Abstract
Obesity represents a heavy burden for modern healthcare. The main challenge facing obesity research progress is the unknown underlying pathways, which limits our understanding of the pathogenesis and developing therapies. Obesity induces specific biochemical environments that impact the different cells and tissues. In this piece of writing, we suggest mimicking obesity-induced in vivo biochemical environments including pH, lipids, hormones, cytokines, and glucose within an in vitro environment. The concept is to reproduce such biochemical environments and use them to treat the tissue cultures, explant cultures, and cell cultures of different biological organs. This will allow us to clarify how the obesity-induced biochemistry impacts such biological entities. It would also be important to try different environments, in terms of the compositions and concentrations of the constitutive elements, in order to establish links between the effects (impaired regeneration, cellular inflammation, etc.) and the factors constituting the environment (hormones, cytokines, etc.) as well as to reveal dose-dependent effects. We believe that such approaches will allow us to elucidate obesity mechanisms, optimize animal models, and develop therapies as well as novel tissue engineering applications.
1. Modern Health Challenges and Obesity
Modern life is characterized by diseases and health problems, some of which are emerging, and others that already existed but have worsened. We see the emergence of new pathologies such as viral and bacterial infections with new microbiological agents or new variants of those agents. On the other hand, another category of previously existing diseases is worsening. These diseases include obesity, cancer, and cardiovascular diseases. Such an epidemiological profile is mainly due to the unhealthy lifestyle developed by our societies during the last decades. This poor lifestyle includes the lack of physical activity [1] resulting from sedentary habits, as well as the technological and scientific developments that have made it easier to complete our daily tasks with limited or no effort. Psychological and mental health issues are also among the factors that worsen our lifestyle. Importantly, the contribution of dietary choices—in terms of both quality and quantity—to lifestyle quality is tremendous [2]. Within this context, we focus on obesity and the related biochemical environment it induces in vivo to build in vitro models of the disease.
Obesity, as a health problem [3,4] that has reached pandemic levels [5], is a disease with complex pathogenesis and complications [6]. Its basic definition is an abnormal fat accumulation as a consequence of energy imbalances between caloric intake and energy expenditure [7,8]. Obesity is characterized by adipocytes proliferation [8,9] and adipose tissue growth and remodeling [10,11,12]. Obesity properties have even been compared to cancer [13], and the disease has also been described as neuroendocrine reprogramming [14].
Beyond the impacts that obesity has via biomechanical mechanisms, such as on joints (cartilage loss) [15] and lungs (mechanics changes) [16], obesity has also been suggested either as a cause or a risk factor for impaired regeneration [17], arterial hypertension [18,19], type 2 diabetes mellitus [20,21], nonalcoholic fatty liver disease [22,23], heart failure [24,25], kidney diseases [26,27], cancer [28,29,30], dyslipidemia [31,32], etc. Functional genomics explorations of obesity and the factors that impact its development (diet, exercise, etc.) [33,34,35,36,37,38,39,40,41] have revealed specific related genes and improved our molecular understanding of obesity development.
It is worth mentioning that diet not only contributes to obesity development via increased energy intake, but also via its indirect effects [42]. Indeed, various food elements (vitamins, antioxidants, etc.) can impact the performance of the metabolic functions and biochemical homeostasis. In addition, a diet that results in obesity also contributes to the metabolic consequences and biological environments (blood contents in lipids, glucose, etc.) developed once obesity and the obesity-related homeostasis impairments are established.
2. In Vitro Obesity-Related Environment
The impacts that obesity-related chemical and biological environments have on homeostasis, as well as on the cellular and tissular functions, along with the underlying pathways, remain less known. Importantly, understanding the homeostatic patterns related to obesity-induced biochemistry and molecular changes would help to better explain how obesity leads to or increases the risks of the pathological phenotypes seen in diverse tissues and organs. Indeed, obesity status is also characterized by a biological environment created from the changes in secretion and biomolecules levels changes seen in the disease. These include dyslipidemia [43]; impaired acid-base balance, as reflected in the urine pH and the small intestine pH [44,45,46,47,48,49]; chronic inflammation [50,51], including gut microbial-related low-grade inflammation [52]; pro-inflammatory cytokines production [53,54,55]; hyperinsulinemia [56,57,58]; thyroid hormones and gender steroids (among other endocrine changes) [59]; increased leptin [60,61]; metabolic dysfunction [62,63]; decreased adipose tissue oxygenation [64]; and hyperuricemia [65,66,67,68].
Such biochemical and molecular changes reported in obesity could represent an important component of the mechanistic links between obesity and its consequences on cells and tissues. Therefore, building in vitro models to explore such mechanistic pathways would allow us to develop studies that are geared towards a deeper understanding of the obesity pathogenesis, development, and the impacts that it has on the diverse cells, tissues, and organs. The purpose is to investigate and explore the changes that such obesity-induced biochemical environments induce on cells and tissues in vitro and explore the modified mechanisms based on the observed in vitro changes.
The concept is to place different types of cell [69] or tissue cultures within environments (culture medium) that mimic the in vivo obesity environments in terms of pH [70,71], inflammatory factors, insulin [72,73], hormonal factors [74,75], lipids content, glucose, etc. (Figure 1). Testing different types of cells/tissues cultures would allow us to build a database towards a molecular mapping of the effects of each set of conditions (one or more factors within the culture medium) on different types of cells/tissues (adipocytes, renal cells, hepatocytes, myocytes, muscles, etc.) and identify the cellular and molecular outcomes. Furthermore, to obtain “dose-dependent” patterns, we can use different compositions of the medium in terms of concentrations of diverse elements within the media. The factors to use (hormones, cytokines, biochemicals, etc.) could also be optimized according to the biological samples, especially depending on the receptors of these factors on the cells/tissues of the culture. The time of exposure should also be a variable of focus, in order to distinguish acute exposure outcomes and chronic effects (more relevant to obesity).
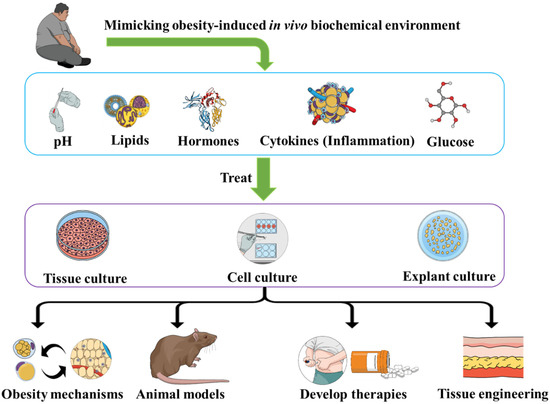
Figure 1.
Treating cells and tissues with an obesity-like bioenvironment allows us to study obesity, develop animal models and therapies, as well as optimize tissue engineering methods.
Such approaches would allow us to better understand how obesity-related biochemistry induces modifications within the biology of the cells and tissues. By studying different media compositions, including media in which we add only one parameter (for instance cytokines such as interleukin-1 beta (IL)-1β and IL-6 and tumor necrosis factor-alpha (TNF-α) [53]), we could be able to link each effect of obesity to one or more factors. Therefore, we could develop therapies to manage obesity consequences depending on the symptoms and target the observed phenotype, especially with the importance of cell culture in drug development [76,77,78]. Such therapy would be more specific since we would know which part of the obesity-induced biochemistry (hormone, pH, etc.) is responsible for the pathological phenotype (kidney, liver, etc.). Interestingly, applying such approaches on embryonic and stems cells could help in understanding the effects of obesity-related biochemistry on embryos (whose mothers develop obesity since they share the blood circulation for which the biochemistry is modified by obesity), and on the biology of development in general. The other key point is that such in vitro methodologies (mainly cell cultures, explant cultures, and tissue cultures) would allow us to study and observe a specific obesity-induced cellular phenotype independently from the other factors, and thus, specifically understand its pathogenesis and the influencing factors. As practical illustrations, recent research papers applied similar concepts to generate fat-on-a-chip models [79], an in vitro model for hypertrophic adipocytes [80], and inflammation in an in-vitro model of central obesity [81], all for applications in obesity discovery and research. These examples are practical illustrations of in vitro models of obesity that would provide strong data to support, confirm, and complete the data collected from animal models. These in vitro models can also allow us to overcome certain limitations reported in animal models by allowing us to study the interactions between one type of cell, tissue, or organ with one factor or one set of factors (signalizing molecules, cytokines, etc.), independently from the other factors. This is not always possible in the animal models since an in vivo environment includes all the factors that are also in variable concentrations with continuous dynamic changes.
3. Biomedical and Clinical Perspectives
Biomedical and clinical perspectives would add important knowledge to the diverse animal models of obesity [82,83,84,85,86], since precise knowledge on the changes that obesity induces in vivo can be applied to further optimize these animal models. This can be achieved, for instance, by injecting chemicals to maximize the biochemical similarities between the patients suffering from obesity and the in vivo patterns of the obesity animal models. In addition, a good example of clinical application would be in regenerative medicine. Indeed, understanding the links between the obesity-induced environment and tissues development could allow us to optimize the tissue culture conditions towards more regenerative medicine-produced tissues for therapies, especially for patients with obesity. This is specifically important due to the specific factors (such as AMP-activated protein kinase and mitochondrial biogenesis) and the related effects of impaired regeneration in the context of obesity [87,88,89,90].
The above-explained principle can be extrapolated to other diseases/conditions that could induce biochemical changes impacting cells, organs, and tissues, such as diabetes, and ageing that shares patterns with obesity [91,92]. Further, it would allow us to explain how the biochemical changes induced by these health conditions could mechanistically lead to the known phenotypes in the diverse tissues (metabolic decline or disorder, cellular senescence, etc.). As illustrated, exposing cell cultures to oxidative stress would allow us to deeply explore the theory of ageing related to free radicals [93,94].
However, it is important to mention the limitations, because the in vitro investigation of cell, tissue, or explant cultures may not completely mimic the in vivo obesity environment, and differences between in vitro and in vivo environments exist. For instance, the surrounding environments’ constitutive elements (hormones, cytokines, etc.) have fixed concentrations in the in vitro cultures, whereas in vivo, their production and concentration changes and are under the control of different regulating factors. Unlike the in vitro conditions, the in vivo mechanisms ensure that both signaling molecules (hormones, cytokines, etc.), as well as the biometabolites (fatty acids, glucose, etc.), have variable patterns of production and secretion depending on food intake, controlling signals, and feedback control pathway among a regulating biological network.
We hope that the knowledge we gain through these approaches will provide additional tools to manage obesity in an era where this disease [95,96] impacts continue to worsen, as illustrated by the COVID-19 crisis [97,98,99,100], since gaining such molecular and genetic knowledge about obesity is the main obstacle towards a deep understanding of obesity-related pathways and, therefore, developing efficient therapies. Building this kind of database would allow us to develop new treatments or improve those already in use. Moreover, the biochemicals that comprise the obesity environment could also be a target of the therapies (develop pharmacotherapies) or be modified by anti-obesity approaches such as exercise [101], which could allow a better understanding of how exercise improves obesity beyond caloric balance and weight loss. This could not only explain obesity pathogenesis, but also provide mechanistic explanations of how anti-obesity therapies work. Importantly, the significance and implications of the present piece of writing in clinical applications and therapies is related to the fact that obesity is a growing problem worldwide and is associated with a range of health complications, including diabetes and hyperlipidemia. Moreover, these kinds of in vitro models represent suitable models for pharmacodynamics-related studies aiming to explore the changes of drug–target interactions, depending on whether the surrounding medium mimics the obesity-induced bioenvironment. Therefore, we could provide data towards optimized therapies for patients with obesity, as well as potentially improve the pharmacovigilance of obesity therapies.
Author Contributions
A.G. structured and wrote the manuscript. A.G., M.Y. and J.S.-A. discussed the content and exchanged ideas and suggestions (concepts to add, the figures, references selection, etc.) throughout the writing process, as well as edited and critically revised the paper. J.S.-A. gave the final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Abdelaziz Ghanemi received a scholarship under the Merit Scholarship Program for foreign students from the Ministry of Education and Higher Education of Quebec, Canada. The Fonds de recherche du Québec—Nature et technologies (FRQNT) is responsible for managing the program (Bourses d’excellence pour étudiants étrangers du Ministère de l’Éducation et de l’Enseignement supérieur du Québec, Le Fonds de recherche du Québec—Nature et technologies (FRQNT) est responsable de la gestion du programme). Abdelaziz Ghanemi received the scholarship Bourse Tremplin-Stage en milieu de pratique (Internship scholarship) from the Fonds de recherche du Québec–Sante (FRQS), Quebec, Canada The graphical abstract was created using images from https://mindthegraph.com/ (accessed on 18 July 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, X.Y.; Han, L.H.; Zhang, J.H.; Luo, S.; Hu, J.W.; Sun, K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS ONE 2017, 12, e0187668. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, A.; El-Sherif, S.; Taylor, A.H.; Ayakannu, T. Ovarian Cancer: Lifestyle, Diet and Nutrition. Nutr. Cancer 2021, 73, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Shamseddeen, H.; Getty, J.Z.; Hamdallah, I.N.; Ali, M.R. Epidemiology and economic impact of obesity and type 2 diabetes. Surg. Clin. N. Am. 2011, 91, 1163–1172, vii. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef]
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001, 2, 239–254. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue remodeling in pathophysiology of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 371–376. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Role of extracellular matrix remodelling in adipose tissue pathophysiology: Relevance in the development of obesity. Histol. Histopathol. 2012, 27, 1515–1528. [Google Scholar] [PubMed]
- Boubertakh, B.; Silvestri, C.; Di Marzo, V. Obesity: The Fat Tissue Disease Version of Cancer. Cells 2022, 11, 1872. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, K.S.; Radakovich, L.B.; Fouts, J.; Foster, M.T. Pathophysiology of obesity on knee joint homeostasis: Contributions of the infrapatellar fat pad. Horm. Mol. Biol. Clin. Investig. 2016, 26, 97–108. [Google Scholar] [CrossRef]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Regeneration during Obesity: An Impaired Homeostasis. Animals 2020, 10, 2344. [Google Scholar] [CrossRef]
- Outón, S.; Galceran, I.; Pascual, J.; Oliveras, A. Central blood pressure in morbid obesity and after bariatric surgery. Nefrologia 2020, 40, 217–222. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of The Obesity Society and the American Society of Hypertension. J. Clin. Hypertens 2013, 15, 14–33. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Triposkiadis, F.; Xanthopoulos, A.; Starling, R.C.; Iliodromitis, E. Obesity, inflammation, and heart failure: Links and misconceptions. Heart. Fail. Rev. 2022, 27, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, G.B.; Bentes, A.C.; Daher, E.F.; Matos, S.M. Obesity and kidney disease. J. Bras. Nefrol. 2017, 39, 65–69. [Google Scholar] [CrossRef]
- Nehus, E. Obesity and chronic kidney disease. Curr. Opin. Pediatr. 2018, 30, 241–246. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Franssen, R.; Monajemi, H.; Stroes, E.S.; Kastelein, J.J. Obesity and dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2008, 37, 623–633, viii. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.; Monajemi, H.; Stroes, E.S.; Kastelein, J.J. Obesity and dyslipidemia. Med. Clin. N. Am. 2011, 95, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2 Expression as an Indicator of the Severity of the High-Fat Diet-Induced Obesity. Genes 2021, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target. Metabolites 2021, 11, 536. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as a Molecular Physiological and Pathological Biomarker. Biomolecules 2021, 11, 1689. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Measuring Exercise-Induced Secreted Protein Acidic and Rich in Cysteine Expression as a Molecular Tool to Optimize Personalized Medicine. Genes 2021, 12, 1832. [Google Scholar] [CrossRef]
- Mucunguzi, O.; Melouane, A.; Ghanemi, A.; Yoshioka, M.; Boivin, A.; Calvo, E.L.; St-Amand, J. Identification of the principal transcriptional regulators for low-fat and high-fat meal responsive genes in small intestine. Nutr. Metab. 2017, 14, 66. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites 2022, 12, 125. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Genetic Expression between Ageing and Exercise: Secreted Protein Acidic and Rich in Cysteine as a Potential “Exercise Substitute” Antiageing Therapy. Genes 2022, 13, 950. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as an Exercise-Induced Gene: Towards Novel Molecular Therapies for Immobilization-Related Muscle Atrophy in Elderly Patients. Genes 2022, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications. Biomolecules 2021, 11, 1830. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizad Farhangi, M.; Nikniaz, L.; Nikniaz, Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: An updated systematic review and meta-analysis. PLoS ONE 2019, 14, e0216547. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Body size and 24-h urine composition. Am. J. Kidney Dis. 2006, 48, 905–915. [Google Scholar] [CrossRef]
- Najeeb, Q.; Masood, I.; Bhaskar, N.; Kaur, H.; Singh, J.; Pandey, R.; Sodhi, K.S.; Prasad, S.; Ishaq, S.; Mahajan, R. Effect of BMI and urinary pH on urolithiasis and its composition. Saudi J. Kidney Dis. Transplant. 2013, 24, 60–66. [Google Scholar] [CrossRef]
- Steenackers, N.; Wauters, L.; Van der Schueren, B.; Augustijns, P.; Falony, G.; Koziolek, M.; Lannoo, M.; Mertens, A.; Meulemans, A.; Raes, J.; et al. Effect of obesity on gastrointestinal transit, pressure and pH using a wireless motility capsule. Eur. J. Pharm. Biopharm. 2021, 167, 1–8. [Google Scholar] [CrossRef]
- Lee, J.; Chang, H.K.; Lee, S. Association of low urine pH as a metabolic feature with abdominal obesity. J. Int. Med. Res. 2020, 48, 300060519898615. [Google Scholar] [CrossRef]
- Song, J.H.; Doo, S.W.; Yang, W.J.; Song, Y.S. Influence of obesity on urinary pH with respect to sex in healthy Koreans. Urology 2011, 78, 1244–1247. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Moghbeli, M.; Khedmatgozar, H.; Yadegari, M.; Avan, A.; Ferns, G.A.; Ghayour Mobarhan, M. Cytokines and the immune response in obesity-related disorders. Adv. Clin. Chem. 2021, 101, 135–168. [Google Scholar] [PubMed]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Skovsø, S.; Page, M.M.; Lim, G.E.; Johnson, J.D. A causal role for hyperinsulinemia in obesity. J. Endocrinol. 2017, 232, R173–R183. [Google Scholar] [CrossRef]
- Poddar, M.; Chetty, Y.; Chetty, V.T. How does obesity affect the endocrine system? A narrative review. Clin. Obes. 2017, 7, 136–144. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Carreira, M.C.; Cabia, B.; Andrade, S.; Amil, M.; Casanueva, F.F. Leptin resistance in obesity: An epigenetic landscape. Life Sci. 2015, 140, 57–63. [Google Scholar] [CrossRef]
- Moraes Ados, S.; Pisani, L.P.; Corgosinho, F.C.; Carvalho, L.O.; Masquio, D.C.; Jamar, G.; Sanches, R.B.; Oyama, L.M.; Dâmaso, A.R.; Belote, C.; et al. The role of leptinemia state as a mediator of inflammation in obese adults. Horm. Metab. Res. 2013, 45, 605–610. [Google Scholar] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Beeman, S.C.; Smith, G.I.; Yoshino, J.; Morozov, D.; Beals, J.W.; Kayser, B.D.; Watrous, J.D.; Jain, M.; Patterson, B.W.; et al. Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J. Clin. Investig. 2020, 130, 6688–6699. [Google Scholar] [CrossRef]
- Remedios, C.; Shah, M.; Bhasker, A.G.; Lakdawala, M. Hyperuricemia: A reality in the Indian obese. Obes. Surg. 2012, 22, 945–948. [Google Scholar] [CrossRef]
- Tang, L.; Kubota, M.; Nagai, A.; Mamemoto, K.; Tokuda, M. Hyperuricemia in obese children and adolescents: The relationship with metabolic syndrome. Pediatr. Rep. 2010, 2, e12. [Google Scholar] [CrossRef]
- Özalp Kızılay, D.; Şen, S.; Ersoy, B. Associations Between Serum Uric Acid Concentrations and Cardiometabolic Risk and Renal Injury in Obese and Overweight Children. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 262–269. [Google Scholar] [CrossRef]
- Civantos Modino, S.; Guijarro de Armas, M.G.; Monereo Mejías, S.; Montaño Martínez, J.M.; Iglesias Bolaños, P.; Merino Viveros, M.; Ladero Quesada, J.M. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol. Nutr. 2012, 59, 533–538. [Google Scholar] [CrossRef]
- Baust, J.M.; Buehring, G.C.; Campbell, L.; Elmore, E.; Harbell, J.W.; Nims, R.W.; Price, P.; Reid, Y.A.; Simione, F. Best practices in cell culture: An overview. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 669–672. [Google Scholar] [CrossRef]
- Higashino, N.; Takayama, T.; Ito, H.; Horade, M.; Yamaguchi, Y.; Dylan Tsai, C.H.; Kaneko, M. LED-CT Scan for pH Distribution on a Cross-Section of Cell Culture Medium. Sensors 2018, 18, 191. [Google Scholar] [CrossRef]
- Jang, J.; Moon, S.J.; Hong, S.H.; Kim, I.H. Colorimetric pH measurement of animal cell culture media. Biotechnol. Lett. 2010, 32, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Sabry, M.; Nour, M.; Abdelrahman, M.Y.; Roshdy, E.; Magdi, Y.; Abdelghafar, H. Integrating insulin into single-step culture medium regulates human embryo development in vitro. Fertil. Steril. 2017, 107, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Mesalam, A.; Song, S.H.; Kong, I.K. Supplementation of insulin-transferrin-sodium selenite in culture medium improves the hypothermic storage of bovine embryos produced in vitro. Theriogenology 2020, 152, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mather, J.P.; Zhuang, L.Z.; Perez-Infante, V.; Phillips, D.M. Culture of testicular cells in hormone-supplemented serum-free medium. Ann. N. Y. Acad. Sci. 1982, 383, 44–68. [Google Scholar] [CrossRef]
- Snyder, E.Y.; Kim, S.U. Hormonal requirements for neuronal survival in culture. Neurosci. Lett. 1979, 13, 225–230. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Ghanemi, A. Cell cultures in drug development: Applications, challenges and limitations. Saudi. Pharm. J. 2015, 23, 453–454. [Google Scholar] [CrossRef]
- Jaroch, K.; Jaroch, A.; Bojko, B. Cell cultures in drug discovery and development: The need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J. Pharm. Biomed. Anal. 2018, 147, 297–312. [Google Scholar] [CrossRef]
- McCarthy, M.; Brown, T.; Alarcon, A.; Williams, C.; Wu, X.; Abbott, R.D.; Gimble, J.; Frazier, T. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng. Part B Rev. 2020, 26, 586–595. [Google Scholar] [CrossRef]
- Qiao, Q.; Bouwman, F.G.; Renes, J.; Mariman, E.C.M. An in vitro model for hypertrophic adipocytes: Time-dependent adipocyte proteome and secretome changes under high glucose and high insulin conditions. J. Cell. Mol. Med. 2020, 24, 8662–8673. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Misto, A.; Vozzi, F.; Magliaro, C.; Mattei, G.; Marescotti, M.C.; Avogaro, A.; Iori, E. Systemic and vascular inflammation in an in-vitro model of central obesity. PLoS ONE 2018, 13, e0192824. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obese Animals as Models for Numerous Diseases: Advantages and Applications. Medicina 2021, 57, 399. [Google Scholar] [CrossRef]
- Rosini, T.C.; Silva, A.S.; Moraes, C. Diet-induced obesity: Rodent model for the study of obesity-related disorders. Rev. Assoc. Med. Bras. (1992) 2012, 58, 383–387. [Google Scholar]
- Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Katsinelos, P.; Grigoriadis, N.; Srivastava, D.S.; Kountouras, J. Rodent models of obesity. Minerva Endocrinol. 2020, 45, 243–263. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; Lima, N.V.; Rezende, K.S.; Santos, I.C.; Silva, I.S.; Guimarães, R.C. Animal models of obesity in rodents. An integrative review. Acta Cir. Bras. 2016, 31, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e6. [Google Scholar] [CrossRef]
- Geiger, A.E.; Daughtry, M.R.; Yen, C.N.; Kirkpatrick, L.T.; Shi, H.; Gerrard, D.E. Dual effects of obesity on satellite cells and muscle regeneration. Physiol. Rep. 2020, 8, e14511. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, M.; Zhang, S.; Foretz, M.; Viollet, B.; Du, M. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes 2016, 65, 188–200. [Google Scholar] [CrossRef]
- Niu, W.; Wang, H.; Wang, B.; Mao, X.; Du, M. Resveratrol improves muscle regeneration in obese mice through enhancing mitochondrial biogenesis. J. Nutr. Biochem. 2021, 98, 108804. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Exercise, Diet and Sleeping as Regenerative Medicine Adjuvants: Obesity and Ageing as Illustrations. Medicines 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, L.C.D.; Davies, K.J.A. Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Yu, W.; Rohli, K.E.; Yang, S.; Jia, P. Impact of obesity on COVID-19 patients. J. Diabetes Complicat. 2021, 35, 107817. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Will an obesity pandemic replace the coronavirus disease-2019 (COVID-19) pandemic? Med. Hypotheses 2020, 144, 110042. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis: Losing Our Immunity When We Need It the Most. Biology 2021, 10, 545. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).