Examining the Effects of Dasatinib, Sorafenib, and Nilotinib on Vascular Smooth Muscle Cells: Insights into Proliferation, Migration, and Gene Expression Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Vascular Smooth Muscles
2.3. Cell Culture and Experimental Design
2.4. Cell Viability Assay
2.5. Scratch Assay
2.6. Flow Cytometry Analysis of Apoptosis
2.7. RNA Isolation and Gene Expression
2.8. Statistical Analysis & Graphs
3. Results
3.1. Cell Viability
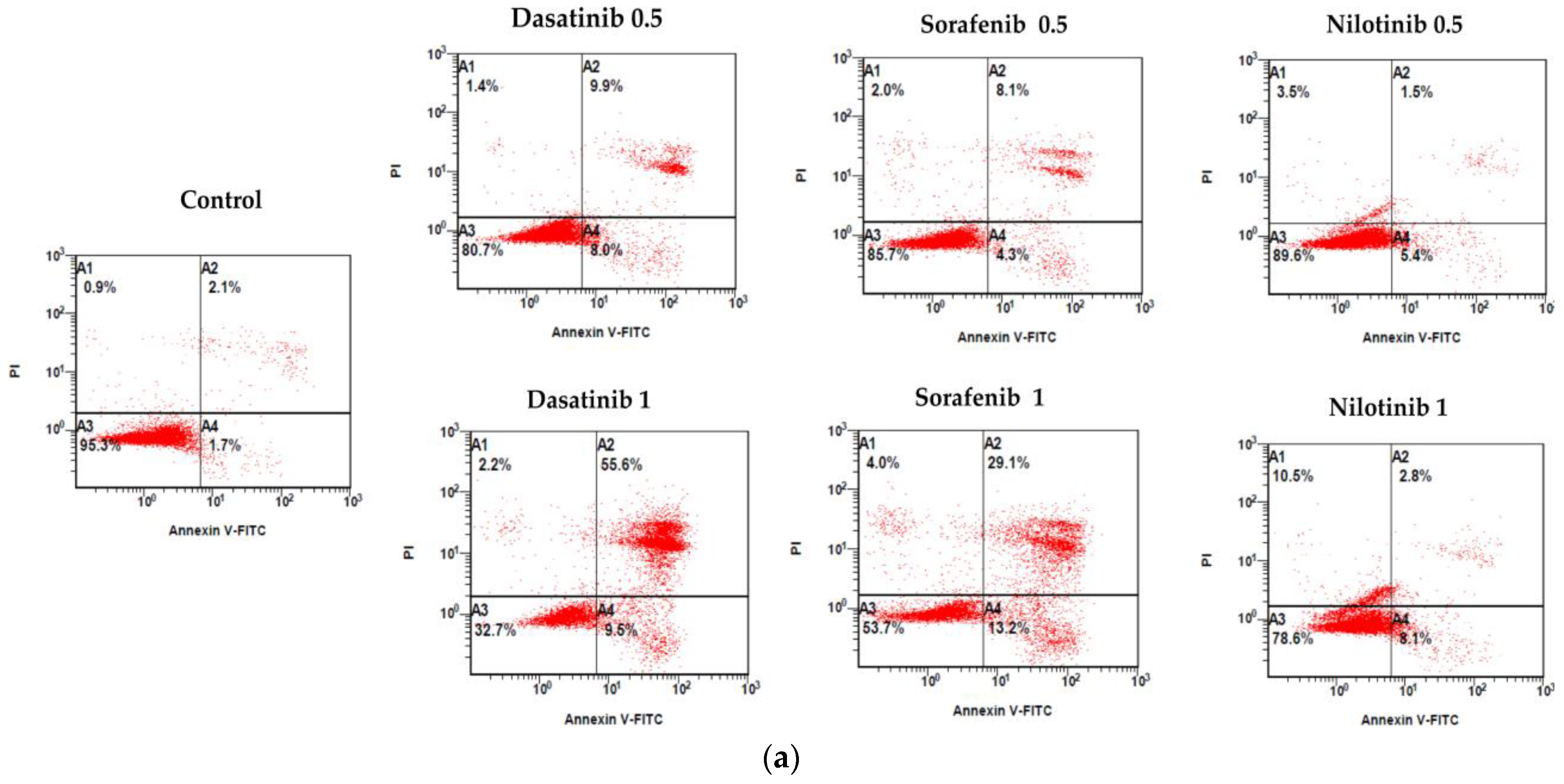
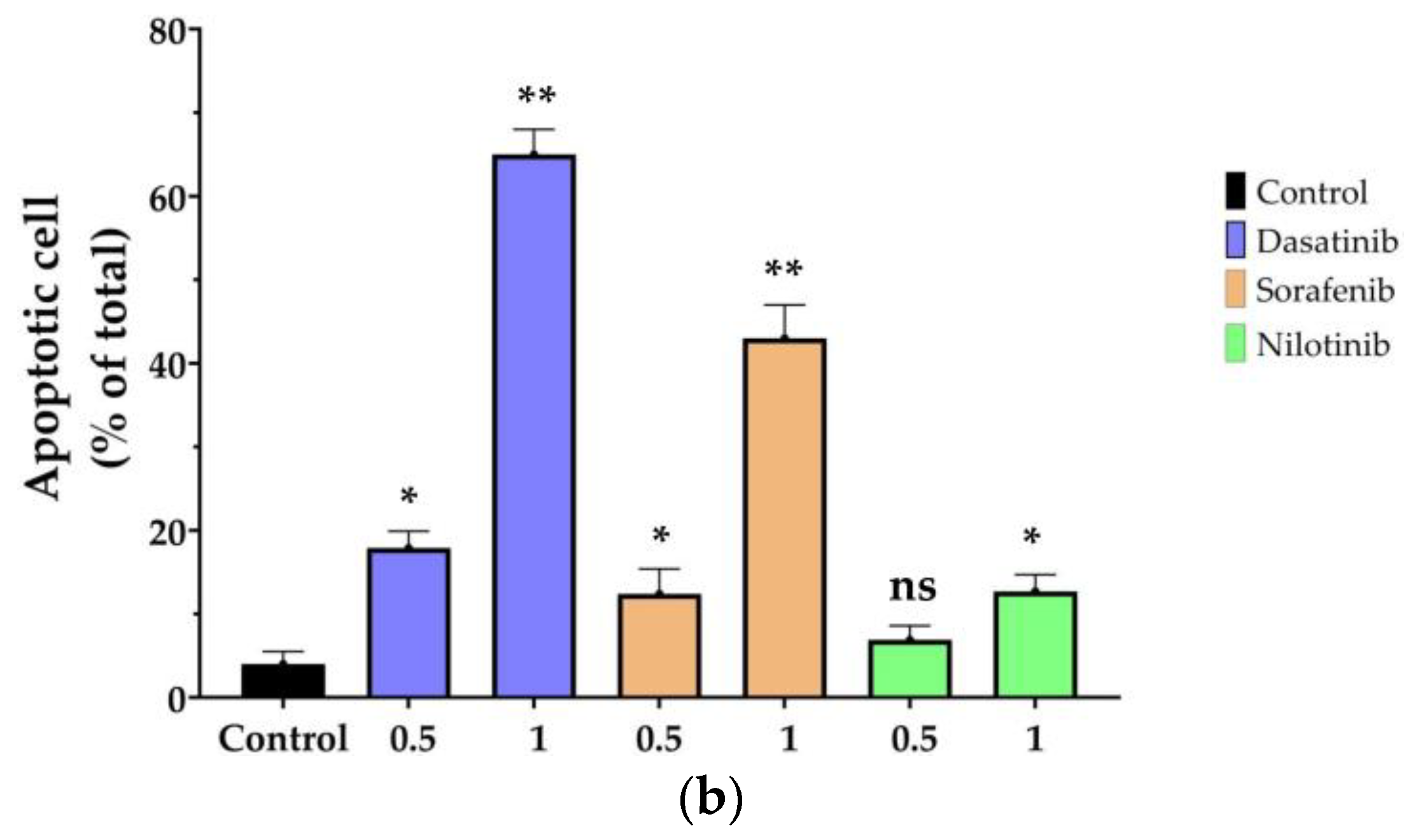
3.2. Cell Apoptosis
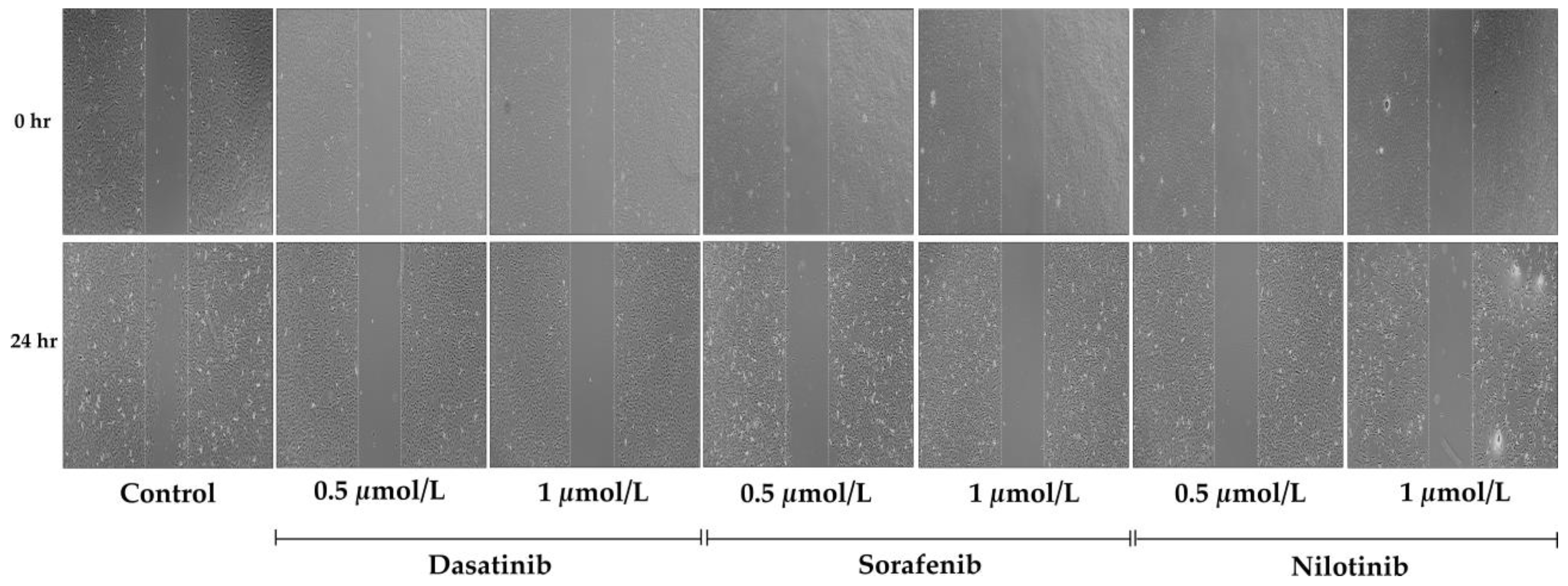
3.3. Cellular Migration Assay
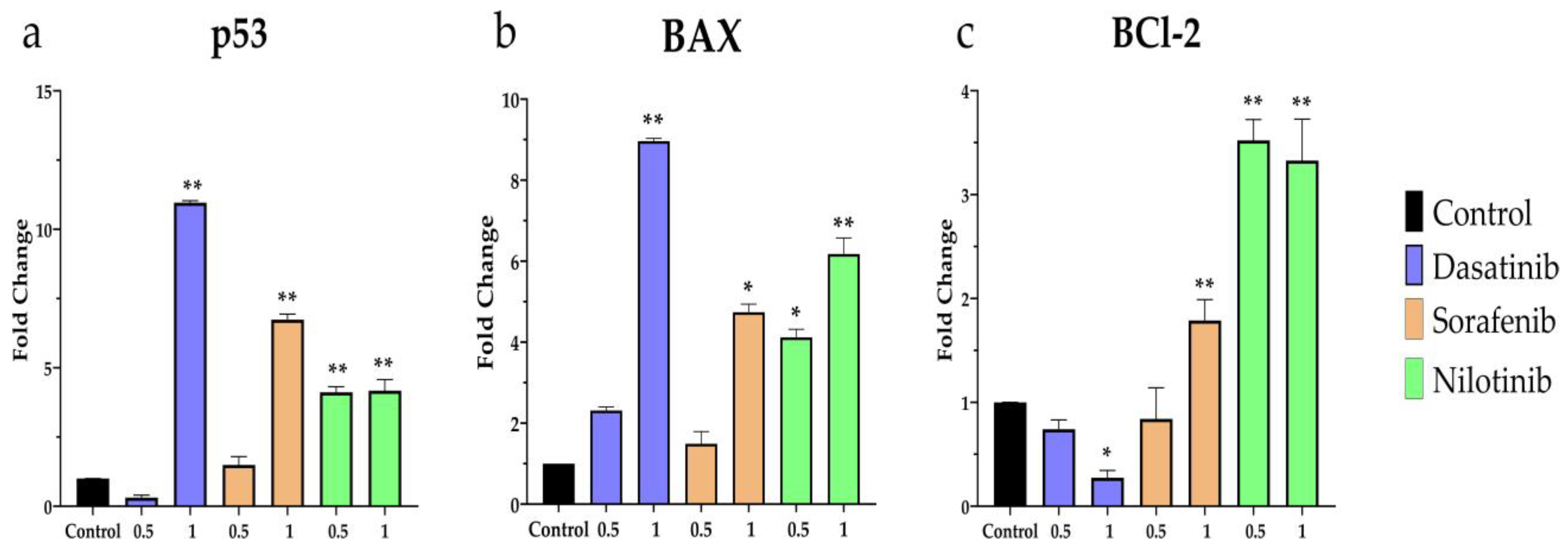
3.4. Apoptosis-Related Gene Expression
3.5. Inflammation-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Force, T.; Krause, D.S.; Van Etten, R.A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 2007, 7, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Hafen, B.B.; Burns, B. Physiology, Smooth Muscle. 2023. Available online: https://europepmc.org/article/NBK/nbk526125 (accessed on 19 October 2023).
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Uraizee, I.; Cheng, S.; Moslehi, J. Reversible Cardiomyopathy Associated with Sunitinib and Sorafenib. N. Engl. J. Med. 2011, 365, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Naib, T.; Steingart, R.M.; Chen, C.L. Sorafenib-associated multivessel coronary artery vasospasm. Herz 2011, 36, 348–351. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Mandrioli, A.; Saponara, M.; Nannini, M.; Erente, G.; Lolli, C.; Biasco, G. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer 2012, 12, 231. [Google Scholar] [CrossRef]
- Hermel, D.J.; Chiu, V.; Hermel, M.H.; Tulpule, A.; Akhtari, M. Cardiac birth defects in a twin infant born to a woman with chronic myeloid leukemia on dasatinib. J. Oncol. Pharm. Pract. 2019, 25, 699–702. [Google Scholar] [CrossRef]
- Chaar, M.; Kamta, J.; Ait-Oudhia, S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco. Targets Ther. 2018, 11, 6227–6237. [Google Scholar] [CrossRef]
- Le Coutre, P.; Rea, D.; Abruzzese, E.; Dombret, H.; Trawinska, M.M.; Herndlhofer, S.; Dorken, B.; Valent, P. Severe Peripheral Arterial Disease During Nilotinib Therapy. JNCI J. Natl. Cancer Inst. 2011, 103, 1347–1348. [Google Scholar] [CrossRef]
- García-Lledó, J.; Cortejoso, L.; Tenorio Núñez, M.; Giménez-Manzorro, Á.; Matilla-Peña, A.; Salcedo-Plaza, M.; Sanjurjo-Sáez, M. Cardiovascular Toxicity and Sorafenib. Am. J. Ther. 2014, 21, e169–e170. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Bojic, M.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac Toxicity of Sunitinib and Sorafenib in Patients with Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.Z.; Clark, M.A. Angiotensin III Induces JAK2/STAT3 Leading to IL-6 Production in Rat Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2019, 20, 5551. [Google Scholar] [CrossRef]
- Alaseem, A.M.; Alhazzani, K.; Alanazi, A.Z.; Alqarni, Y.; Algahtani, M.M.; Alhamed, A.S.; Alasiri, G.; Alotaibi, F.T.; Jawaid, T.; Aldali, J.A. Preclinical In Vitro Investigation of MDM2 Inhibition in Combination with Antiangiogenic Therapy for Breast Cancer Treatment. Sci. Pharm. 2023, 91, 12. [Google Scholar] [CrossRef]
- Alanazi, A.Z.; Alhazzani, K.; Alrewily, S.Q.; Aljerian, K.; Algahtani, M.M.; Alqahtani, Q.H.; Haspula, D.; Alhamed, A.S.; Alqinyah, M.; Raish, M. The Potential Protective Role of Naringenin against Dasatinib-Induced Hepatotoxicity. Pharmaceuticals 2023, 16, 921. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Deininger, M.; Buchdunger, E.; Druker, B.J. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005, 105, 2640–2653. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib Blocks the RAF/MEK/ERK Pathway, Inhibits Tumor Angiogenesis, and Induces Tumor Cell Apoptosis in Hepatocellular Carcinoma Model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Bian, Z.-Y.; Fan, Q.-M.; Li, G.; Xu, W.-T.; Tang, T.-T. Human mesenchymal stem cells promote growth of osteosarcoma: Involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci 2010, 101, 2554–2560. [Google Scholar] [CrossRef]
- Xia, W.; Mullin, R.J.; Keith, B.R.; Liu, L.-H.; Ma, H.; Rusnak, D.W.; Owens, G.; Alligood, K.J.; Spector, N.L. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002, 21, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, S.; Qiu, Y.; Zhang, J.; Rios, F.J.; Zou, Z.; Touyz, R.M. Cardiovascular toxicity of tyrosine kinase inhibitors during cancer treatment: Potential involvement of TRPM7. Front. Cardiovasc. Med. 2023, 10, 1002438. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Beg, A.A.; Baltimore, D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Villar-Fincheira, P.; Sanhueza-Olivares, F.; Norambuena-Soto, I.; Cancino-Arenas, N.; Hernandez-Vargas, F.; Troncoso, R.; Gabrielli, L.; Chiong, M. Role of Interleukin-6 in Vascular Health and Disease. Front. Mol. Biosci. 2021, 8, 641734. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Cleary, M.; Charo, I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394, 894–897. [Google Scholar] [CrossRef]
- Bennett, M.R.; Evan, G.I.; Schwartz, S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Investig. 1995, 95, 2266–2274. [Google Scholar] [CrossRef]
- Romano, M.; Sironi, M.; Toniatti, C.; Polentarutti, N.; Fruscella, P.; Ghezzi, P.; Faggioni, R.; Luini, W.; van Hinsbergh, V.; Sozzani, S.; et al. Role of IL-6 and Its Soluble Receptor in Induction of Chemokines and Leukocyte Recruitment. Immunity 1997, 6, 315–325. [Google Scholar] [CrossRef]
- Schieffer, B.; Schieffer, E.; Hilfiker-Kleiner, D.; Hilfiker, A.; Kovanen, P.T.; Kaartinen, M.; Nussberger, J.; Harringer, W.; Drexler, H. Expression of Angiotensin II and Interleukin 6 in Human Coronary Atherosclerotic Plaques. Circulation 2000, 101, 1372–1378. [Google Scholar] [CrossRef]
- Grote, K.; Luchtefeld, M.; Schieffer, B. JANUS under stress—Role of JAK/STAT signaling pathway in vascular diseases. Vascul. Pharmacol. 2005, 43, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Delombaerde, D.; De Sutter, J.; Croes, L.; Vervloet, D.; Moerman, V.; Van de Veire, N.; Willems, A.-M.; Wouters, K.; Peeters, M.; Prenen, H.; et al. Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study. Pharmaceuticals 2023, 16, 625. [Google Scholar] [CrossRef] [PubMed]


| Gene | 5′-Forward Sequence-3′ | 5′-Reverse Sequence-3′ |
|---|---|---|
| P53 | ACATGACTGAGGTCGTGAGA | GATTTCCTTCCACCCGGATAAG |
| BAX | GATGGCCTCCTTTCCTACTTC | CTTCTTCCAGATGGTGAGTGAG |
| BCL-2 | GGAGGATTGTGGCCT TCT TT | GTGAGCTGAGTGGAGAAGAAG |
| TNF-α | TCCTTCAGACACCCTCAACC | AGGCCCCAGTTTGAATTCTT |
| IL-6 | TCTGGAGTTCCGTTTCTACCTGG | CATAGCACACTAGGTTTGCCGAG |
| IL-1β | CTATGGCAACTG TCCCTGAA | GGCTTGGAAGCAATCCTTAATC |
| GAPDH | GTATTGGGCGCCTGGTCACC | CGCTCCTGGAAGATGGTGATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazzani, K.; Almangour, A.; Alsalem, A.; Alqinyah, M.; Alhamed, A.S.; Alhamami, H.N.; Alanazi, A.Z. Examining the Effects of Dasatinib, Sorafenib, and Nilotinib on Vascular Smooth Muscle Cells: Insights into Proliferation, Migration, and Gene Expression Dynamics. Diseases 2023, 11, 147. https://doi.org/10.3390/diseases11040147
Alhazzani K, Almangour A, Alsalem A, Alqinyah M, Alhamed AS, Alhamami HN, Alanazi AZ. Examining the Effects of Dasatinib, Sorafenib, and Nilotinib on Vascular Smooth Muscle Cells: Insights into Proliferation, Migration, and Gene Expression Dynamics. Diseases. 2023; 11(4):147. https://doi.org/10.3390/diseases11040147
Chicago/Turabian StyleAlhazzani, Khalid, Abdullah Almangour, Abdulaziz Alsalem, Mohammed Alqinyah, Abdullah S. Alhamed, Hussain N. Alhamami, and Ahmed Z. Alanazi. 2023. "Examining the Effects of Dasatinib, Sorafenib, and Nilotinib on Vascular Smooth Muscle Cells: Insights into Proliferation, Migration, and Gene Expression Dynamics" Diseases 11, no. 4: 147. https://doi.org/10.3390/diseases11040147
APA StyleAlhazzani, K., Almangour, A., Alsalem, A., Alqinyah, M., Alhamed, A. S., Alhamami, H. N., & Alanazi, A. Z. (2023). Examining the Effects of Dasatinib, Sorafenib, and Nilotinib on Vascular Smooth Muscle Cells: Insights into Proliferation, Migration, and Gene Expression Dynamics. Diseases, 11(4), 147. https://doi.org/10.3390/diseases11040147







