Comparing Efficacy of Erlotinib and Bevacizumab Combination with Erlotinib Monotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Bottom of Form Statistical Analysis
3. Results
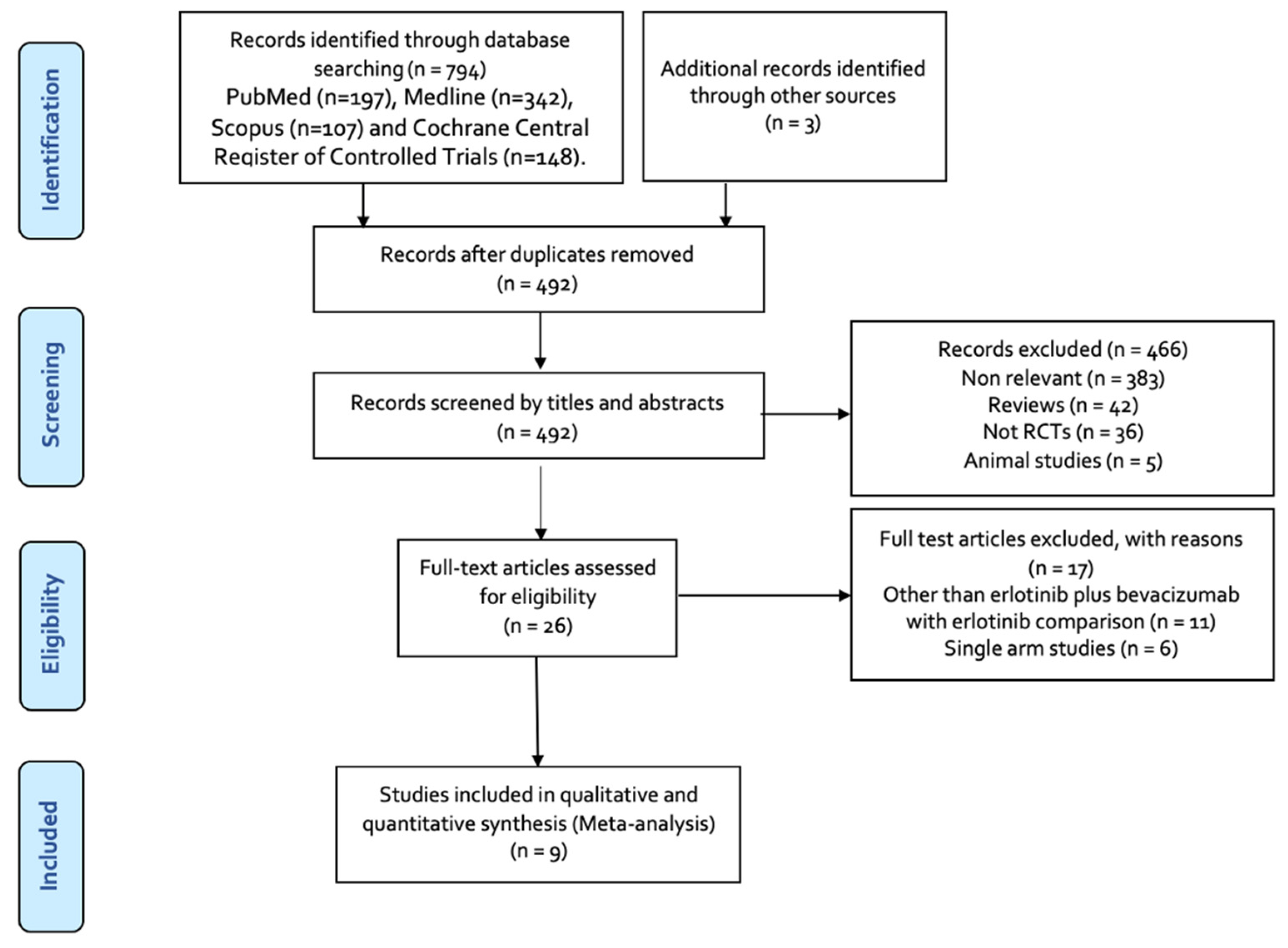
3.1. Results of the Literature Search
3.2. Characteristics of the Included Studies
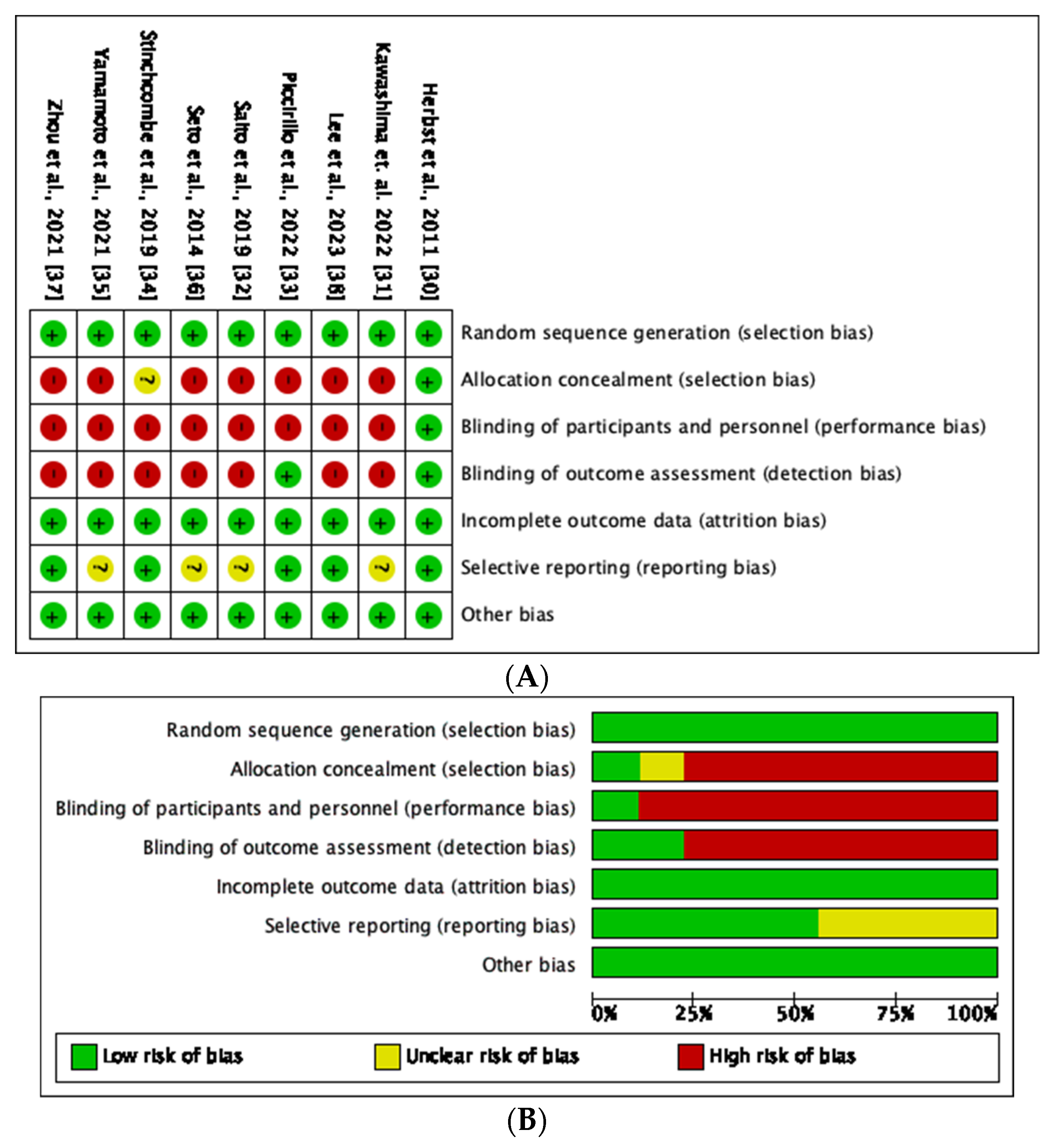
3.3. Risk of Bias and Quality Assessment
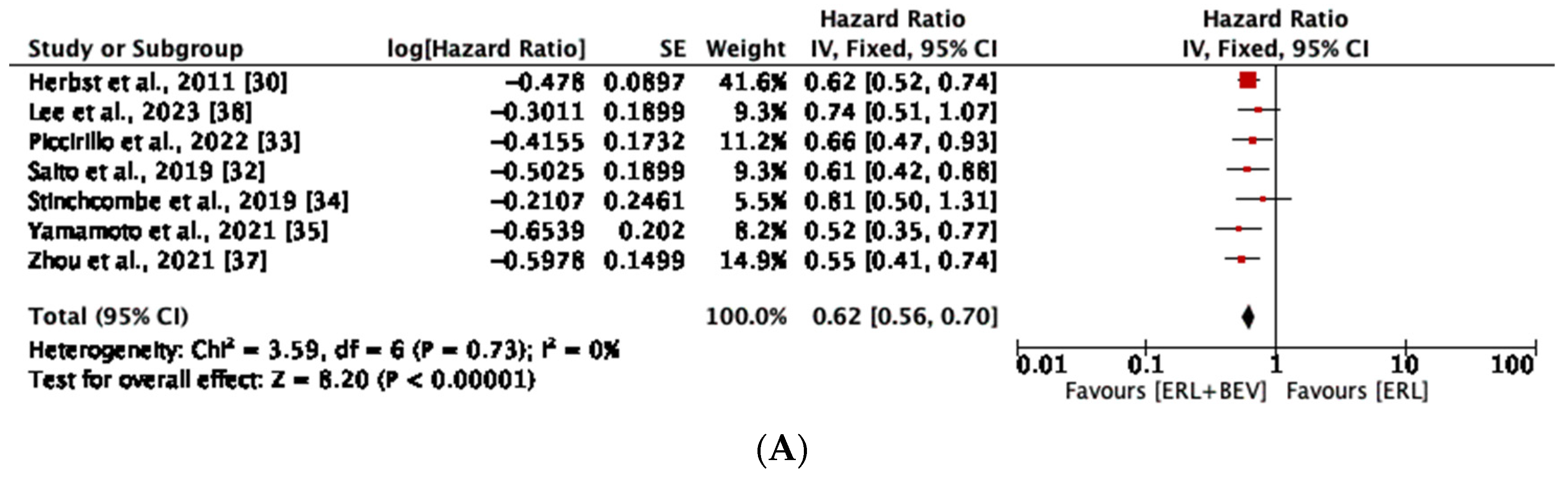
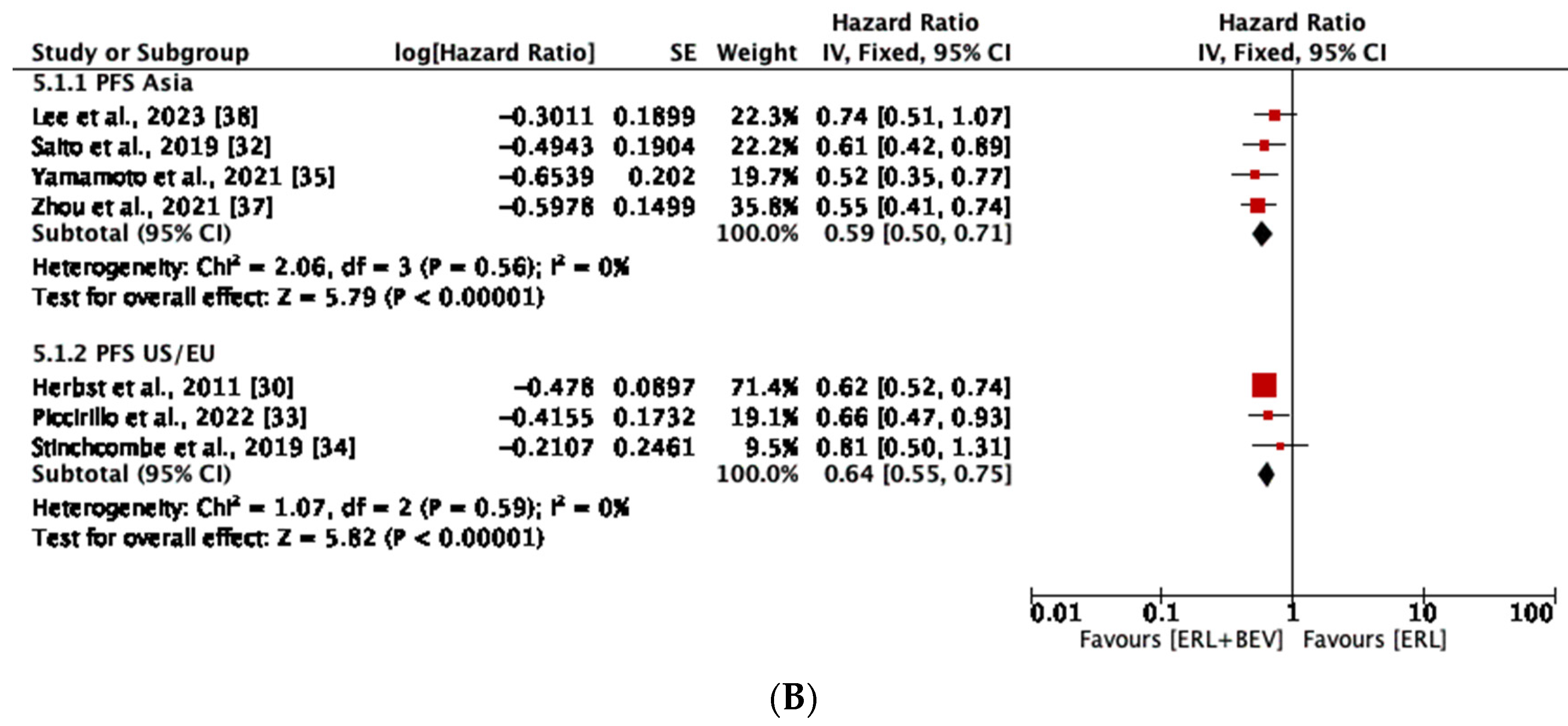
3.4. Progression-Free Survival
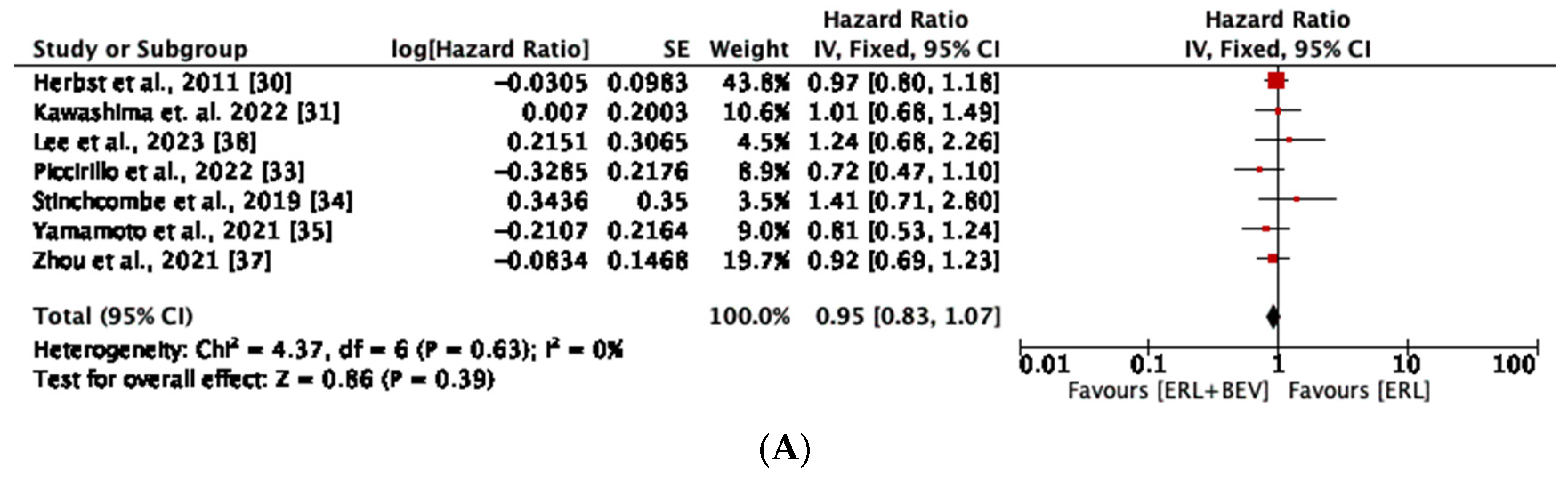
3.5. Overall Survival
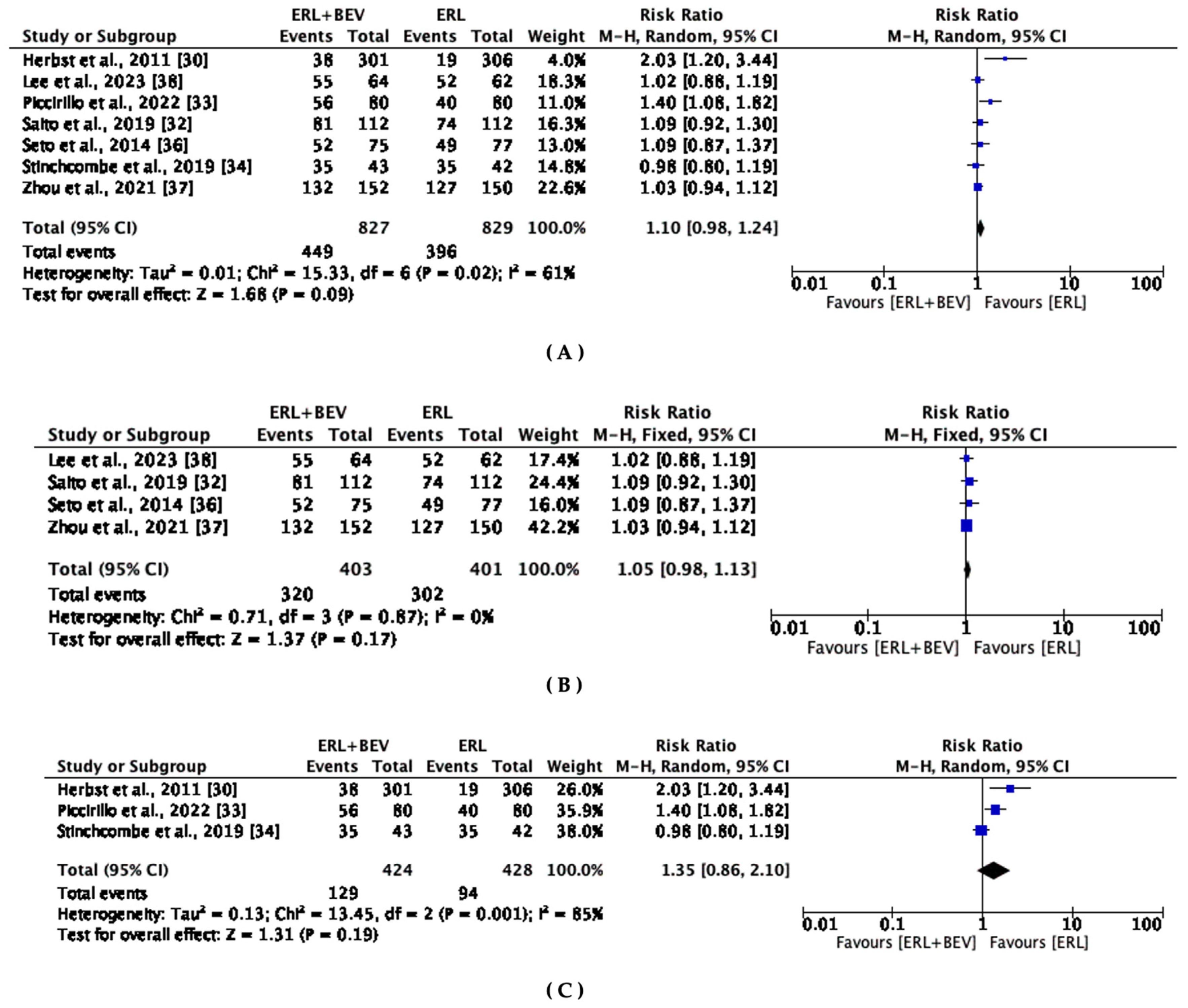
3.6. Objective Response Rate
3.7. Adverse Events
3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 12 April 2023).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 730. [Google Scholar] [CrossRef] [PubMed]
- Kuan, F.C.; Kuo, L.T.; Chen, M.C.; Yang, C.T.; Shi, C.S.; Teng, D.; Lee, K.-D. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: A systematic review and meta-analysis. Br. J. Cancer 2015, 113, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Davies, L.; Wu, Y.L.; Mitsudomi, T.; Inoue, A.; Rosell, R.; Zhou, C.; Nakagawa, K.; Thongprasert, S.; Fukuoka, M.; et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. J. Natl. Cancer Inst. 2017, 109, djw279. [Google Scholar] [CrossRef]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v1–v27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Y.; Li, X.; Zhao, C.; Chen, X.; Su, C.; Ren, S.; Yang, N.; Zhou, C. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs monotherapy in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur. J. Cancer 2019, 121, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Lester, J.F. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Clash of the Generations. Clin. Lung Cancer 2020, 21, e216–e228. [Google Scholar] [CrossRef]
- Castellanos, E.; Feld, E.; Horn, L. Driven by mutations: The predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 612–623. [Google Scholar] [CrossRef]
- Lima, A.B.; Macedo, L.T.; Sasse, A.D. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2011, 6, e22681. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Liu, X.; Zhu, Y.; Lu, S.; Feng, J.; He, J.; Han, B.; Wang, J.; et al. BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent non-squamous non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2197–2204. [Google Scholar] [CrossRef]
- Rolff, J.; Becker, M.; Merk, J.; Hoffmann, J.; Fichtner, I. Preclinical Study of a Combination of Erlotinib and Bevacizumab in Early Stages of Unselected Non-Small Cell Lung Cancer Patient-Derived Xenografts. Target. Oncol. 2016, 11, 507–514. [Google Scholar] [CrossRef]
- Li, H.; Takayama, K.; Wang, S.; Shiraishi, Y.; Gotanda, K.; Harada, T.; Furuyama, K.; Iwama, E.; Ieiri, I.; Okamoto, I.; et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother. Pharmacol. 2014, 74, 1297–1305. [Google Scholar] [CrossRef]
- Rosell, R.; Dafni, U.; Felip, E.; Curioni-Fontecedro, A.; Gautschi, O.; Peters, S.; Massuti, B.; Palmero, R.; Aix, S.P.; Carcereny, E.; et al. Erlotinib and bevacizumab in patients with advanced non-small- cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med. 2017, 5, 435–444. [Google Scholar] [CrossRef]
- Wang, Y.; Schmid-Bindert, G.; Zhou, C. Erlotinib in the treatment of advanced non-small cell lung cancer: An update for clinicians. Ther. Adv. Med. Oncol. 2012, 4, 19–29. [Google Scholar] [CrossRef]
- Kenmotsu, H.; Wakuda, K.; Mori, K.; Kato, T.; Sugawara, S.; Kirita, K.; Yoneshima, Y.; Azuma, K.; Nishino, K.; Teraoka, S.; et al. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients with Nonsquamous NSCLC Harboring EGFR Mutations: WJOG9717L Study. J. Thorac. Oncol. 2022, 17, 1098–1108. [Google Scholar] [CrossRef]
- Akamatsu, H.; Toi, Y.; Hayashi, H.; Fujimoto, D.; Tachihara, M.; Furuya, N.; Otani, S.; Shimizu, J.; Katakami, N.; Azuma, K.; et al. Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients with EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated with Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.A.; Han, J.Y.; Dafni, U.; Cho, B.C.; Yeo, C.M.; Nadal, E.; Carcereny, E.; de Castro, J.; Sala, M.A.; Bernabé, R.; et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: The European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann. Oncol. 2022, 33, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Johnson, D.H.; Mininberg, E.; Carbone, D.P.; Henderson, T.; Kim, E.S.; Blumenschein, G.; Lee, J.J.; Liu, D.D.; Truong, M.T.; et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 2544–2555. [Google Scholar] [PubMed]
- Dingemans, A.M.; de Langen, A.D.; den Boogart, V.V.; Marcus, J.; Backes, W.; Scholten, H.; Tinteren, H.V.; Hoekstra, O.S.; Pruim, J.; Brans, B.; et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: A phase II study including molecular imaging. Ann. Oncol. 2010, 22, 559–566. [Google Scholar] [CrossRef]
- Zappa, F.; Droege, C.; Betticher, D.; von Moos, R.; Bubendorf, L.; Ochsenbein, A.; Gautschi, O.; Leibundgut, E.O.; Froesch, P.; Stahel, R.; et al. Bevacizumab and erlotinib (BE) first-line therapy in advanced non-squamous non-small-cell lung cancer (NSCLC) (stage IIIB/IV) followed by platinum-based chemotherapy (CT) at disease progression: A multicenter phase II trial (SAKK 19/05). Lung Cancer 2012, 78, 239–244. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, S.; Wu, Y.; Shi, L.; Xu, X.; Shen, B. Efficacy and safety of first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) combined with chemotherapy or antiangiogenic therapy as first-line treatment in patients with EGFR-mutant non-small cell lung cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103393. [Google Scholar] [CrossRef]
- Chen, F.; Chen, N.; Yu, Y.; Cui, J. Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients with Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 904. [Google Scholar] [CrossRef]
- Deng, W.; Wang, K.; Jiang, Y.; Li, D.; Bao, C.; Luo, J.; Liu, L.; Huang, B.; Kong, J. Erlotinib plus bevacizumab versus erlotinib monotherapy in patients with EGFR-positive advanced non-small-cell lung cancer: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e062036. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Aki, E.; Brennen, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kanters, S. Fixed- and Random-Effects Models. Methods Mol. Biol. 2022, 2345, 41–65. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Chu, H.; Lin, L.; Wang, Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid. Based Med. 2018, 23, 84–86. [Google Scholar] [CrossRef]
- Herbst, R.S.; Ansari, R.; Bustin, F.; Flynn, P.; Hart, L.; Otterson, G.A.; Vlahovic, G.; Soh, C.H.; O’Connor, P.; Hainsworth, J. Efficacy of bevacizumab plus erlotinib versus erlotinib monotherapy in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1846–1854. [Google Scholar] [CrossRef]
- Kawashima, Y.; Fukuhara, T.; Saito, H.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; et al. Bevacizumab plus erlotinib versus erlotinib monotherapy in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): Overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir. Med. 2022, 10, 72–82. [Google Scholar] [CrossRef]
- Saito, H.; Fukuhara, T.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; Yoshimori, K.; et al. Erlotinib plus bevacizumab versus erlotinib monotherapy in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019, 20, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, M.C.; Bonanno, L.; Garassino, M.C.; Esposito, G.; Dazzi, C.; Cavanna, L.; Burgio, M.A.; Roseti, F.; Rizzato, S.; Morgillo, F.; et al. Addition of Bevacizumab to Erlotinib as First-Line Treatment of Patients With EGFR-Mutated Advanced Nonsquamous NSCLC: The BEVERLY Multicenter Randomized Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 1086–1097. [Google Scholar] [CrossRef]
- Stinchcombe, T.E.; Jänne, P.A.; Wang, X.; Bertino, E.M.; Weiss, J.; Bazhenova, L.; Gu, L.; Lau, C.; Pawaletz, C.; Jaslowski, A.; et al. Effect of Erlotinib Plus Bevacizumab vs Erlotinib Monotherapy on Progression-Free Survival in Patients with Advanced EGFR-Mutant Non-Small Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Seto, T.; Nishio, M.; Goto, K.; Yamamoto, N.; Okamoto, I.; Yamanaka, T.; Tanaka, M.; Takahashi, K.; Fukuoka, M. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: Survival follow-up results of the randomized JO25567 study. Lung Cancer 2021, 151, 20–24. [Google Scholar] [CrossRef]
- Seto, T.; Kato, T.; Nishio, M.; Goto, K.; Atagi, S.; Hosomi, Y.; Yamamoto, N.; Hida, T.; Maemondo, M.; Nakagawa, K.; et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014, 15, 1236–1244. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.R.; Cheng, Y.; Liu, Y.P.; Chen, G.Y.; Cui, J.W.; Yang, N.; Song, Y.; Li, X.-L.; Lu, S. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell 2021, 39, 1279–1291.e3. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.R.; Hong, M.H.; Lee, K.H.; Park, K.U.; Lee, G.K.; Kin, H.Y.; Lee, S.H.; Lim, K.Y.; Yoon, S.G.; et al. A randomized Phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with EGFR mutation. Cancer 2023, 129, 405–414. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol. Res. 2021, 167, 105583. [Google Scholar] [CrossRef]
- Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, R.; Daneille, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. [Google Scholar] [CrossRef] [PubMed]
- Shukuya, T.; Mori, K.; Amann, J.M.; Bertino, E.M.; Otterson, G.A.; Shields, P.G.; Morita, S.; Carbone, D. Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies. J. Thorac. Oncol. 2016, 11, 1927–1939. [Google Scholar] [CrossRef]
- Harris, S.J.; Brown, J.; Lopez, J.; Yap, T.A. Immuno-oncology combinations: Raising the tail of the survival curve. Cancer Biol. Med. 2016, 13, 171–193. [Google Scholar] [CrossRef]
- Inno, A.; Lo Russo, G.; Salgarello, M.; Corrao, G.; Casolino, R.; Galli, G.; Modena, A.; Romano, L.; Pusceddu, S.; Greco, F.G.; et al. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori J. 2018, 104, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Duran, R.; Bai, W.; Sahu, S.; Wang, W.; Kabus, S.; Lin, M.; Han, G.; Geschwind, J.F. Which Criteria Applied in Multi-Phasic CT Can Predict Early Tumor Response in Patients with Hepatocellular Carcinoma Treated Using Conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc. Interv. Radiol. 2018, 41, 433–442. [Google Scholar] [CrossRef]
- Brandes, A.A.; Bartolotti, M.; Tosoni, A.; Poggi, R.; Franceschi, E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist 2015, 20, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Maitland, M.L.; Bakris, G.L.; Black, H.R.; Chen, H.X.; Durand, J.B.; Elliott, W.J.; Ivy, S.P.; Leier, C.V.; Lindenfeld, J.; Liu, G.; et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J. Natl. Cancer Inst. 2010, 102, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Latcha, S.; Jaimes, E.A.; Gutgarts, V.; Seshan, S. Case of Proteinuria, Worsening Hypertension, and Glomerular Endotheliosis with Erlotinib and Gefitinib. Kidney Int. Rep. 2018, 3, 1477–1481. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mitsudomi, T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016, 107, 1179–1186. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Liu, J.; Yang, C.; Zhang, L.; Bao, H.; Yeng, Y. Comparative Efficacy and Safety of TKIs alone or in Combination with Antiangiogenic Agents in Advanced EGFR-Mutated NSCLC as the First-Line Treatment: A Systematic Review and Meta-Analysis. Clin. Lung Cancer 2022, 23, 159–169. [Google Scholar] [CrossRef]
- Landre, T.; Des Guetz, G.; Chouahnia, K.; Duchemann, B.; Assié, J.B.; Chouaid, C. First-line angiogenesis inhibitor plus erlotinib versus erlotinib monotherapy for advanced non-small-cell lung cancer harboring an EGFR mutation. J. Cancer Res. Clin. Oncol. 2020, 146, 3333–3339. [Google Scholar] [CrossRef]

| Author, Year | Trial Name | Patients (N) | Phase | Study Region | Clinical Stage | Line of Treatment | Patients in (ERL + BEV) Group (n) | Patients in (ERL) Group (n) | Age (yrs.) (Median) | Female (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Herbst et al., 2011 [30] | BeTa | 636 | III | US | I–IV | Second line | 319 | 317 | 65 | 46 | PFS, OS, ORR |
| Kawashima et. al., 2022 [31] (a) | NEJ026 | 224 | III | Japan | IIIb–IV | First-line | 112 | 112 | 67 | 64 | OS |
| Saito et al., 2019 [32] (a) | NEJ026 | 224 | III | Japan | IIIb–IV | First-line | 112 | 112 | 67 | 64 | PFS, ORR |
| Piccirillo et al., 2022 [33] | BEVERLY | 160 | III | Italy | IV | First-line | 80 | 80 | 67 | 62.5 | PFS, OS, ORR |
| Stinchcombe et al., 2019 [34] | NCT01532089 | 88 | II | US | IV | First-line | 43 | 45 | 63 | 70 | PFS, OS, ORR |
| Yamamoto et al., 2021 [35] (b) | JO25567 | 152 | II | Japan | IIIb–IV | First-line | 75 | 77 | 67 | 63 | PFS, OS |
| Seto et al., 2014 [36] (b) | JO25567 | 152 | II | Japan | IIIb–IV | First-line | 75 | 77 | 67 | 63 | ORR |
| Zhou et al., 2021 [37] | ARTEMIS CTOG1509 | 311 | III | China | IIIb–IV | First-line | 157 | 154 | 58 | 62 | PFS, OS, ORR |
| Lee et al., 2023 [38] | NCT03126799 | 127 | II | Republic of Korea | IIIb–IV | First-line | 64 | 63 | 63 | 66.1 | PFS, OS, ORR |
| Author, Year | ORR: ERL + BEV (n) | ERL + BEV (N) | ORR: ERL (n) | ERL (N) | ORR—p-Value | PFS: ERL + BEV (Months) | PFS: ERL (Months) | PFS–HR, (95% CI) | OS: ERL + BEV (Months) | OS: ERL (Months) | OS–HR, (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Herbst et al., 2011 [30] | 38 | 301 | 19 | 306 | 3.4 | 1.7 | 0.62 (0.52, 0.75) | 50.7 | 46.2 | 0.97 (0.80, 1.18) | |
| Kawashima et. al., 2022 [31] (a) | 1.007 (0.68, 1.49) | ||||||||||
| Saito et al., 2019 [32] (a) | 81 | 112 | 74 | 112 | 0.31 | 16.9 | 13.3 | 0.61 (0.42, 0.88) | |||
| Piccirillo et al., 2022 [33] | 56 | 80 | 40 | 80 | 0.01 | 15.4 | 9.6 | 0.66 (0.47, 0.92) | 33.3 | 22.8 | 0.72 (0.47, 1.10) |
| Stinchcombe et al., 2019 [34] | 35 | 43 | 35 | 42 | 0.81 | 17.9 | 13.5 | 0.81 (0.50, 1.31) | 32.4 | 50.6 | 1.41 (0.71, 2.81) |
| Yamamoto et al., 2021 [35] (b) | 16.4 | 9.8 | 0.52 (0.35, 0.76) | 47 | 47.4 | 0.81 (0.53, 1.23) | |||||
| Seto et al., 2014 [36] (b) | 52 | 75 | 49 | 77 | 0.49 | ||||||
| Zhou et al., 2021 [37] | 132 | 152 | 127 | 150 | 0.56 | 17.9 | 11.2 | 0.55 (0.41, 0.73) | 36.2 | 31.6 | 0.92 (0.69, 1.23) |
| Lee et al. 2023 [38] | 55 | 64 | 52 | 62 | 0.476 | 17.5 | 12.4 | 0.74 (0.51, 1.08) | 1.24 (0.68, 2.26) |
| Author, Year | AE ≥ G3 (ERL + BEV) | AE ≥ G3 (ERL) | Skin Rash (ERL + BEV) | Rash (ERL) | HTN (ERL + BEV) | HTN (ERL) | Proteinuria (ERL + BEV) | Proteinuria (ERL) | Diarrhea (ERL + BEV) | Diarrhea (ERL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Herbst et al., 2011 [30] | 208 (66%) | 165 (53%) | 49 (16%) | 19 (6%) | 15 (5%) | 4 (1%) | ||||
| Saito et al., 2019 [32] | 98 (88%) | 53 (46%) | 23 (21%) | 24 (21%) | 26 (23%) | 1 (1%) | 8 (7%) | 1 (1%) | 6 (5%) | 2 (2%) |
| Piccirillo et al., 2022 [33] | 45 (56%) | 39 (49%) | 73 (92%) | 70 (88%) | 19 (24%) | 4 (5%) | 5 (6%) | 1 (1%) | 4 (5%) | 3 (4%) |
| Stinchcombe et al., 2019 [34] | 26 (60%) | 15 (33%) | 11 (26%) | 7 (16%) | 17 (40%) | 9 (20%) | 5 (12%) | 0 (0%) | 4 (9%) | 6 (13%) |
| Seto et al., 2014 [36] | 71 (95%) | 24 (31%) | 19 (25%) | 15 (19%) | 45 (60%) | 8 (10%) | 6 (8%) | 0 (0%) | 1 (1%) | 1 (1%) |
| Zhou et al., 2021 [37] | 63 (40%) | 15 (9%) | 8 (5%) | 5 (3%) | 29 (19%) | 5 (3%) | 11 (7%) | 0 (0%) | 4 (3%) | 0 (0%) |
| Lee et al., 2023 [38] | 29 (45%) | 6 (9.5%) | 11 (17.2%) | 3 (4.8%) | 9 (14.1%) | 0 (0%) | 5 (7.8%) | 0 (0%) | 4 (6.3%) | 3 (4.8%) |
| Adverse Events | ERL + BEV Event/Total | ERL Event/Total | RR (95%CI) | p-Value | Heterogeneity | Model Type | |
|---|---|---|---|---|---|---|---|
| I2 | p-Value | ||||||
| Grade 3 AEs | 540/844 | 317/844 | 2.09 (1.47, 2.97) | <0.00001 | 90% | <0.00001 | Random effect |
| Skin rash | 194/844 | 143/844 | 1.53 (0.92, 2.52) | 0.10 | 84% | <0.00001 | Random effect |
| Hypertension | 160/844 | 31/844 | 5.15 (3.59, 7.39) | <0.00001 | 47% | 0.08 | Fixed effect |
| Diarrhea | 23/531 | 15/531 | 1.53 (0.48, 2.86) | 0.18 | 0% | 0.56 | Fixed effect |
| Proteinuria | 46/531 | 2/531 | 12.03 (4.37, 33.17) | <0.00001 | 0% | 0.94 | Fixed effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakharkar, P.; Kurup, S. Comparing Efficacy of Erlotinib and Bevacizumab Combination with Erlotinib Monotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis. Diseases 2023, 11, 146. https://doi.org/10.3390/diseases11040146
Sakharkar P, Kurup S. Comparing Efficacy of Erlotinib and Bevacizumab Combination with Erlotinib Monotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis. Diseases. 2023; 11(4):146. https://doi.org/10.3390/diseases11040146
Chicago/Turabian StyleSakharkar, Prashant, and Sonali Kurup. 2023. "Comparing Efficacy of Erlotinib and Bevacizumab Combination with Erlotinib Monotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis" Diseases 11, no. 4: 146. https://doi.org/10.3390/diseases11040146
APA StyleSakharkar, P., & Kurup, S. (2023). Comparing Efficacy of Erlotinib and Bevacizumab Combination with Erlotinib Monotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis. Diseases, 11(4), 146. https://doi.org/10.3390/diseases11040146





