1 nCoV-19 vaccination. Mult. Scler. 2022, 28, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Mumoli, L.; Vescio, V.; Pirritano, D.; Russo, E.; Bosco, D. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol. Sci. 2022, 43, 763–766. [Google Scholar] [CrossRef]
- Sehgal, V.; Bansal, P.; Arora, S.; Kapila, S.; Bedi, G.S. Myelin Oligodendrocyte Glycoprotein Antibody Disease After COVID-19 Vaccination—Causal or Incidental? Cureus 2022, 14, e27024. [Google Scholar] [CrossRef]
- Garg, R.K.; Malhotra, H.S.; Kumar, N.; Pandey, S.; Patil, M.R.; Uniyal, R.; Rizvi, I. Tumefactive Demyelinating Brain Lesion Developing after Administration of Adenovector-Based COVID-19 Vaccine: A Case Report. Neurol. India 2022, 70, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Ballout, A.A.; Babaie, A.; Kolesnik, M.; Li, J.Y.; Hameed, N.; Waldman, G.; Chaudhry, F.; Saba, S.; Harel, A.; Najjar, S. A Single-Health System Case Series of New-Onset CNS Inflammatory Disorders Temporally Associated With mRNA-Based SARS-CoV-2 Vaccines. Front. Neurol. 2022, 13, 796882. [Google Scholar] [CrossRef]
- Raknuzzaman, D.M.; Jannaty, T.; Hossain, D.M.B.; Saha, D.B.; Dey, D.S.K.; Shahidullah, D.M. Post COVID-19 Vaccination Acute Disseminated Encephalomyelitis: A Case Report in Bangladesh. Int. J. Med. Sci. And. Clin. Res. Stud. 2021, 1, 31–36. [Google Scholar]
- Nagaratnam, S.A.; Ferdi, A.C.; Leaney, J.; Lee, R.L.K.; Hwang, Y.T.; Heard, R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, F.; Iranpour, P.; Haseli, S.; Poursadeghfard, M.; Yarmahmoodi, F. Acute disseminated encephalomyelitis (ADEM) after SARS- CoV-2 vaccination: A case report. Radiol. Case Rep. 2022, 17, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Ohara, Y.; Ouchi, T.; Sasaki, R.; Maki, F.; Mizuno, J. Cervical Transverse Myelitis Following COVID-19 Vaccination. NMC Case Rep. J. 2022, 9, 145–149. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Timmermans, V.M.; Dakakni, T. Acute Disseminated Encephalomyelitis After SARS-CoV-2 Vaccination. Am. J. Case Rep. 2022, 23, e936574. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Lotlikar, R.S.; Sukumaran, S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol. Sci. 2022, 43, 3503–3507. [Google Scholar] [CrossRef]
- Al-Quliti, K.; Qureshi, A.; Quadri, M.; Abdulhameed, B.; Alanazi, A.; Alhujeily, R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases 2022, 10, 13. [Google Scholar] [CrossRef]
- Mousa, H.; Patel, T.H.; Meadows, I.; Ozdemir, B. Acute Disseminated Encephalomyelitis (ADEM) After Consecutive Exposures to Mycoplasma and COVID Vaccine: A Case Report. Cureus 2022, 14, e26258. [Google Scholar] [CrossRef]
- Motahharynia, A.; Naghavi, S.; Shaygannejad, V.; Adibi, I. Fulminant neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccination: A need for reconsideration? Mult. Scler. Relat. Disord. 2022, 66, 104035. [Google Scholar] [CrossRef] [PubMed]
- Anamnart, C.; Tisavipat, N.; Owattanapanich, W.; Apiwattanakul, M.; Savangned, P.; Prayoonwiwat, N.; Siritho, S.; Rattanathamsakul, N.; Jitprapaikulsan, J. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review. Mult. Scler. Relat. Disord. 2022, 58, 103414. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Saab, G.; Schneider, R. Antibody-Positive Neuromyelitis Optica Spectrum Disorder After Second COVID-19 Vaccination: A Case Report. SN Compr. Clin. Med. 2022, 4, 130. [Google Scholar] [CrossRef]
- Caliskan, I.; Bulus, E.; Afsar, N.; Altintas, A. A Case With New-Onset Neuromyelitis Optica Spectrum Disorder Following COVID-19 mRNA BNT162b2 Vaccination. Neurologist 2022, 27, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Netravathi, M.; Dhamija, K.; Gupta, M.; Tamborska, A.; Nalini, A.; Holla, V.V.; Nitish, L.K.; Menon, D.; Pal, P.K.; Seena, V.; et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult. Scler. Relat. Disord. 2022, 60, 103739. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Interim Recommendations for Use of the ChAdOx1-S [Recombinant] Vaccine against COVID-19 (AstraZeneca COVID-19 Vaccine AZD1222 Vaxzevria™, SII COVISHIELD™): Interim Guidance, First Issued 10 February 2021, Updated 21 April 2021, Updated 30 July 2021, Latest Update 15 March 2022; World Health Organization: Geneva, Switzerland, 2022.
- Marignier, R.; Hacohen, Y.; Cobo-Calvo, A.; Pröbstel, A.K.; Aktas, O.; Alexopoulos, H.; Amato, M.P.; Asgari, N.; Banwell, B.; Bennett, J.; et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021, 20, 762–772. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef]
- Lotan, I.; Romanow, G.; Levy, M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult. Scler. Relat. Disord. 2021, 55, 103189. [Google Scholar] [CrossRef]
- Taiwan Food and Drug Administration. The Report of Adverse Effects after COVID-19 Vaccines in Taiwan. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f637814781667990040&type=2&cid=39989 (accessed on 10 May 2023).
- Huang, C.T.; Hsu, S.Y.; Wang, C.H.; Tseng, W.J.; Yang, C.Y.; Ng, C.J.; Warkentin, T.E.; Cheng, M.H. Double high-dose immunoglobulin for ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia. Thromb. Res. 2021, 206, 14–17. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Smith, H.; Ma, Y.; Baum, H.; Mieli-Vergani, G.; Vergani, D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin. Dev. Immunol. 2005, 12, 217–224. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.S.; Gomes-Neto, F.; Aguiar-Oliveira, M.L.; Burlandy, F.M.; Tavares, F.N.; da Silva, E.E.; Sousa, I.P., Jr. Analysis of Coxsackievirus B5 Infections in the Central Nervous System in Brazil: Insights into Molecular Epidemiology and Genetic Diversity. Viruses 2022, 14, 899. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Wingerchuk, D.M.; Vukusic, S.; Linbo, L.; Pittock, S.J.; Lucchinetti, C.F.; Lennon, V.A. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann. Neurol. 2006, 59, 566–569. [Google Scholar] [CrossRef] [PubMed]
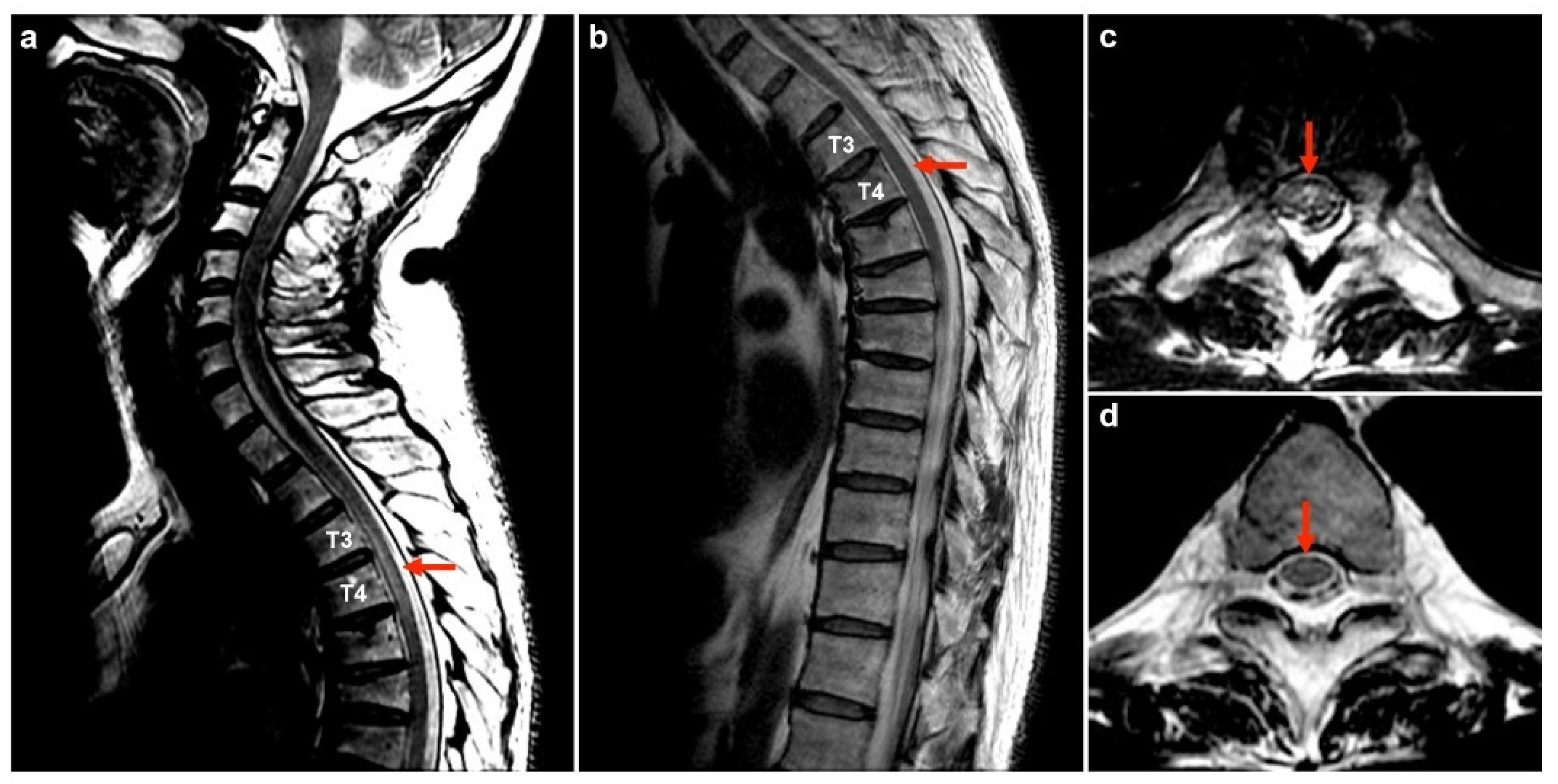
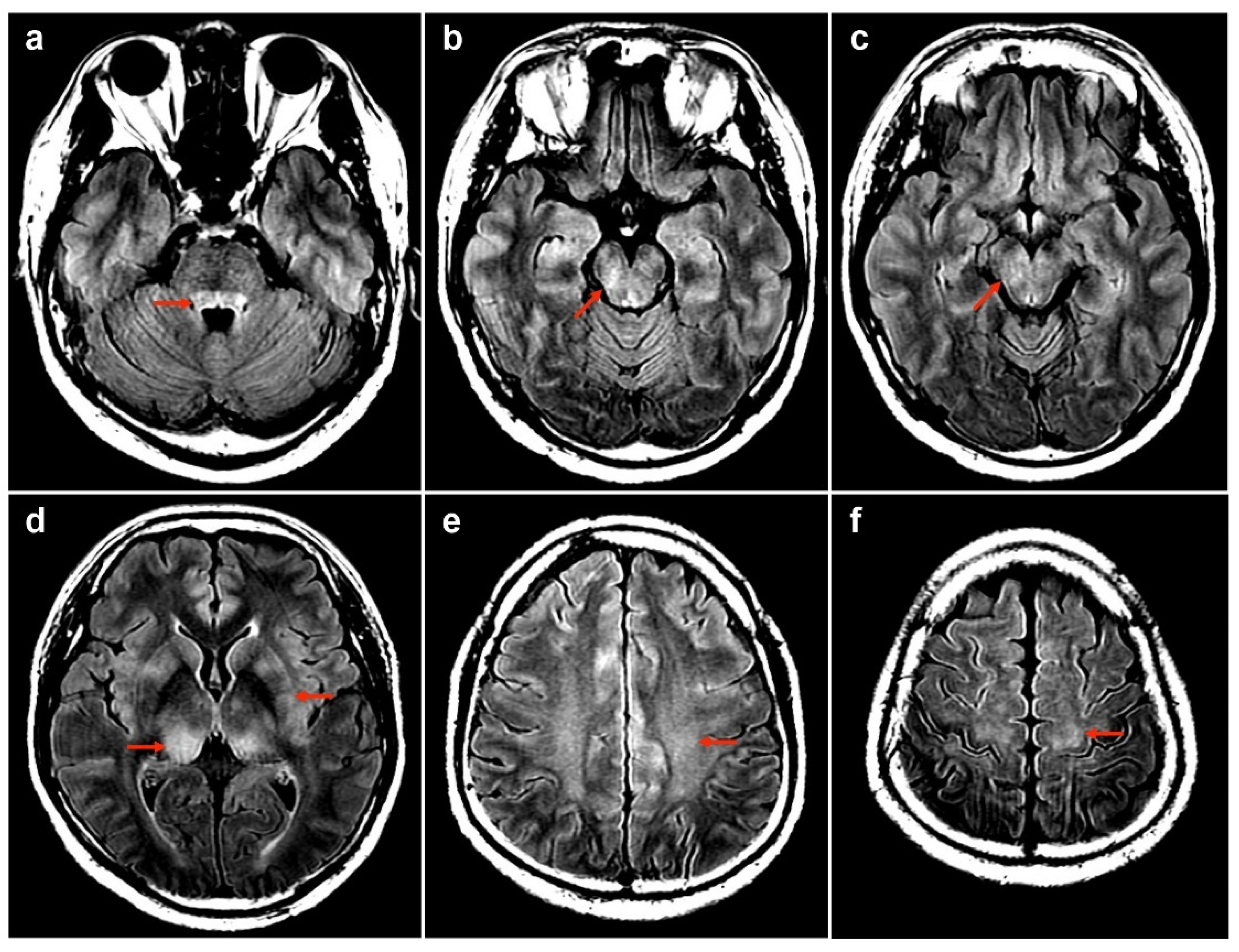
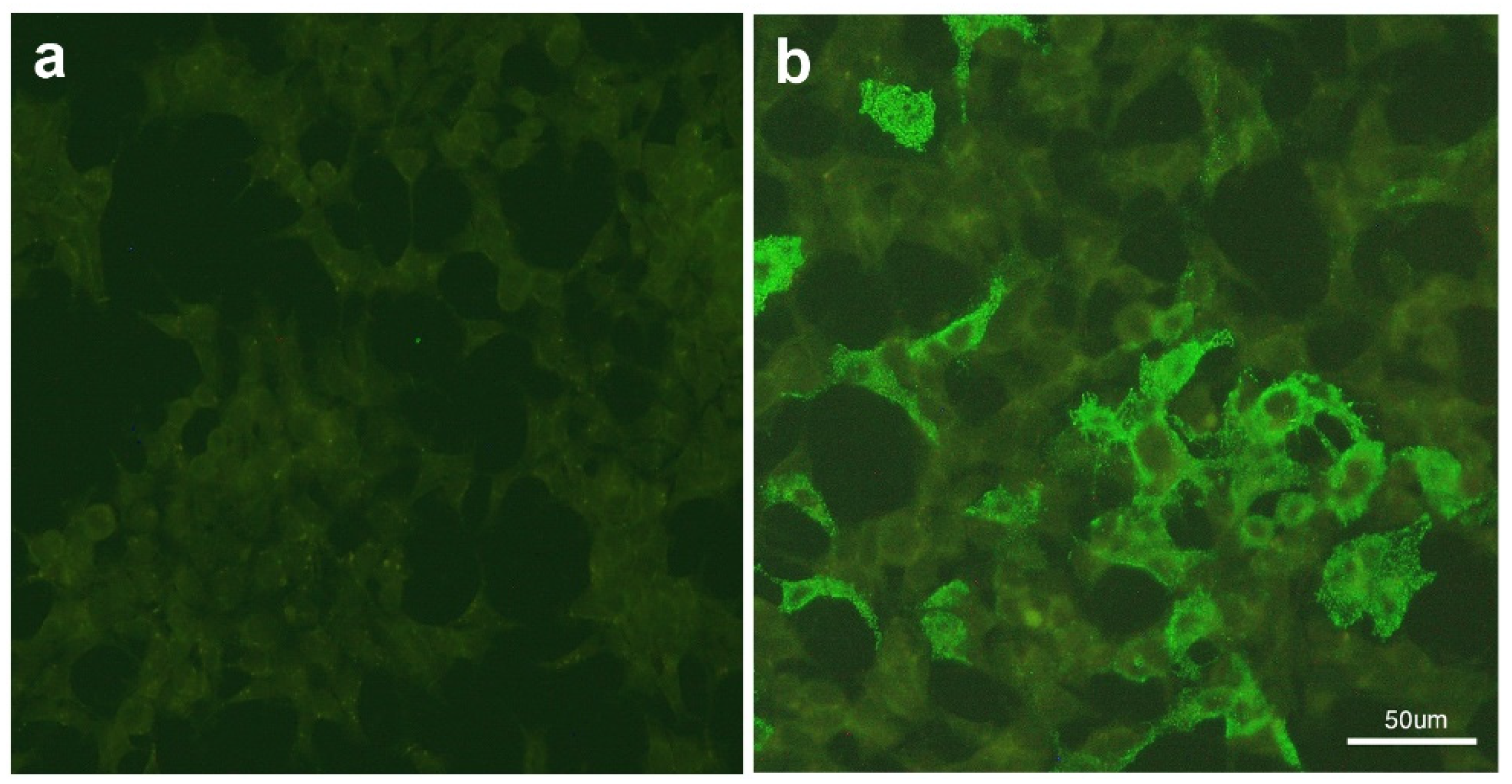
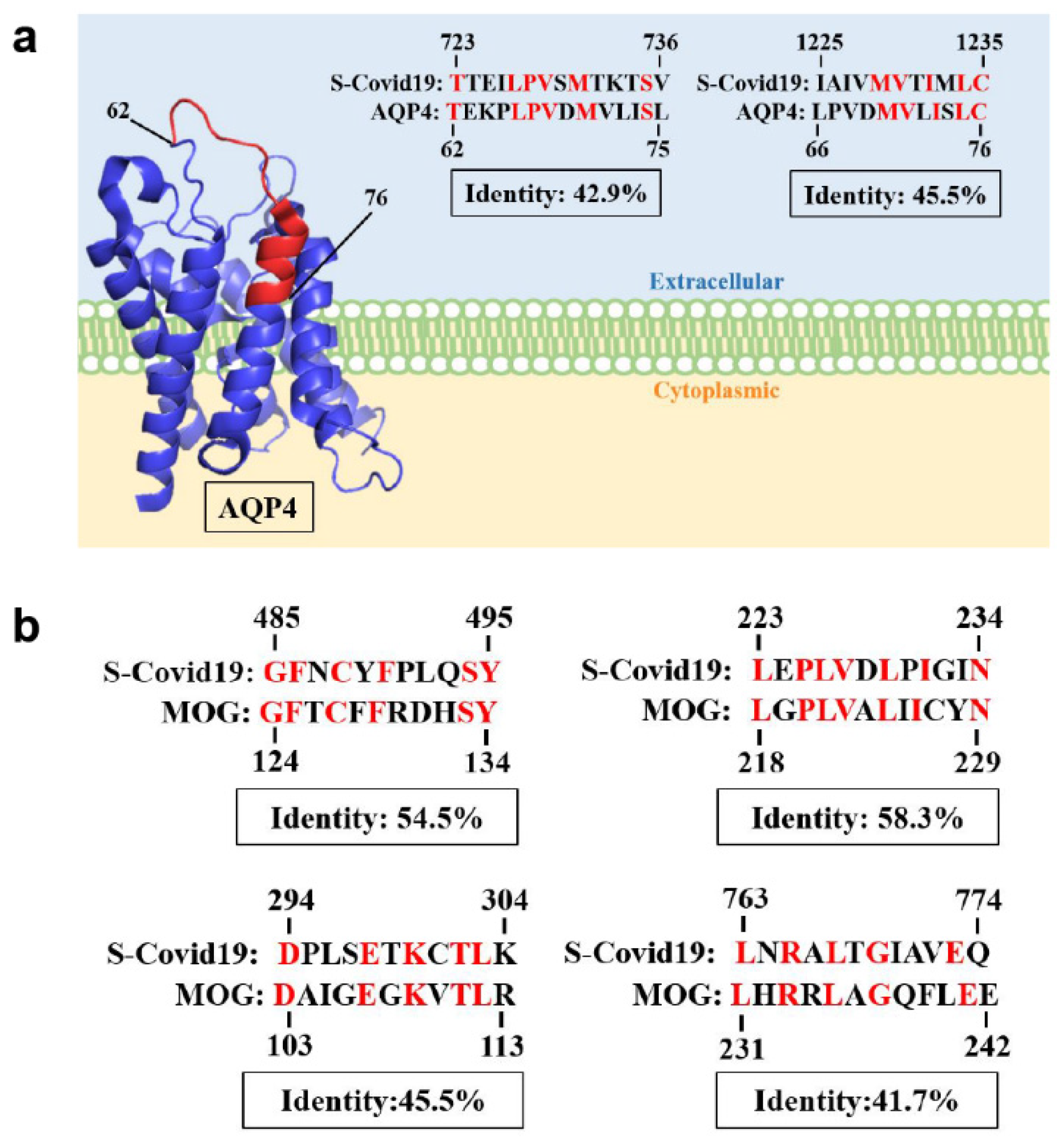
| No | Age/Sex/PH | Vaccine/ Interval (D) | Dx | Serum | CSF | MRI | Tx | Outcome | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQP4 | MOG | WBC | Lym (%) | Neut (%) | TP | Glu Ratio | OCB | Brain | Spine (Myelitis) | |||||||
| 1 | 50M/chronic HBV | 1st AZ a/13 | MOGAD | − | + | 175 | 99 | 0 | 78.1 | 0.47 | NA | Bil thalami, pu, subcortical WM, brainstem | T3–T4 | PT | R | Index case |
| 2 | 45M | 1st AZ/12 | ADEM + SSM | − | − | 44 | P | NA | N | NA | + | Pons, R MCP, R thalamus; C+ | Cervical, thoracic, conus medullaris; C+ | PT | R | [21] |
| 3 | 63M/DM, HL, IHD, Af | 1st AZ/12 | ADEM | − | − | 2 | NA | NA | 69 | N | + | Bil WM, bil CC, L thalamus, IC, L midbrain, lower pons, R MCP | N | PT + PP | E(D20) | [22] |
| 4 | 45M/atopic dermatitis | 1st AZ/8 | LETM | − | − | 481 | NA | 67 | 140 | 0.43 | − | N | C3–T2 | PT | I | [23] |
| 5 | 25F | 1st AZ/12 | LETM | − | − | NA | NA | NA | 54.6 | 0.55 | − | N | T3–T5, T7–T8, T11–L1; C+ in T7–T8 | PT | I | [24] |
| 6 | 58M/DM, pulmonary sarcoidosis | 1st AZ/7 | LETM | − | − | 11 | 100 | 0 | 162 | 0.54 | + | NA | C1–T10; C+ in T3–T4, T9–T10 | PT + PE | I | [25] |
| 7 | 41M/DM | 1st AZ/14 | LETM | − | NA | 11 | 100 | 0 | 44.3 | NA | NA | N | T1–T6; C+ | PT | R | [26] |
| 8 | 44F | 1st AZ/4 | SSM | − | − | ↑ | P | NA | 76.7 | NA | − | N | T7–T8, T10–T11; C+ in T7–T8 | PT | R | [27] |
| 9 | 36M | 1st AZ/8 | SSM | − | − | NA | NA | NA | 54 | NA | NA | N | C6–C7; C+ | PT | R | [28] |
| 10 | 44F | 1st Janssen b/10 | LETM | − | NA | 227 | 96 | 0 | 43 | N | − | N | C2 to upper thoracic | PT + PE | R | [29] |
| 11 | 34M | 2nd Sputnik V c/21 | NMOSD | + | − | ↑ | P | NA | ↑ | NA | − | 3rd, 4th PV, thalamus, CC, optic chiasma | N | PP | I | [30] |
| 12 | 88F/DM, AD | 2nd Pfizer d/29 | ADEM | NA | NA | NA | NA | NA | NA | NA | − | Bil MCPs | NA | PT | R | [31] |
| 13 | 56F/Post-infectious rhombencephalitis | 1st Pfizer/14 | ADEM-like i | − | − | N | NA | NA | N | N | − | L cerebellar peduncle and L centrum semiovale | NA | Oral steroid | R | [32] |
| 14 | 64M | 1st Pfizer/18 | NMOSD | + | NA | N | NA | NA | N | N | − | CC, L frontal and parietal WM; C− | Cervical to the conus; C+ | PT + PE + RTX | I | [33] |
| 15 | 38M | 1st Pfizer/2 | SSM | NA | NA | NA | NA | NA | 62.1 | N | NA | NA | T11–T12; C+ | NA | NA | [34] |
| 16 | 69F/cervical ca, HL, hypothyroidism | 1st Pfizer/2 | LETM | − | − | N | NA | NA | N | N | + | N | C3–C4 to T2–T3 | PT | I | [35] |
| 17 | 19F/atopic dermatitis, depression | 1st Moderna e/14 | ADEM + LETM | − | − | 294 | 91 | 1 | 64.8 | NA | − | Bil hemispheres, pons, medulla, cerebellum; C+ | Medulla to T11; C+ | PT + PE | R | [36] |
| 18 | 46F/vit B12 deficiency | 1st Moderna/2 | NMOSD | − | NA | NA | NA | NA | NA | NA | NA | N | C6–T2 | PT | R | [37] |
| 19 | 76F/HTN, vit B12 deficiency | 1st Moderna/2 | LETM | − | NA | 15 | NA | 73 | 57.2 | NA | − | N | C2–C5; C+ in C3 | PT | I | [38] |
| 20 | 67F/CAD, CKD, neuropathy | 1st Moderna/1 | LETM | − | − | 2 | NA | NA | 56 | 0.61 | + | Nonspecific WM | C1–C3; C+ | PT + PP | I | [39] |
| 21 | 63M | 2nd Moderna/1 | SSM | − | − | 3 | NA | NA | 37 | N | NA | Nonspecific bil corona radiata | Conus medullaris; C+ | IVIG + PT | I | [40] |
| 22 | 46F/Hashimoto’s thyroiditis | 2nd Sinovac f/30 | ADEM-like | − | − | 0 | 0 | 0 | 45 | NA | − | L thalamus, bil corona radiata, L diencephalon, R parietal cortex | NA | PT | R | [41] |
| 23 | 78F/DM, HTN, breast ca | 2nd Sinovac/21 | LETM | − | − | 2 | NA | NA | 56 | 0.69 | − | N | C1–T3 | PT | I | [42] |
| 24 | 24F | 1st Sinopharm g/14 | ADEM | − | − | 51 | NA | NA | NA | NA | − | Bil temporal | NA | IVIG | I | [43] |
| 25 | 71M/DM, HTN, IHD | 1st Sinopharm/5 | LETM | − | − | 0 | 0 | 0 | N | N | − | N | Cervico–medullary junction to C3 | PT | I | [44] |
| 26 | 50F | 1st inactivated/3 | NMOSD | + | − | 31 | NA | NA | N | N | − | Area postrema, bil hypothalamus | N | PT | I | [45] |
| 27 | 65M | 1st AZ/8 | LETM | − | − | N | NA | NA | 70 | NA | − | NA | C4–C6 | PT | R | [46] |
| 28 | 68F/HTN, pancreatic ca | 2nd Moderna/14 | MOGAD | − | + | 0 | 0 | 0 | 32 | NA | + | R lateral pons, trigeminal nerve, MCP | NA | PT | I | [47] |
| 29 | 59M | 1st AZ/14 | MOGAD | − | + | 110 | NA | NA | 625 | N | + | N | T7–L1 | PT + PE | I | [48] |
| 30 | 45M/allergic asthma | 1st AZ/7 | MOGAD | − | + | 43 | NA | NA | 40.6 | N | − | Bil subcortical, gray–white matter | T10–conus | PT | I | [49] |
| 31 | 26M | 1st AZ/20 | MOGAD | − | + | 184 | NA | NA | 88 | NA | − | Bil MCPs, pons | C3–C6 | PT | I | [50] |
| 32 | 56F/HTN | 1st AZ/2 | ADEM-like | NA | NA | NA | NA | NA | NA | NA | NA | L parietal WM, CC | NA | Oral steroid | I | [51] |
| 33 | 81M | 1st Moderna/13 | ADEM | NA | − | 69 | 83% | NA | 52 | N | NA | R dorsal medulla, L pons, midbrain, thalami | NA | PT + IVIG + PP | E(D26) | [52] |
| 34 | 63F/HL, hypothyroidism | 1st Pfizer/7 | NMOSD | + | − | 33 | 91% | NA | 57 | NA | − | L thalamus | T6–T12 | PT + PP | R | |
| 35 | 54F/ITP | 2nd Moderna/3 | NMOSD | + | − | 26 | 86% | NA | 71 | NA | − | N | T2–T9 | PT | I | |
| 36 | 55M | 1st mRNA/21 | ADEM | NA | NA | 200 | 95% | NA | 75 | N | NA | Bil WM | NA | PT | R | [53] |
| 37 | 36F | 1st AZ/14 | ADEM | − | − | 59 | NA | NA | 40 | N | + | Subcortical WM, PIC, pons, L MCP | N | PT | R | [54] |
| 38 | 37M | 1st Sinopharm/30 | ADEM | NA | NA | 2 | NA | NA | 56 | 0.61 | − | L cerebral peduncle, bil pons, medulla | N | PT | R | [55] |
| 39 | 27F/Rectovaginal fistula | 2nd Pfizer/4 | LETM | − | − | 7 | NA | NA | 43 | N | − | N | C5–C7 | PT | I | [56] |
| 40 | 61F/HTN, anxiety | 1st Pfizer/5 | ADEM | NA | − | N | NA | NA | 61 | N | − | Deep WM, brainstem, cerebellum | N | PT + IVIG | I | [57] |
| 41 | 64M | 1st AZ/10 | ADEM | − | − | 25 | P | NA | NA | N | NA | Bil mesial temporal, hippocampus, MCPs | NA | PT + PP + RTX | R | [58] |
| 42 | 64M | 2nd AZ/20 | ADEM + SSM | − | − | N | NA | NA | N | N | NA | Bil perirolandic cortex, corona radiata | T8–T9 dorsal | PT + IVIG + RTX | I | |
| 43 | 46M | 1st AZ/5 | ADEM + LETM | − | − | 63 | NA | NA | 52 | N | NA | Bil MCP, pons, R paramedian medulla, L thalamocapsular | LETM | PT + PE | I | |
| 44 | 42F | 1st AZ/5 | ADEM-like | − | − | N | NA | NA | N | N | NA | R temporal | NA | Oral steroid | R | |
| 45 | 56F | 1st AZ/10 | ADEM-like | NA | NA | 1 | 16% | 20% | ↑ | N | NA | Subcortical WM, basal ganglia | NA | PT | R | [59] |
| 46 | 44F/HL, hypothyroidism, renal stone, anxiety | 1st mRNA/6 | ADEM + LETM | − | NA | 105 | NA | NA | 98 | N | − | Multifocal PV lesions; C+ in L frontal WM | C3–C4 to thoracic with sparing C5–C6; C+ in T7–T8 | PT + PP | I | [60] |
| 47 | 70F | 3rd Sinovac/ 7 | NMOSD | + | NA | N | N | N | N | N | − | NA | C1–C7 and T1–T3 | PT + PE + CP | E(M2) | [61] |
| 48 | 26F | 1st Sinovac/10 | NMOSD | + | NA | N | N | N | N | N | − | N | C4–C5 | PT + PE + RTX | I | [62] |
| 49 | 46F | 1st AZ/10 | NMOSD | + | NA | N | N | N | N | N | − | R lateral medulla, PV | C2–C3 | PT +AZT | I | |
| 50 | 80M | 2nd Pfizer/2 | NMOSD | + | + | 39 | 93% | NA | N | N | − | N | T3–T10 | PT + PE + MMF | I | [63] |
| 51 | 43F | 2nd Pfizer/1 | NMOSD | + | − | 6 | NA | NA | 40.1 | N | + | R ON, R periatrium, L crus cerebri | C1 to mid-thoracic | PT + PE + RTX | R | [64] |
| 52 | 29F | 1st AZ/11 | MOGAD | NA | + | 0 | NA | NA | 18 | N | − | Long intraorbital segment of R ON | NA | PT + PP | NA | [65] |
| 53 | 26F | 1st Covaxin h/11 | LETM | − | − | 207 | NA | P | 95.8 | N | NA | NA | C2–L1 | PT + PP | NA | |
| 54 | 54F | 1st AZ/14 | ADEM-like | − | − | 8 | P | NA | 77 | N | NA | CC, PV, subcortical WM, infratentorial | NA | PT + PP | NA | |
| 55 | 44M | 1st AZ/7 | MOGAD | NA | + | 130 | P | NA | 38 | N | NA | NA | Cervical and dorsal cord, conus | PT + PP | NA | |
| 56 | 50F | 1st AZ/28 | SSM | − | − | 2 | P | NA | 28 | N | NA | NA | C6 | PT | NA | |
| 57 | 39M | 1st AZ/14 | MOGAD | NA | + | NA | NA | NA | NA | NA | NA | Long intraorbital segment of R ON | NA | PT | NA | |
| 58 | 54M | 1st AZ/14 | MOGAD | NA | + | NA | NA | NA | NA | NA | NA | R pons | N | PT | NA | |
| 59 | 34M | 1st AZ/1 | ON | − | − | 2 | P | NA | 26 | N | NA | R ON | NA | PT | NA | |
| 60 | 35M | 1st AZ/9 | MOGAD | NA | + | 58 | P | NA | 47.4 | N | NA | Midbrain, pons, L MCP, PICs, thalamus, bil centrum semiovale | Cervical to conus | PT | NA | |
| 61 | 20F | 1st AZ/3 | ADEM-like | − | − | NA | NA | NA | NA | NA | NA | Pericallosal, callososeptal, PV, fronto-parietal | NA | PT | NA | |
| 62 | 31M | 1st AZ/14 | LETM | − | − | 370 | NA | P | 174 | N | NA | NA | Cervico–dorsal long segment | PT + PP + RTX | NA | |
| 63 | 20F | 1st Covaxin/1 | ADEM + SSM | − | − | 8 | P | NA | 24.9 | N | − | Juxtacortical | C5 | PT + PP | NA | |
| 64 | 45F | 1st AZ/21 | MOGAD | NA | + | 2 | P | NA | 52.3 | N | + | Bil ON | N | PT + PP | NA | |
| 65 | 33F | 1st AZ/14 | MOGAD | NA | + | 105 | P | NA | 28.12 | N | NA | Bil fronto-parietal | NA | PT | NA | |
| 66 | 53F | 2nd AZ/1 | ADEM + LETM | − | − | 6 | P | NA | 54.2 | N | NA | Bil subcortical, PV, insular, cerebellum, brainstem | C5–C7 and T6–T7 | PT | NA | |
| 67 | 38M | 2nd AZ/6 | ADEM-like | − | − | 6 | NA | NA | 67.8 | N | NA | L MCP, R corona radiata | NA | PT | NA | |
| 68 | 30M | 1st AZ/14 | ADEM + ON | − | − | 4 | 50 | NA | 26.8 | N | + | Bil subcortical, bil ON | NA | PT + PP + RTX | NA | |
| 69 | 30F | 1st AZ/15 | ADEM + SSM | − | − | 4 | NA | NA | 36 | N | + | CC | C3 | PT + PP + MMF | NA | |
| 70 | 36M | 2nd AZ/32 | MOGAD | NA | + | 720 | 80 | NA | 144.4 | N | NA | Bil trigeminal n, pons | Obex to conus | PT + PP | NA | |
| 71 | 27F | 1st AZ/8 | ADEM-like | − | − | Clear | NA | NA | 27.7 | N | NA | Bil PV | N | PT | NA | |
| 72 | 60M | 2nd AZ/14 | ADEM | − | − | 9 | 90 | NA | 68.3 | N | − | R pons, midbrain, temporal, parietal, CC | NA | PT + MMF | NA | |
| 73 | 23F | 2nd AZ/7 | ADEM + LETM | − | − | NA | NA | NA | NA | NA | − | R frontal horn and bil lateral ventricles | C2–C5 and T4 myelitis | PT | NA | |
| 74 | 40M | 1st AZ/10 | MOGAD | NA | + | 8 | 100 | 0 | 32 | N | + | Pons, bil thalami, and R frontal cortex | C4–T3 | PT + MMF | NA | |
| 75 | 45M | 1st AZ/10 | MOGAD | − | + | 44 | 44 | NA | 90.9 | N | NA | Brainstem, supratentorial | Cervicodorsal cord | PT + PP | NA | |
| 76 | 34F | 2nd AZ/36 | NMOSD | + | − | 1 | NA | NA | 15.3 | N | − | Dorsal aspect of medulla | NA | PT + PP + RTX | NA | |
| 77 | 31M | 1st AZ/42 | ADEM + LETM | − | − | 32 | 100 | 0 | 49.2 | N | NA | Cervico-medullary junction, R frontal subcortical | C2–C5 | PT + PP + MMF | NA | |
| 78 | 52F | 1st AZ/35 | ADEM-like | − | − | 2 | NA | NA | 40.5 | N | NA | L frontal, insular, midbrain | NA | PT + PP + RTX | NA | |
| 79 | 65F | 1st AZ/42 | NMOSD | + | − | 17 | NA | NA | 49 | N | NA | Frontal subcortical WM | T2–T11 | PT + PP + MMF | NA | |
| Vaccine Types | ||||
|---|---|---|---|---|
| Viral Vector (n = 49) | mRNA (n = 20) | Inactivated (n = 10) | p Value | |
| Sex, n (%) | 0.027 * | |||
| Male | 28 (57) | 6 (30) | 2 (20) | |
| Female | 21 (43) | 14 (70) | 8 (80) | |
| Mean age of onset (S.D.) | 44.3 (12.2) | 58.1 (17.9) | 44.8 (21.8) | 0.002 #* |
| Doses (1st/2nd/3rd) | 41/8/0 | 13/7/0 | 7/2/1 | 0.042 * |
| Post-vaccination onset time (days) | 13.6 | 8.1 | 13.6 | 0.086 |
| Clinical presentations, n (%) | 0.203 | |||
| ADEM | 13 (27) | 5 (25) | 3 (30) | |
| Pure myelitis | 10 (20) | 6 (30) | 3 (30) | |
| MOGAD | 14 (29) | 1 (5) | 0 (0) | |
| NMOSD | 4 (8) | 6 (30) | 3 (30) | |
| ADEM with myelitis | 7 (14) | 2 (10) | 1 (10) | |
| ON | 1 (2) | 0 (0) | 0 (0) | |
| Serum Autoantibodies , n | 0.044 * | |||
| Negative | 31 | 14 | 7 | |
| MOG | 14 | 1 | 0 | |
| AQP4 | 4 | 4 | 3 | |
| MOG + AQP4 | 0 | 1 | 0 | |
| CSF, n | ||||
| WBC count | 74.2 | 57.1 | 30.1 | 0.605 |
| Lym predominant (yes/no) | 23/3 | 6/2 | 1/4 | 0.004 * |
| Elevated total protein (yes/no) @ | 24/19 | 10/8 | 4/5 | 0.817 |
| CSF/serum glu ratio (<0.6/>0.6) | 4/33 | 0/13 | 0/8 | 0.295 |
| Oligoclonal bands (+/−) | 9/13 | 4/11 | 0/9 | 0.071 |
| Brain MRI lesions, n | ||||
| Cortex (+/−) | 7/33 | 1/16 | 2/5 | 0.329 |
| Deep grey matters (+/−) | 8/32 | 2/15 | 2/5 | 0.598 |
| Subcortical white matters (+/−) | 18/22 | 5/12 | 2/5 | 0.454 |
| Periventricular white matters (+/−) | 16/24 | 6/11 | 1/6 | 0.424 |
| Periaqueductal white matters (+/−) | 3/37 | 0/17 | 0/7 | 0.389 |
| Brainstem (+/−) | 20/20 | 7/10 | 2/5 | 0.532 |
| Gadolinium enhanced (+/−) | 1/9 | 2/9 | 0/5 | 0.562 |
| Spine MRI, n | ||||
| Segments of cord lesions (≥3/<3) | 20/8 | 12/2 | 4/2 | 0.143 |
| Gadolinium enhanced (+/−) | 6/6 | 7/1 | 0/4 | 0.015 * |
| Treatment, n | 0.754 | |||
| First-line immunotherapy | 43 | 17 | 8 | |
| First- and second-line immunotherapy | 6 | 2 | 2 | |
| Outcome, n | 0.762 | |||
| Recovery | 11 | 7 | 2 | |
| Improved | 11 | 11 | 5 | |
| Expired | 1 | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-Y.; Ho, H.-C.; Hsu, J.-L.; Wang, Y.; Chen, L.; Lim, S.-N.; Liao, M.-F.; Ro, L.-S. Clinical Research into Central Nervous System Inflammatory Demyelinating Diseases Related to COVID-19 Vaccines. Diseases 2024, 12, 60. https://doi.org/10.3390/diseases12030060
Cheng M-Y, Ho H-C, Hsu J-L, Wang Y, Chen L, Lim S-N, Liao M-F, Ro L-S. Clinical Research into Central Nervous System Inflammatory Demyelinating Diseases Related to COVID-19 Vaccines. Diseases. 2024; 12(3):60. https://doi.org/10.3390/diseases12030060
Chicago/Turabian StyleCheng, Mei-Yun, Hsuan-Chen Ho, Jung-Lung Hsu, Yi Wang, Linyi Chen, Siew-Na Lim, Ming-Feng Liao, and Long-Sun Ro. 2024. "Clinical Research into Central Nervous System Inflammatory Demyelinating Diseases Related to COVID-19 Vaccines" Diseases 12, no. 3: 60. https://doi.org/10.3390/diseases12030060
APA StyleCheng, M.-Y., Ho, H.-C., Hsu, J.-L., Wang, Y., Chen, L., Lim, S.-N., Liao, M.-F., & Ro, L.-S. (2024). Clinical Research into Central Nervous System Inflammatory Demyelinating Diseases Related to COVID-19 Vaccines. Diseases, 12(3), 60. https://doi.org/10.3390/diseases12030060






