Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Study Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Bardan, R.; Muntean, C.; Olariu, A.; Olariu, S. Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned. Medicina 2022, 58, 1322. [Google Scholar] [CrossRef]
- Gaertner, W.B.; Kwaan, M.R.; Madoff, R.D.; Melton, G.B. Rectal cancer: An evidence-based update for primary care providers. World J. Gastroenterol. 2015, 21, 7659–7671. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Bardan, R.; Muntean, C.; Feier, O.; Olariu, A.; Olariu, S. The consequences of the COVID-19 pandemic on elective surgery for colon cancer. Ann. Ital. Chir. 2022, 93, 599–605. [Google Scholar]
- Lo Bianco, S.; Lanzafame, K.; Piazza, C.D.; Piazza, V.G.; Provenzano, D.; Piazza, D. Total mesorectal excision laparoscopic versus transanal approach for rectal cancer: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 74, 103260. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique-Past, Present, and Future. Clin. Colon Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.B.; Hawkins, A.T. Non-operative management of rectal cancer. Semin. Colon Rectal Surg. 2019, 30, 79–84. [Google Scholar] [CrossRef]
- Nicolescu, C.; Pop, A.; Mihu, A.; Pilat, L.; Bedreag, O.; Nicolescu, L. The Evaluation of the Role of the Cytokines TNF- alfa and IL 6 in the Production of Hypoalbuminemia in Patients Undergoing Major Surgical Interventions. Rev. Chim. 2018, 69, 1830–1837. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.; Pandey, A.; Singh, N.K.; Srivastava, S. Molecular subtypes of colorectal cancer: An emerging therapeutic opportunity for personalized medicine. Genes Dis. 2019, 8, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Feier, C.V.I.; Faur, A.M.; Muntean, C.; Blidari, A.; Contes, O.E.; Streinu, D.R.; Olariu, S. The Challenges of Gastric Cancer Surgery during the COVID-19 Pandemic. Healthcare 2023, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Muntean, C.; Faur, A.M.; Blidari, A.; Contes, O.E.; Streinu, D.R.; Olariu, S. The Changing Landscape of Thyroid Surgery during the COVID-19 Pandemic: A Four-Year Analysis in a University Hospital in Romania. Cancers 2023, 15, 3032. [Google Scholar] [CrossRef]
- Aabed, H.; Bloanca, V.; Crainiceanu, Z.; Bratosin, F.; Citu, C.; Diaconu, M.M.; Ciorica, O.; Bratu, T. The Impact of SARS-CoV-2 Pandemic on Patients with Malignant Melanoma at a Romanian Academic Center: A Four-Year Retrospective Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8499. [Google Scholar] [CrossRef]
- Hamilton, A.C.; Donnelly, D.W.; Loughrey, M.B.; Turkington, R.C.; Fox, C.; Fitzpatrick, D.; O’neill, C.E.; Gavin, A.T.; Coleman, H.G. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: A population-based study. Br. J. Cancer 2021, 125, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.-O.; Prodan, M.; Reddyreddy, A.R.; Seclaman, E.; Crainiceanu, Z.; Bloanca, V.; Bratosin, F.; Dumitru, C.; Pilut, C.N.; Alambaram, S.; et al. The Epidemiology of Malignant Melanoma during the First Two Years of the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Brajcich, B.C.; Benson, A.B.; Gantt, G.; Eng, O.S.; Marsh, R.W.; Mulcahy, M.F.; Polite, B.N.; Shogan, B.D.; Yang, A.D.; Merkow, R.P. Management of colorectal cancer during the COVID-19 pandemic: Recommendations from a statewide multidisciplinary cancer collaborative. J. Surg. Oncol. 2022, 125, 560–563. [Google Scholar] [CrossRef]
- Gallo, G.; Sturiale, A.; De Simone, V.; Di Tanna, G.L.; Giani, I.; Grossi, U.; ProctoLock 2020 Working Group. A worldwide survey on proctological practice during COVID-19 lockdown (ProctoLock 2020): A cross-sectional analysis. Colorectal Dis. 2021, 23, 246–264. [Google Scholar] [CrossRef]
- Pirvu, C.A.; Bratosin, F.; Nica, C.; Cârțu, D.; Patrascu, Ș.; Șurlin, V.; Sapalidis, K.; Georgescu, E.; Georgescu, I.; Pantea, S. The Utility of Biomarkers in Diagnosing and Predicting Outcomes in Acute Mesenteric Ischemia. J. Surg. 2021, 17, 119–126. [Google Scholar] [CrossRef]
- Duncan, R.; Szabo, B.; Jackson, Q.L.; Crain, M.; Lett, C.; Masters, C.; Spinks, R.; Uhrig, L.K.; Gullatte, M.M. Care and Coping During COVID-19: Practice Changes and Innovations in the Oncology Setting. Clin. J. Oncol. Nurs. 2021, 25, 48–55. [Google Scholar] [CrossRef]
- Popescu, A.; Craina, M.; Pantea, S.; Pirvu, C.; Radu, D.; Marincu, I.; Bratosin, F.; Bogdan, I.; Hosin, S.; Citu, C.; et al. COVID-19 Pandemic Impact on Surgical Treatment Methods for Early-Stage Cervical Cancer: A Population-Based Study in Romania. Healthcare 2022, 10, 639. [Google Scholar] [CrossRef]
- Omboni, S.; Padwal, R.S.; Alessa, T.; Benczúr, B.; Green, B.B.; Hubbard, I.; Kario, K.; Khan, N.A.; Konradi, A.; Logan, A.G.; et al. The worldwide impact of telemedicine during COVID-19: Current evidence and recommendations for the future. Connect. Health 2022, 1, 7–35. [Google Scholar] [CrossRef]
- Di Martino, G.; Cedrone, F.; Di Giovanni, P.; Romano, F.; Staniscia, T. Impact of COVID-19 Pandemic on Oncological Surgery Activities: A Retrospective Study from a Southern Italian Region. Healthcare 2022, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Craina, M.; Pantea, S.; Pirvu, C.; Chiriac, V.D.; Marincu, I.; Bratosin, F.; Bogdan, I.; Hosin, S.; Citu, C.; et al. COVID-19 Pandemic Effects on Cervical Cancer Diagnosis and Management: A Population-Based Study in Romania. Diagnostics 2022, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Tarta, C.; Marian, M.; Capitanio, M.; Faur, F.I.; Duta, C.; Diaconescu, R.; Oprescu-Macovei, A.M.; Totolici, B.; Dobrescu, A. The Challenges of Colorectal Cancer Surgery during the COVID-19 Pandemic in Romania: A Three-Year Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 14320. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2014, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, T.M.; Abdelrazek, M.E.G.; Fitzpatrick, A.J.; Froud, J.L.J.; Kelly, J.R.; Williamson, J.S.; Williams, G.L. Delay to elective colorectal cancer surgery and implications for survival: A systematic review and meta-analysis. Colorectal Dis. 2021, 23, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, M.; Paradis, T.; Kouyoumdjian, A.; Dumitra, T.; Charlebois, P.; Stein, B.S.; Liberman, A.S.; Schwartzman, K.; Carli, F.; Fried, G.M.; et al. The impact of delays to definitive surgical care on survival in colorectal cancer patients. J. Gastrointest. Surg. 2020, 24, 115–122. [Google Scholar] [CrossRef]
- Suárez, J.; Mata, E.; Guerra, A.; Jiménez, G.; Montes, M.; Arias, F.; Ciga, M.A.; Ursúa, E.; Ederra, M.; Arín, B.; et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. J. Surg. Oncol. 2021, 123, 32–36. [Google Scholar] [CrossRef]
- Aguiar, S., Jr.; Riechelmann, R.P.; de Mello, C.A.L.; da Silva, J.C.F.; Diogenes, I.D.C.; Andrade, M.S.; de Miranda Marques, T.M.D.; Stevanato, P.R.; Bezerra, T.S.; Silva, M.L.G.; et al. Impact of COVID-19 on colorectal cancer presentation. Br. J. Surg. 2021, 108, e81–e82. [Google Scholar] [CrossRef]
- Virzob, C.R.B.; Poenaru, M.; Morar, R.; Horhat, I.D.; Balica, N.C.; Prathipati, R.; Moleriu, R.D.; Toma, A.-O.; Juganaru, I.; Bloanca, V.; et al. Efficacy of Bilateral Cochlear Implantation in Pediatric and Adult Patients with Profound Sensorineural Hearing Loss: A Retrospective Analysis in a Developing European Country. J. Clin. Med. 2023, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Dantes, E.; Lilios, G.; Fildan, A.P. Environmental tobacco smoke exposure–An independent risk factor for lung cancer? J. Environ. Prot. Ecol. JEPE 2015, 16, 1620–1626. [Google Scholar]
- Rajnoveanu, R.M.; Rajnoveanu, A.G.; Ardelean, A.B.; Todea, D.A.; Pop, C.M.; Antoniu, S.A.; Motoc, N.S.; Chis, A.F.; Fildan, A.P.; Man, M.A. Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve. Medicina 2020, 56, 422. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Hadavandsiri, F.; Momenimovahed, Z.; Salehiniya, H. Impact of the COVID-19 Pandemic on Colorectal Cancer Diagnosis and Treatment: A Systematic Review. J. Gastrointest. Cancer 2023, 54, 171–187. [Google Scholar] [CrossRef]
- Buscarini, E.; Benedetti, A.; Monica, F.; Pasquale, L.; Buttitta, F.; Cameletti, M.; Ferrari, C.; Ricciardiello, L.; FISMAD: the FISMAD-ALERT Survey Group. Changes in digestive cancer diagnosis during the SARS-CoV-2 pandemic in Italy: A nationwide survey. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 682–688. [Google Scholar] [CrossRef]
- Brito, M.; Laranjo, A.; Sabino, J.; Oliveira, C.; Mocanu, I.; Fonseca, J. Digestive Oncology in the COVID-19 Pandemic Era. GE Port. J. Gastroenterol. 2021, 28, 303–310. [Google Scholar] [CrossRef]
- Lui, T.K.L.; Leung, K.; Guo, C.G.; Tsui, V.W.M.; Wu, J.T.; Leung, W.K. Impacts of the coronavirus 2019 pandemic on gastrointestinal endoscopy volume and diagnosis of gastric and colorectal cancers: A population-based study. Gastroenterology 2020, 159, 1164–1166.e3. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Cellamare, M.; Visser, O.; de Munck, L.; Elferink, M.A.G.; Westenend, P.J.; Wesseling, J.; Broeders, M.J.M.; Kuipers, E.J.; Merkx, M.A.W.; et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J. Hematol. Oncol. 2020, 13, 147. [Google Scholar] [CrossRef]
- Shinkwin, M.; Silva, L.; Vogel, I.; Reeves, N.; Cornish, J.; Horwood, J.; Davies, M.M.; Torkington, J.; Ansell, J. COVID-19 and the emergency presentation of colorectal cancer. Color. Dis. Off. J. Assoc. Coloproctology G. B. Irel. 2021, 23, 2014–2019. [Google Scholar] [CrossRef]
- Mizuno, R.; Ganeko, R.; Takeuchi, G.; Mimura, K.; Nakahara, H.; Hashimoto, K.; Hinami, J.; Shimomatsuya, T.; Kubota, Y. The number of obstructive colorectal cancers in Japan has increased during the COVID-19 pandemic: A retrospective single-center cohort study. Expert. Rev. Mol. Diagn. 2020, 60, 675–679. [Google Scholar] [CrossRef] [PubMed]
- de Neree Tot Babberich, M.P.M.; van Groningen, J.T.; Dekker, E.; Wiggers, T.; Wouters, M.W.J.M.; Bemelman, W.A.; Tanis, P.J.; Dutch Surgical Colorectal Audit. Laparoscopic conversion in colorectal cancer surgery; is there any improvement over time at a population level? Surg. Endosc. 2018, 32, 3234–3246. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Petrucciani, N. Treatment approach in locally advanced rectal cancer during coronavirus (COVID-19) pandemic: Long course or short course? Color. Dis. Off. J. Assoc. Coloproctology G. B. Irel. 2020, 22, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Allaix, M.E.; Lo Secco, G.; Velluti, F.; De Paolis, P.; Arolfo, S.; Morino, M. Colorectal surgery during the COVID-19 outbreak: Do we need to change? Updates Surg. 2021, 73, 173–177. [Google Scholar] [CrossRef]
- Gurney, J.K.; Millar, E.; Dunn, A.; Pirie, R.; Mako, M.; Manderson, J.; Hardie, C.; Jackson, C.G.; North, R.; Ruka, M.; et al. The impact of the COVID-19 pandemic on cancer diagnosis and service access in New Zealand–a country pursuing COVID-19 elimination. Lancet Reg. Health—West. Pac. 2021, 10, 100127. [Google Scholar] [CrossRef] [PubMed]
- Schots, M.A.S.; Coleman, H.L.S.; Lutwama, G.W.; Straetemans, M.; Jacobs, E. The impact of the COVID-19 pandemic on healthcare access and utilisation in South Sudan: A cross-sectional mixed methods study. BMC Health Serv. Res. 2022, 22, 1559. [Google Scholar] [CrossRef]
- Osakwe, H.I.; Dragomir, C.; Nicolescu, C.; Boia, E.S. The challenges of managing and following-up a case of short bowel in eastern europe. Int. J. Surg. Case Rep. 2016, 26, 187–192. [Google Scholar] [CrossRef][Green Version]
- Licker, M.; Anghel, A.; Moldovan, R.; Hogea, E.; Muntean, D.; Horhat, F.; Seclaman, E.; Tamas, L.; Anghel, M.; Baditoiu, L. Genotype-phenotype correlation in multiresistant Escherichia coli and Klebsiella pneumoniae strains isolated in Western Romania. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1888–1894. [Google Scholar]
- Pujolar, G.; Oliver-Anglès, A.; Vargas, I.; Vázquez, M.L. Changes in Access to Health Services during the COVID-19 Pandemic: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1749. [Google Scholar] [CrossRef]
- Hunt, X.; Hameed, S.; Tetali, S.; Ngoc, L.A.; Ganle, J.; Huq, L.; Shakespeare, T.; Smythe, T.; Ilkkursun, Z.; Kuper, H.; et al. Impacts of the COVID-19 pandemic on access to healthcare among people with disabilities: Evidence from six low- and middle-income countries. Int. J. Equity Health 2023, 22, 172. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Hou, L.; Zhao, E. Short-term and long-term outcomes of patients with gastric cancer during versus before the COVID-19 pandemic: Cohort study using propensity score matching method. BMC Cancer 2023, 23, 913. [Google Scholar] [CrossRef]
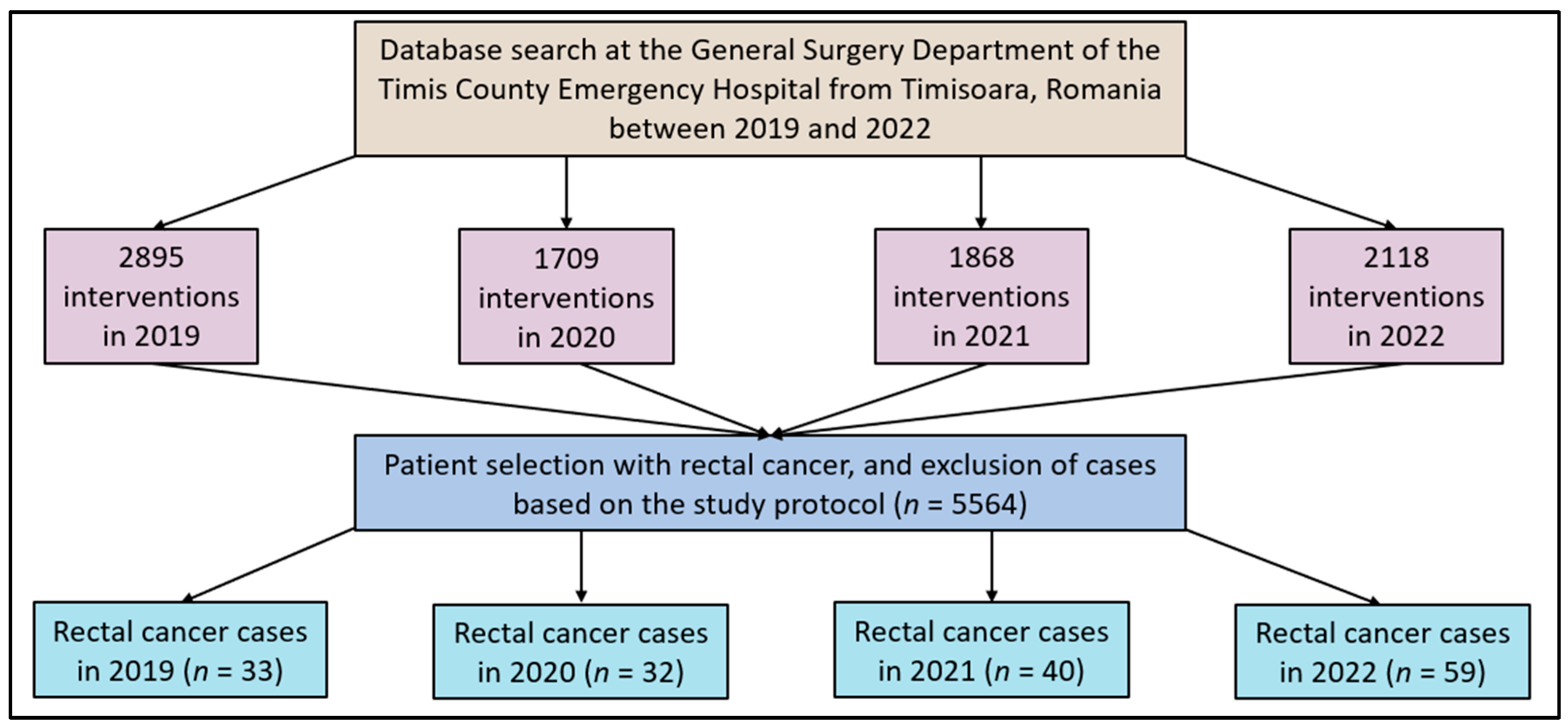
| Variables | Control—2019 (n = 33) | 2020 (n = 32) | 2021 (n = 40) | 2022 (n = 59) | p-Value |
|---|---|---|---|---|---|
| Age (mean ± SD) | 65.5 ± 9.8 | 66.2 ± 10.2 | 64.0 ± 10.8 | 67.4 ± 10.5 | 0.324 |
| Sex (male) | 21 (63.6%) | 20 (62.5%) | 21 (52.5%) | 35 (59.3%) | 0.668 |
| BMI (kg/m2) | 0.760 | ||||
| Normal weight (18.5–25) | 9 (27.3%) | 8 (25.0%) | 7 (17.5%) | 15 (25.4%) | |
| Overweight (25–30) | 12 (36.4%) | 13 (40.6%) | 17 (42.5%) | 19 (32.2%) | |
| Obese (>30) | 12 (36.4%) | 11 (34.4%) | 16 (40.0%) | 25 (42.4%) | |
| SMI | |||||
| Male | 54.7 ± 6.4 | 55.8 ± 6.6 | 53.2 ± 6.0 | 53.9 ± 7.2 | 0.196 |
| Female | 42.0 ± 5.8 | 41.3 ± 5.9 | 42.8 ± 6.2 | 40.5 ± 4.0 | 0.130 |
| Visceral obesity (n, %) | 8 (24.2%) | 9 (28.1%) | 14 (35.0%) | 20 (33.9%) | 0.803 |
| Comorbidities | |||||
| Cardiovascular | 18 (54.5%) | 20 (62.5%) | 18 (45.0%) | 29 (49.2%) | 0.308 |
| Pulmonary | 4 (12.1%) | 5 (15.6%) | 5 (12.5%) | 8 (13.6%) | 0.927 |
| Diabetes mellitus | 8 (24.2%) | 7 (21.9%) | 9 (22.5%) | 11 (18.6%) | 0.878 |
| Cerebrovascular | 3 (9.1%) | 3 (9.4%) | 4 (10.0%) | 8 (13.6%) | 0.788 |
| Renal disease | 2 (6.1%) | 3 (9.4%) | 3 (7.5%) | 6 (10.2%) | 0.901 |
| Previous history of neoplasia | 3 (9.1%) | 3 (9.4%) | 2 (5.0%) | 2 (3.4%) | 0.476 |
| Steroids or immunotherapy during the past 3 months | 1 (3.0%) | 1 (3.1%) | 0 (0.0%) | 2 (3.4%) | 0.507 |
| Variables | Control—2019 (n = 33) | 2020 (n = 32) | 2021 (n = 40) | 2022 (n = 59) | p-Value |
|---|---|---|---|---|---|
| Preoperative laboratory data | |||||
| Hemoglobin | 10.2 ± 3.2 | 10.3 ± 3.3 | 9.8 ± 3.5 | 10.9 ± 9.8 | 0.323 |
| Hematocrit | 38.5 ± 7.0 | 38.7 ± 7.1 | 36.5 ± 8.9 | 39.4 ± 6.8 | 0.170 |
| Albumin | 4.1 ± 0.6 | 4.0 ± 0.7 | 3.8 ± 0.8 | 4.2 ± 0.6 | 0.019 |
| Total proteins | 7.1 ± 0.8 | 7.0 ± 0.9 | 6.8 ± 1.1 | 7.2 ± 0.8 | 0.109 |
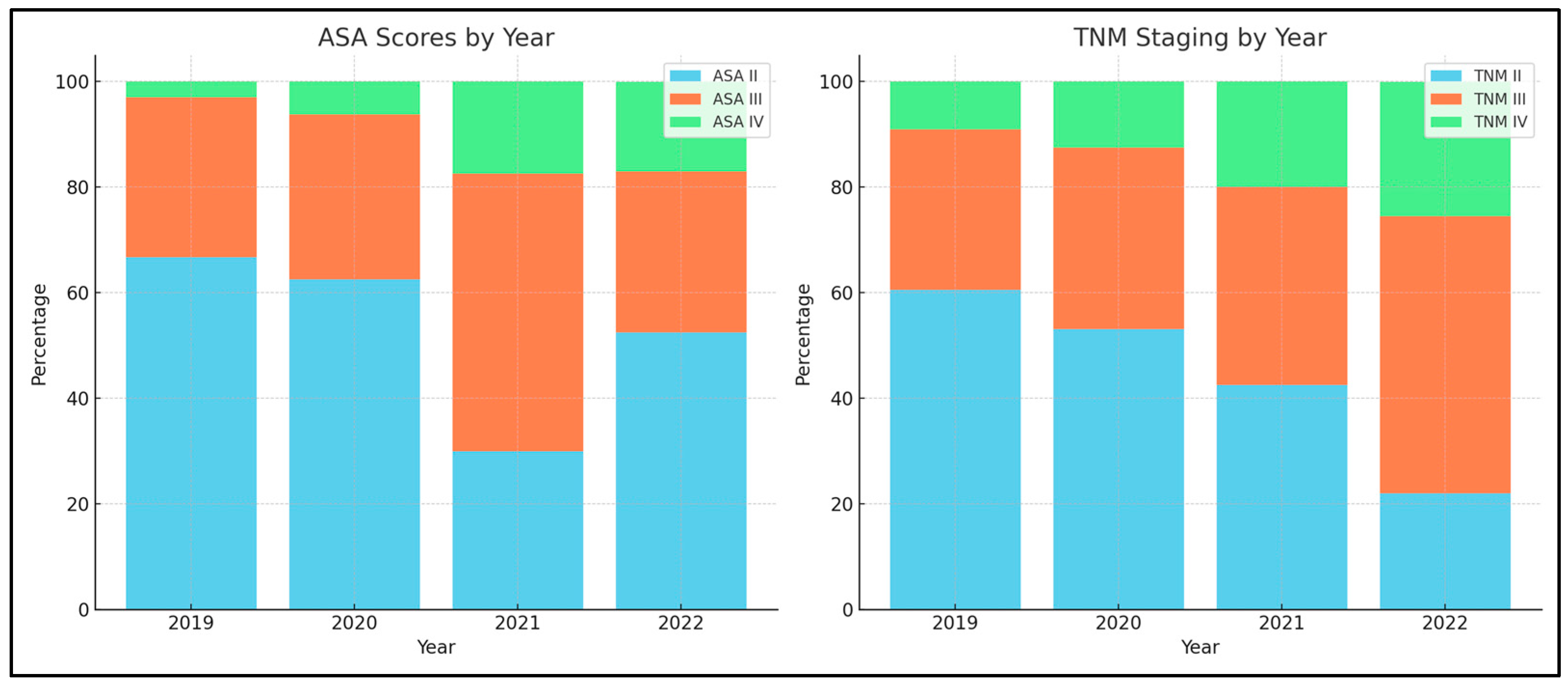
| ASA score | 0.043 | ||||
| II | 22 (66.7%) | 20 (62.5%) | 12 (30.0%) | 31 (52.5%) | |
| III | 10 (30.3%) | 10 (31.3%) | 21 (52.5%) | 18 (30.5%) | |
| IV | 1 (3.0%) | 2 (6.2%) | 7 (17.5%) | 10 (16.9%) | |
| TNM Staging | 0.039 | ||||
| II (all subtypes) | 20 (60.6%) | 17 (53.1%) | 17 (42.5%) | 13 (22.0%) | |
| III (all subtypes) | 10 (30.3%) | 11 (34.4%) | 15 (37.5%) | 31 (52.5%) | |
| IV (all subtypes) | 3 (9.1%) | 4 (12.5%) | 8 (20.0%) | 15 (25.4%) | |
| Metastasis | |||||
| Local | 2 (6.1%) | 1 (3.1%) | 2 (5.0%) | 7 (11.9%) | 0.244 |
| Distant | 3 (9.1%) | 4 (12.5%) | 7 (17.5%) | 12 (20.3%) | 0.643 |
| Local invasion | 3 (9.1%) | 3 (9.4%) | 3 (7.5%) | 9 (15.3%) | 0.450 |
| Metastasis location | 0.867 | ||||
| Lungs | 2 (6.1%) | 1 (3.1%) | 1 (2.5%) | 4 (6.6%) | |
| Liver | 4 (12.1%) | 5 (15.6%) | 7 (17.5%) | 10 (16.9%) | |
| Peritoneal | 3 (9.1%) | 4 (12.5%) | 3 (7.5%) | 7 (11.9%) | |
| Other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (3.4%) | |
| Distance from the anal verge (mean ± SD) | 12.5 ± 6.9 | 12.7 ± 7.1 | 11.9 ± 6.8 | 13.2 ± 6.6 | 0.646 |
| Position based on MRI | 0.626 | ||||
| Low | 6 (18.2%) | 5 (15.6%) | 9 (22.5%) | 12 (20.3%) | |
| Medium | 10 (30.3%) | 8 (25.0%) | 12 (30.0%) | 11 (18.6%) | |
| High | 17 (51.5%) | 19 (59.4%) | 19 (47.5%) | 36 (61.0%) |
| Variables | Control—2019 (n = 33) | 2020 (n = 32) | 2021 (n = 40) | 2022 (n = 59) | p-Value |
|---|---|---|---|---|---|
| Patient presentation | 0.045 | ||||
| Emergency intervention | 10 (30.3%) | 12 (37.5%) | 17 (42.5%) | 12 (20.3%) | |
| Elective surgery | 23 (69.7%) | 20 (62.5%) | 23 (57.5%) | 47 (79.7%) | |
| Neoadjuvant therapy | 12 (36.4%) | 6 (18.8%) | 6 (15.0%) | 21 (35.6%) | 0.043 |
| Type of neoadjuvant therapy | 0.456 | ||||
| Chemo-radiotherapy | 5 (15.2%) | 1 (3.1%) | 3 (7.5%) | 11 (18.6%) | |
| Short course following chemotherapy | 4 (12.1%) | 3 (9.4%) | 1 (2.5%) | 7 (11.9%) | |
| Chemotherapy following short course | 3 (9.1%) | 2 (6.3%) | 2 (5.0%) | 3 (5.1%) | |
| Preoperative colon preparation | 0.031 | ||||
| No preparation | 8 (24.2%) | 12 (37.5%) | 17 (42.5%) | 12 (20.3%) | |
| Laxative | 16 (48.5%) | 13 (40.6%) | 10 (25.0%) | 33 (55.9%) | |
| Enema | 9 (27.3%) | 7 (21.9%) | 13 (32.5%) | 14 (23.7%) | |
| Presence of stoma | 2 (6.1%) | 1 (3.1%) | 1 (2.5%) | 3 (5.1%) | 0.783 |
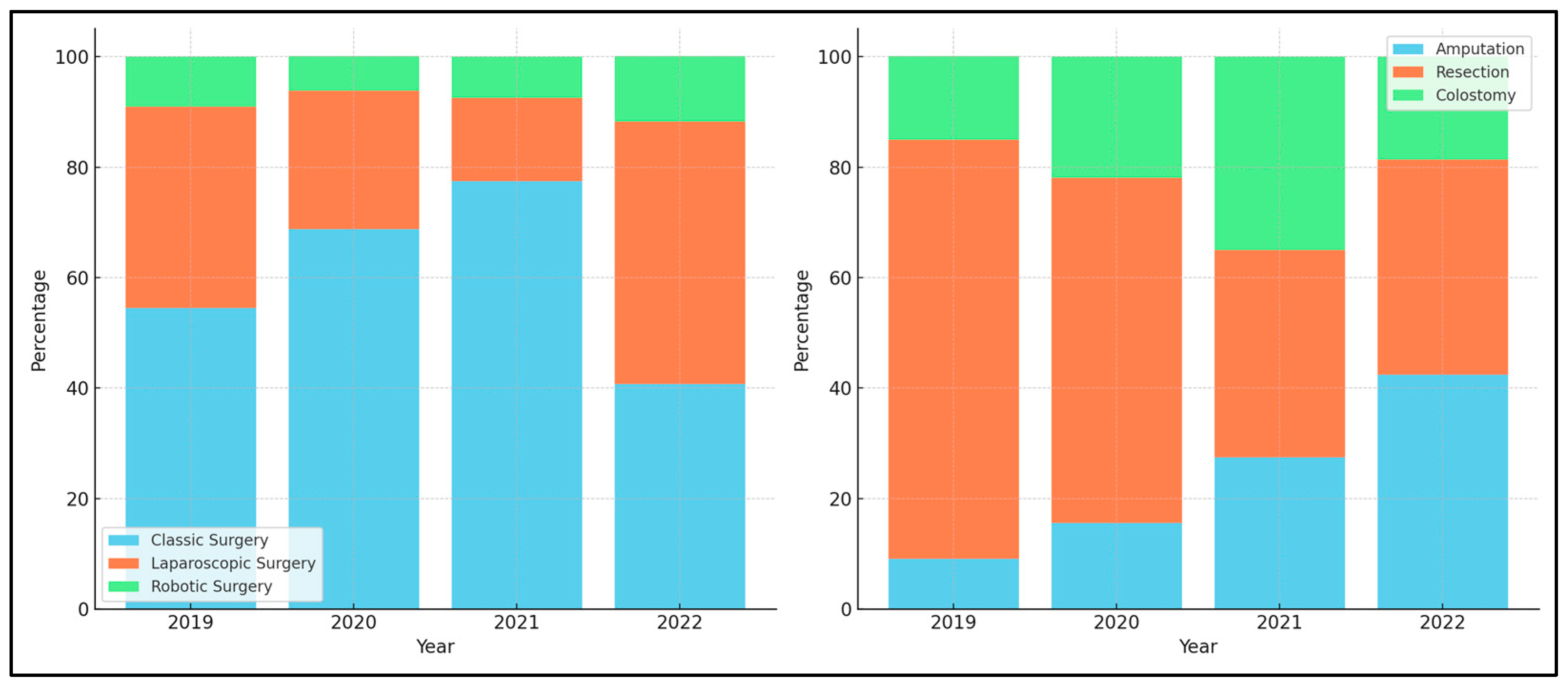
| Type of surgery | 0.004 | ||||
| Classic | 18 (54.5%) | 22 (68.8%) | 31 (77.5%) | 24 (40.7%) | |
| Laparoscopy | 12 (36.4%) | 8 (25.0%) | 6 (15.0%) | 28 (47.5%) | |
| Robotic | 3 (9.1%) | 2 (6.3%) | 3 (7.5%) | 7 (11.9%) | |
| Surgical method | 0.027 | ||||
| Amputation | 3 (9.1%) | 5 (15.6%) | 11 (27.5%) | 25 (42.4%) | |
| Resection | 25 (75.8%) | 20 (62.5%) | 15 (37.5%) | 23 (39.0%) | |
| Colostomy | 5 (15.2%) | 7 (21.9%) | 14 (35.0%) | 11 (18.6%) | |
| Surgical conversion | 2 (6.1%) | 3 (9.4%) | 4 (10.0%) | 9 (15.3%) | 0.627 |
| Vascular ligation of the IMA | 0.393 | ||||
| High tie | 13 (39.4%) | 12 (37.5%) | 24 (60.0%) | 31 (52.5%) | |
| Low tie | 16 (48.5%) | 15 (46.9%) | 12 (30.0%) | 19 (32.2%) | |
| Unknown | 4 (12.1%) | 5 (15.6%) | 4 (10.0%) | 9 (15.3%) | |
| Splenic flexure mobilization | 10 (30.3%) | 9 (28.1%) | 15 (37.5%) | 19 (32.2%) | 0.695 |
| Multiorgan resection | 3 (9.1%) | 2 (6.3%) | 5 (12.5%) | 3 (5.1%) | 0.373 |
| Type of anastomosis | 0.885 | ||||
| End to end | 31 (93.9%) | 30 (93.8%) | 37 (92.5%) | 56 (94.9%) | |
| End to side | 2 (6.1%) | 2 (6.3%) | 3 (7.5%) | 3 (5.1%) | |
| Method of anastomosis | 0.656 | ||||
| Double stapled | 24 (72.7%) | 23 (71.9%) | 35 (87.5%) | 50 (84.7%) | |
| Double purse-string suture | 3 (9.1%) | 2 (6.3%) | 1 (2.5%) | 4 (6.8%) | |
| Manual | 6 (18.2%) | 5 (15.6%) | 4 (10.0%) | 5 (8.5%) | |
| Intraoperative blood loss > 500 mL | 1 (3.0%) | 2 (3.1%) | 3 (7.5%) | 1 (1.7%) | 0.348 |
| Number of positive lymph nodes | 21.8 ± 7.6 | 21.9 ± 7.7 | 22.6 ± 8.0 | 24.7 ± 8.2 | 0.219 |
| Distal resection margin < 1 mm | 2 (6.1%) | 1 (3.1%) | 2 (5.0%) | 3 (5.1%) | 0.902 |
| Variables | Control—2019 (n = 33) | 2020 (n = 32) | 2021 (n = 40) | 2022 (n = 59) | p-Value |
|---|---|---|---|---|---|
| Biological response to radiation therapy | 3 (9.1%) | 4 (12.5%) | 4 (10.0%) | 7 (11.9%) | 0.938 |
| GCS < 15 | 3 (9.1%) | 2 (6.3%) | 4 (10.0%) | 5 (8.5%) | 0.849 |
| Respiratory rate > 22 | 6 (18.2%) | 5 (15.6%) | 7 (17.5%) | 9 (15.3%) | 0.953 |
| Temperature < 36 or >38 | 7 (21.2%) | 6 (18.8%) | 10 (25.0%) | 11 (18.6%) | 0.712 |
| Heart rate > 100 | 9 (27.3%) | 8 (25.0%) | 13 (32.5%) | 13 (22.0%) | 0.502 |
| Systolic BP < 100 mmHg | 6 (18.2%) | 5 (15.6%) | 8 (20.0%) | 10 (16.9%) | 0.877 |
| Pressor support | 2 (6.1%) | 2 (6.3%) | 6 (15.0%) | 4 (6.8%) | 0.306 |
| Mechanical ventilation | 4 (12.1%) | 3 (9.4%) | 5 (12.5%) | 7 (11.9%) | 0.909 |
| Peritoneal contamination | 3 (9.1%) | 4 (12.5%) | 4 (10.0%) | 5 (8.5%) | 0.828 |
| Ischemia of resection margins | 2 (6.1%) | 1 (3.1%) | 2 (5.0%) | 2 (3.4%) | 0.290 |
| Reintervention | 2 (6.1%) | 3 (9.4%) | 3 (7.5%) | 2 (3.4%) | 0.475 |
| Clavien–Dindo score | 0.704 | ||||
| I | 8 (24.2%) | 7 (21.9%) | 6 (15.0%) | 19 (32.2%) | |
| II | 11 (33.3%) | 10 (31.3%) | 9 (22.5%) | 22 (37.3%) | |
| III | 8 (24.2%) | 9 (28.1%) | 17 (42.5%) | 12 (20.3%) | |
| IV | 4 (12.1%) | 4 (12.5%) | 5 (12.5%) | 4 (6.8%) | |
| V | 2 (6.1%) | 2 (6.3%) | 3 (7.5%) | 2 (3.4%) | |
| ICU admissions | 4 (12.1%) | 5 (15.6%) | 6 (15.0%) | 4 (6.8%) | 0.314 |
| Mortality rate | 2 (6.1%) | 3 (9.4%) | 3 (7.5%) | 2 (3.4%) | 0.474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braicu, V.; Fulger, L.; Nelluri, A.; Maganti, R.K.; Shetty, U.S.A.; Verdes, G.; Brebu, D.; Dumitru, C.; Toma, A.-O.; Rosca, O.; et al. Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic. Diseases 2023, 11, 181. https://doi.org/10.3390/diseases11040181
Braicu V, Fulger L, Nelluri A, Maganti RK, Shetty USA, Verdes G, Brebu D, Dumitru C, Toma A-O, Rosca O, et al. Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic. Diseases. 2023; 11(4):181. https://doi.org/10.3390/diseases11040181
Chicago/Turabian StyleBraicu, Vlad, Lazar Fulger, Aditya Nelluri, Ram Kiran Maganti, Uday Shree Akkala Shetty, Gabriel Verdes, Dan Brebu, Catalin Dumitru, Ana-Olivia Toma, Ovidiu Rosca, and et al. 2023. "Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic" Diseases 11, no. 4: 181. https://doi.org/10.3390/diseases11040181
APA StyleBraicu, V., Fulger, L., Nelluri, A., Maganti, R. K., Shetty, U. S. A., Verdes, G., Brebu, D., Dumitru, C., Toma, A.-O., Rosca, O., & Duta, C. (2023). Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic. Diseases, 11(4), 181. https://doi.org/10.3390/diseases11040181






