A Simple Capillary Blood Cell Flow Monitoring System using Magnetic Micro-Sensor: A Simulation Study
Abstract
1. Introduction
2. Modeling and Simulation
2.1. Analysis of the Magnetic Field Generated by Flowing Blood Cells
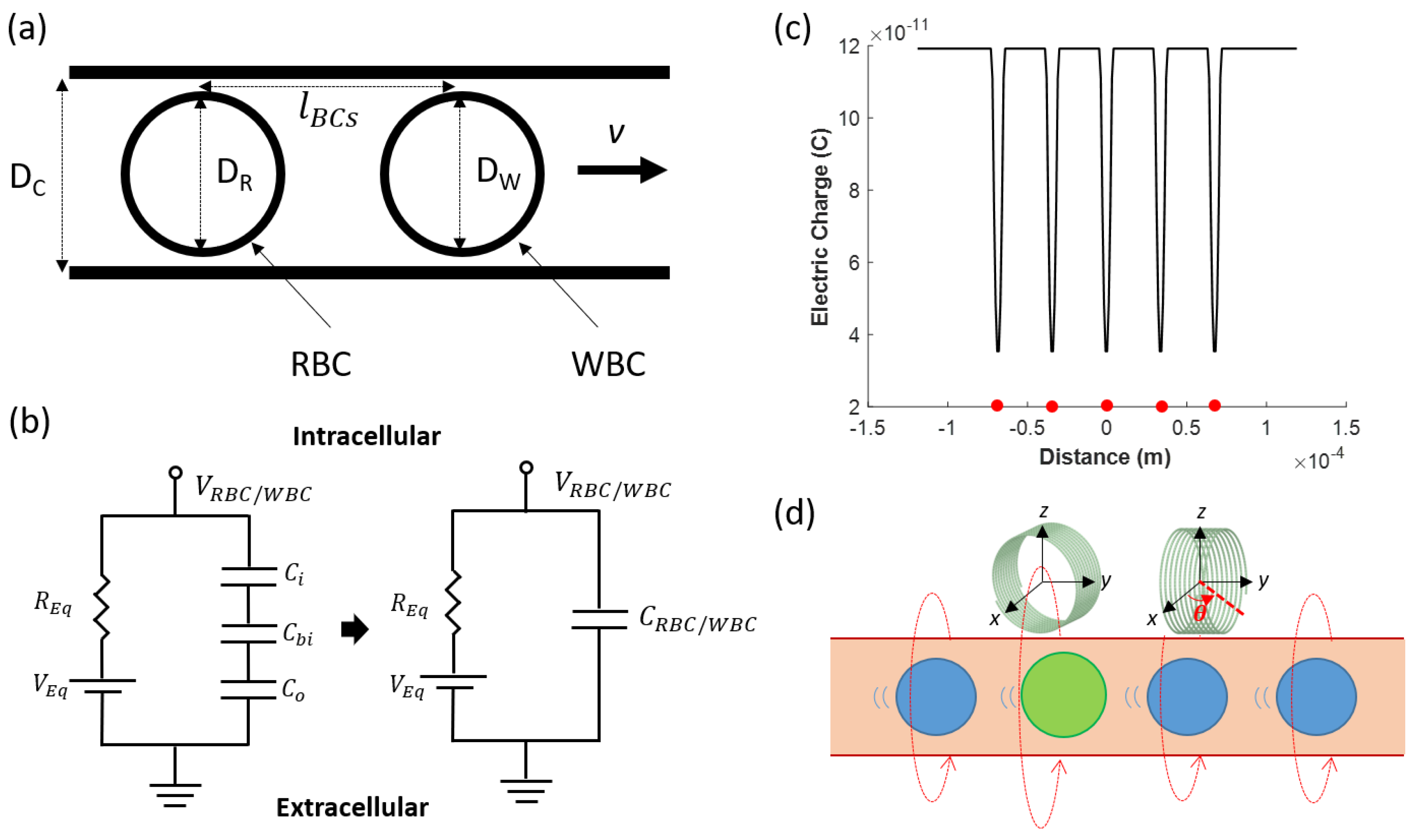
2.2. Modeling of Capillary and Blood Components
2.3. Modeling of the Microcoil
3. Results
3.1. Blood Flow Measurement
3.1.1. An Inductively Coupled Magnetic Sensor for Velocity Measurement
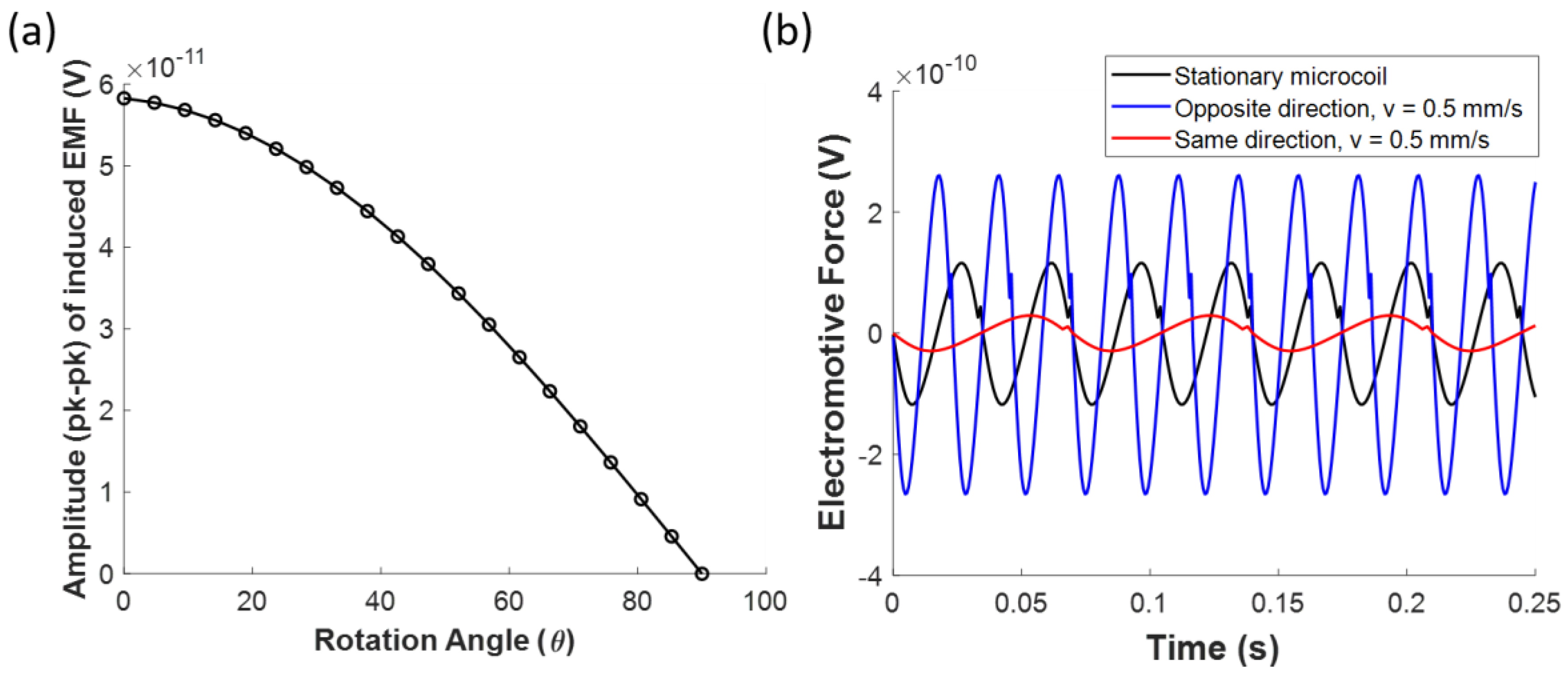
3.1.2. Magnetic Sensor for Directional Flow Measurement
3.2. Other Variations
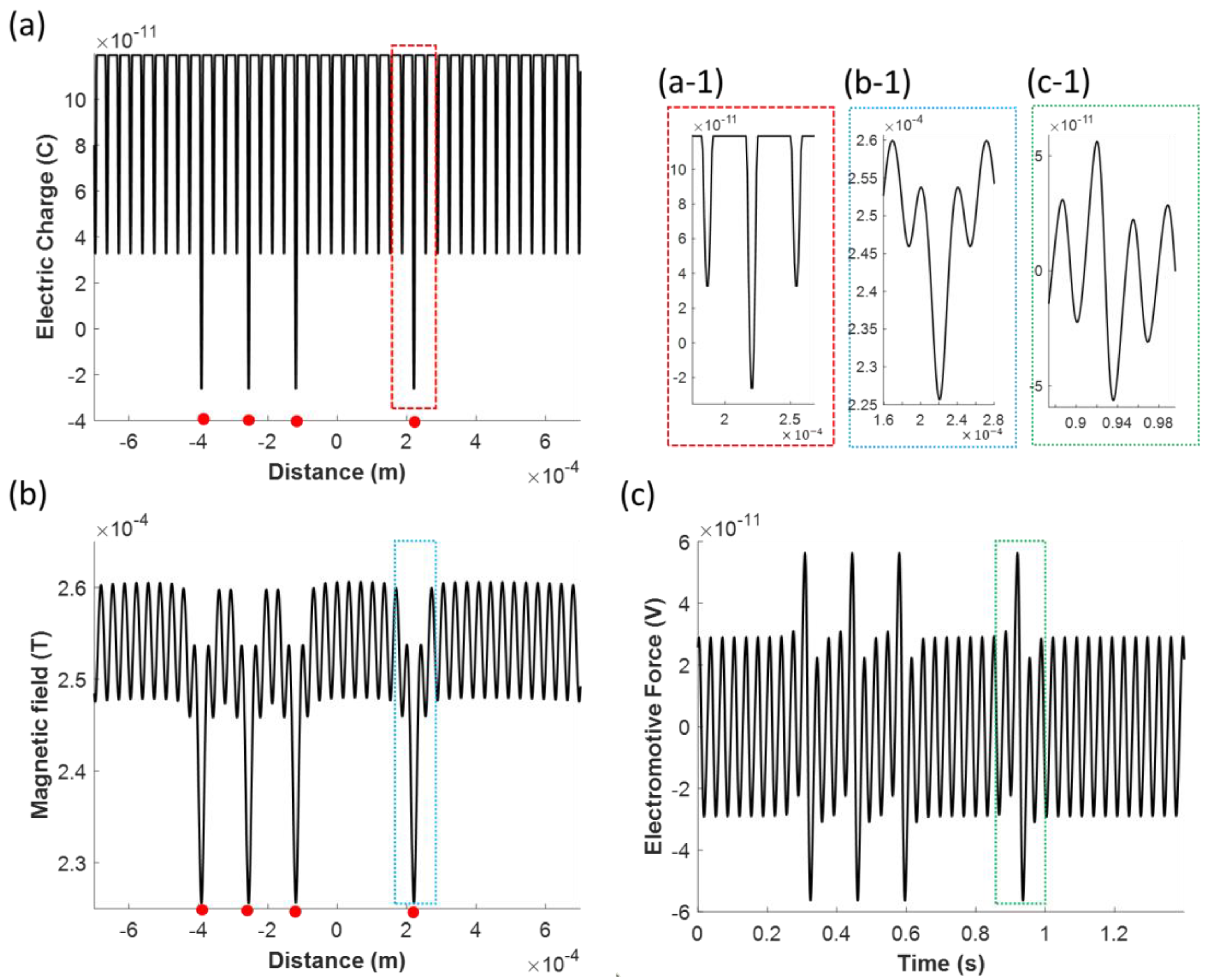
3.2.1. Effects of White Blood Cells on the Induced Electromotive Force
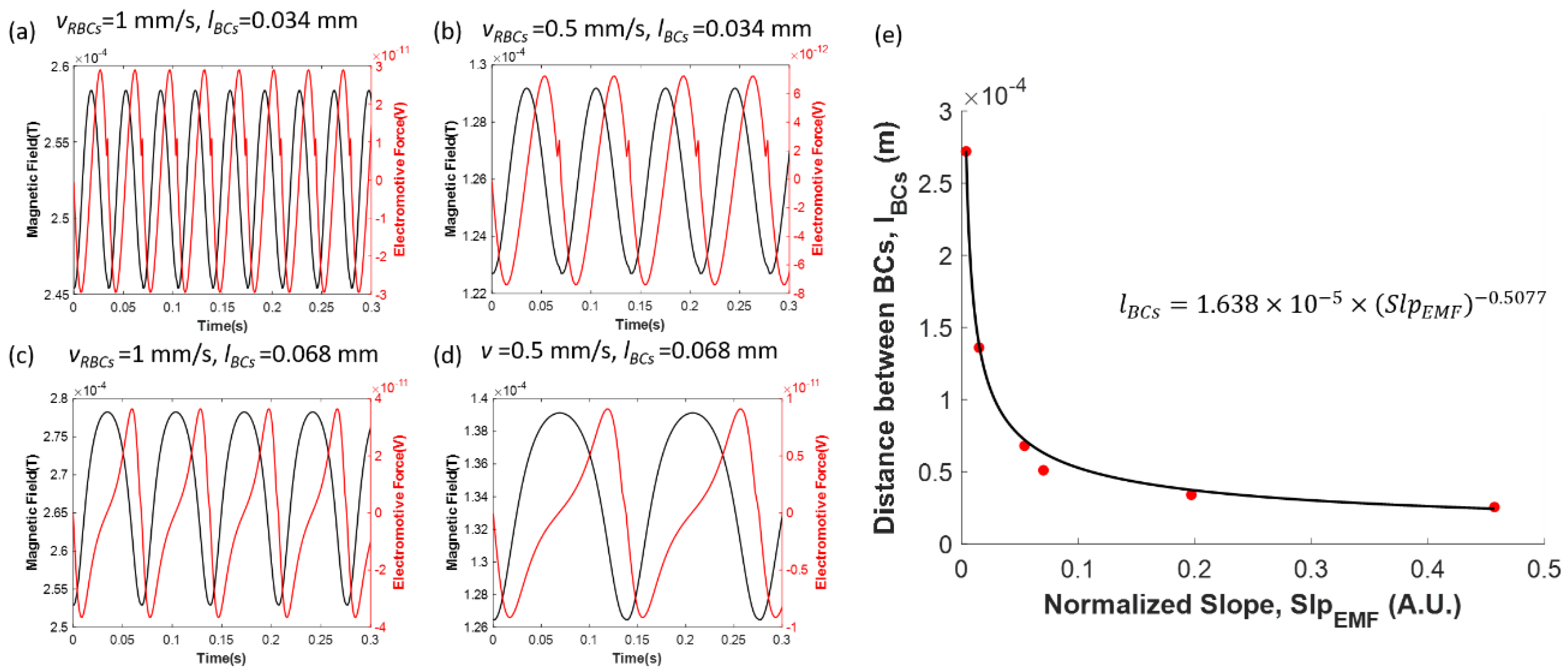
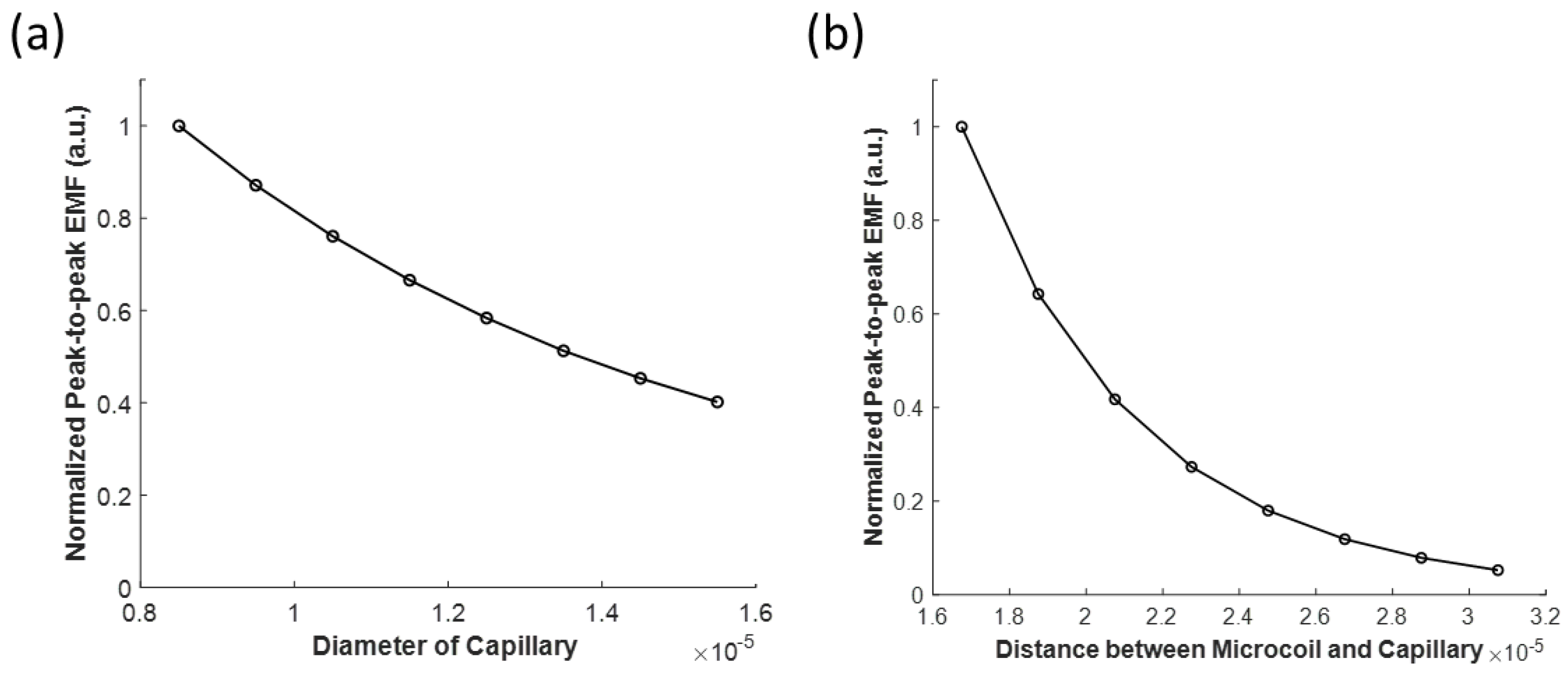
3.2.2. Effects of the Capillary Diameter and the Coil to Capillary Distance on the Induced Electromotive Force
4. Conclusions and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Tabrizchi, R.; Pugsley, M.K. Methods of blood flow measurement in the arterial circulatory system. J. Pharmacol. Toxicol. Methods 2000, 44, 375–384. [Google Scholar] [CrossRef]
- Jayanthy, A.K.; Sujatha, N.; Ramasubba Reddy, M. Measuring blood flow: Techniques and applications. Int. J. Res. Rev. Appl. Sci. 2011, 6, 203–216. [Google Scholar]
- Micheels, J.; Aisbjorn, B.; Sorensen, B. Laser doppler flowmetry. A new non-invasive measurement of microcirculation in intensive care? Resuscitation 1984, 12, 31–39. [Google Scholar] [CrossRef]
- Essex, T.J.H.; Byrne, P.O. A laser Doppler scanner for imaging blood flow in skin. J. Biomed. Eng. 1991, 13, 189–194. [Google Scholar] [CrossRef]
- Briers, D.; Duncan, D.D.; Hirst, E.R.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 1–10. [Google Scholar] [CrossRef]
- Dunn, A.K. Laser Speckle Contrast Imaging of Cerebral Blood Flow. Ann. Biomed. Eng. 2012, 40, 367–377. [Google Scholar] [CrossRef]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef]
- Jochim, K.E. The Development of the Electro-Magnetic Blood Flowmeter. IRE Trans. Biomed. Electron. 1962, 9, 228–235. [Google Scholar] [CrossRef]
- Wyatt, D.G. The electromagnetic blood flowmeter. J. Phys. E. 1968, 1, 1146–1152. [Google Scholar] [CrossRef]
- Teferra, M.N. Electromagnetic Blood Flow meter: Review. Int. J. Latest Res. Eng. Technol. 2017, 3, 21–26. [Google Scholar]
- Raunig, D.L.; Fox, M.D. Doppler ultrasound determination of capillary blood flow. In Proceedings of the IEEE 23rd Northeast Bioengineering Conference, Durham, NH, USA, 21–22 May 1997; pp. 1–2. [Google Scholar]
- Azran, A.; Hirao, Y.; Kinouchi, Y.; Yamaguchi, H.; Yoshizaki, K. Variations of the Maximum Blood Flow Velocity in the Carotid, Brachial and Femoral Arteries in a Passive Postural Changes by a Doppler Ultrasound Method. In Proceedings of the The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 3708–3711. [Google Scholar]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.-P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.; Byun, K.M.; Jun, S.B.; Kim, S.J.; Lee, J. Theoretical Study on Gold-Nanorod-Enhanced Near-Infrared Neural Stimulation. Biophys. J. 2018, 115, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, M.L. Clinical Hematology: Theory and Procedures, 4th ed.; Lippincott Williams & Wilkinsa: Philadelphia, PA, USA, 2005; ISBN 0781750075. [Google Scholar]
- Rodighiero, S.; De Simoni, A.; Formenti, A. The voltage-dependent nonselective cation current in human red blood cells studied by means of whole-cell and nystatin-perforated patch-clamp techniques. Biochim. Biophys. Acta Biomembr. 2004, 1660, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chang, C.-C.; Chiu, T.-K.; Zhao, X.; Chen, D.; Chou, W.-P.; Zhao, Y.; Wang, H.-M.; Wang, J.; Wu, M.-H.; et al. Membrane capacitance of thousands of single white blood cells. J. R. Soc. Interface 2017, 14, 20170717. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Piasecka, A.; Szosland, K.; Bryszewska, M. Human red blood cell membrane potential and fluidity in glucose solutions. Scand. J. Clin. Lab. Invest. 1997, 57, 59–63. [Google Scholar] [CrossRef]
- Whitin, J.C.; Clark, R.A.; Simons, E.R.; Cohen, H.J. Effects of the myeloperoxidase system on fluorescent probes of granulocyte membrane potential. J. Biol. Chem. 1981, 256, 8904–8906. [Google Scholar]
- Baran, U.; Shi, L.; Wang, R.K. Capillary blood flow imaging within human finger cuticle using optical microangiography. J. Biophotonics 2015, 8, 46–51. [Google Scholar] [CrossRef]
- Lee, J.; Wu, W.; Lesage, F.; Boas, D.A. Multiple-capillary measurement of RBC speed, flux, and density with optical coherence tomography. J. Cereb. Blood Flow Metab. 2013, 33, 1707–1710. [Google Scholar] [CrossRef]
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S. Surface reactivity and in vitro biological evaluation of sol gel derived silver/calcium silicophosphate bioactive glass. Biotechnol. Bioprocess Eng. 2012, 17, 746–754. [Google Scholar] [CrossRef]
| Variable | Definition | Value | Unit |
|---|---|---|---|
| Diameter of red blood cell | 7.2 [17] | [μm] | |
| Diameter of white blood cell | 7.5 | [μm] | |
| Membrane capacitance of red blood cell | 1.5 [18] | [pF] | |
| Membrane capacitance of white blood cell | 4.6 [19] | [pF] | |
| Transmembrane potential of red blood cell | −10 [20] | [mV] | |
| Transmembrane potential of white blood cell | −60 [21] | [mV] | |
| Velocity of blood | 1 [22] | [mm/s] | |
| Distance between adjacent blood cells | [23] | [mm] | |
| Diameter of capillary | 7.5 | [μm] | |
| Concentration of in blood plasma | 142 [24] | [mmol/L] | |
| Concentration of in blood plasma | 5 [24] | [mmol/L] | |
| Concentration of in blood plasma | 1.5 [24] | [mmol/L] | |
| Concentration of in blood plasma | 2.5 [24] | [mmol/L] | |
| Concentration of in blood plasma | 103 [24] | [mmol/L] | |
| Concentration of in blood plasma | 27 [24] | [mmol/L] | |
| Concentration of in blood plasma | 1 [24] | [mmol/L] | |
| Concentration of in blood plasma | 0.5 [24] | [mmol/L] | |
| Valence of | 1 | N.A. | |
| Valence of | 1 | N.A. | |
| Valence of | 2 | N.A. | |
| Valence of | 2 | N.A. | |
| Valence of | −2 | N.A. | |
| Valence of | −1 | N.A. | |
| Valence of | −2 | N.A. | |
| Valence of | −2 | N.A. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Eom, K. A Simple Capillary Blood Cell Flow Monitoring System using Magnetic Micro-Sensor: A Simulation Study. Electronics 2020, 9, 618. https://doi.org/10.3390/electronics9040618
Jo S, Eom K. A Simple Capillary Blood Cell Flow Monitoring System using Magnetic Micro-Sensor: A Simulation Study. Electronics. 2020; 9(4):618. https://doi.org/10.3390/electronics9040618
Chicago/Turabian StyleJo, Seonghoon, and Kyungsik Eom. 2020. "A Simple Capillary Blood Cell Flow Monitoring System using Magnetic Micro-Sensor: A Simulation Study" Electronics 9, no. 4: 618. https://doi.org/10.3390/electronics9040618
APA StyleJo, S., & Eom, K. (2020). A Simple Capillary Blood Cell Flow Monitoring System using Magnetic Micro-Sensor: A Simulation Study. Electronics, 9(4), 618. https://doi.org/10.3390/electronics9040618




