Recognition of Gait Phases with a Single Knee Electrogoniometer: A Deep Learning Approach
Abstract
1. Introduction
2. Related Works
3. Materials and Methods
3.1. Participants
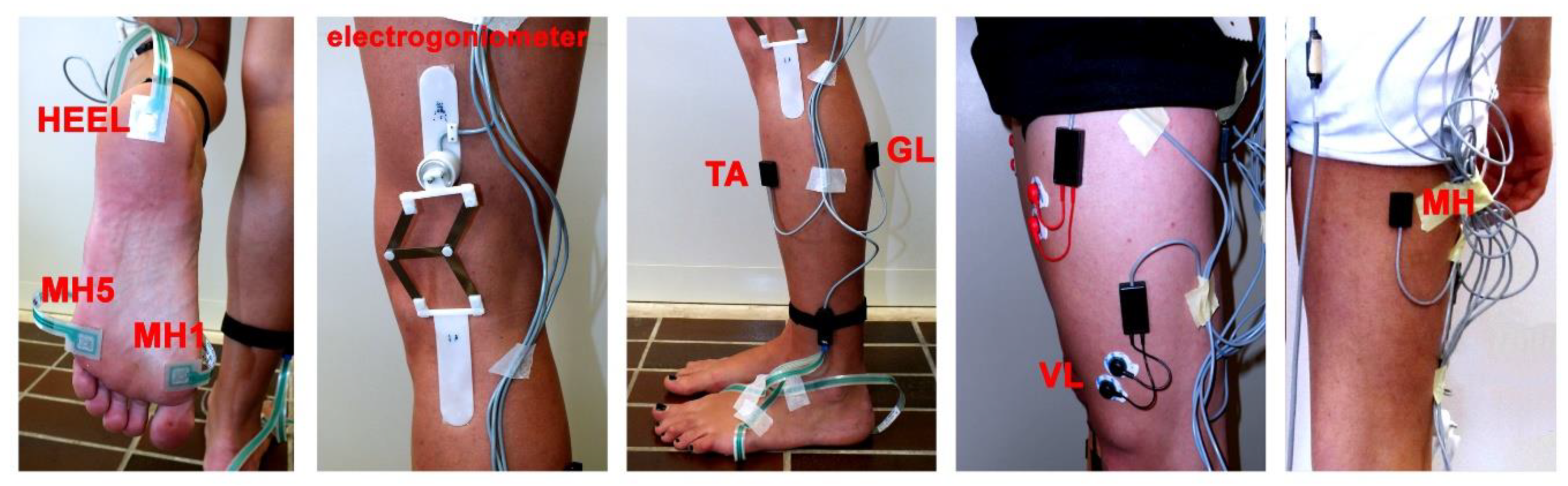
3.2. Signal Acquisition
3.3. Signal Pre-Processing
3.4. Data Preparation
3.4.1. Knee Approach
3.4.2. KEMG Approach
3.4.3. Reference Approach
3.5. Training the Classifier
3.6. Neural Network
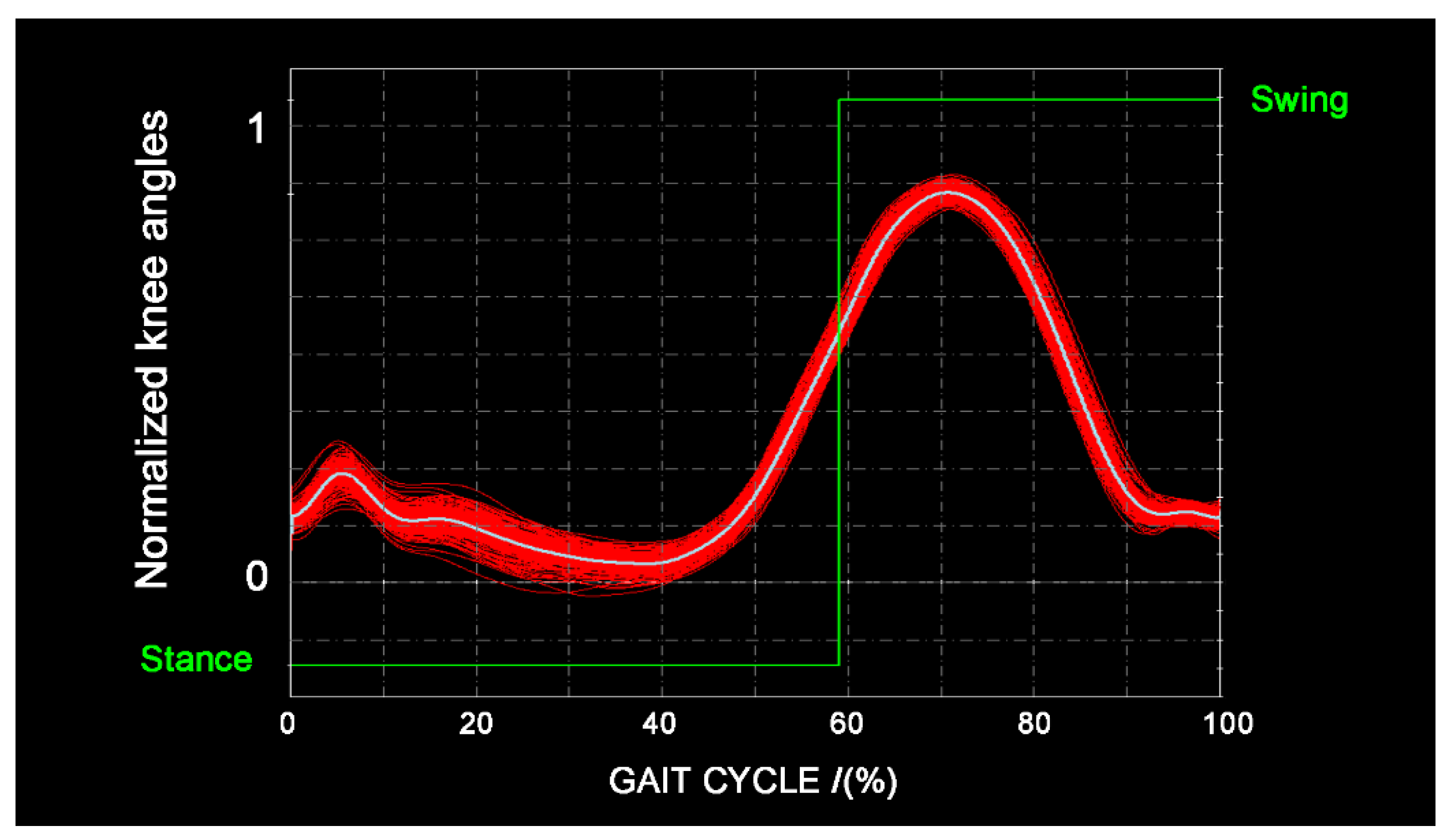
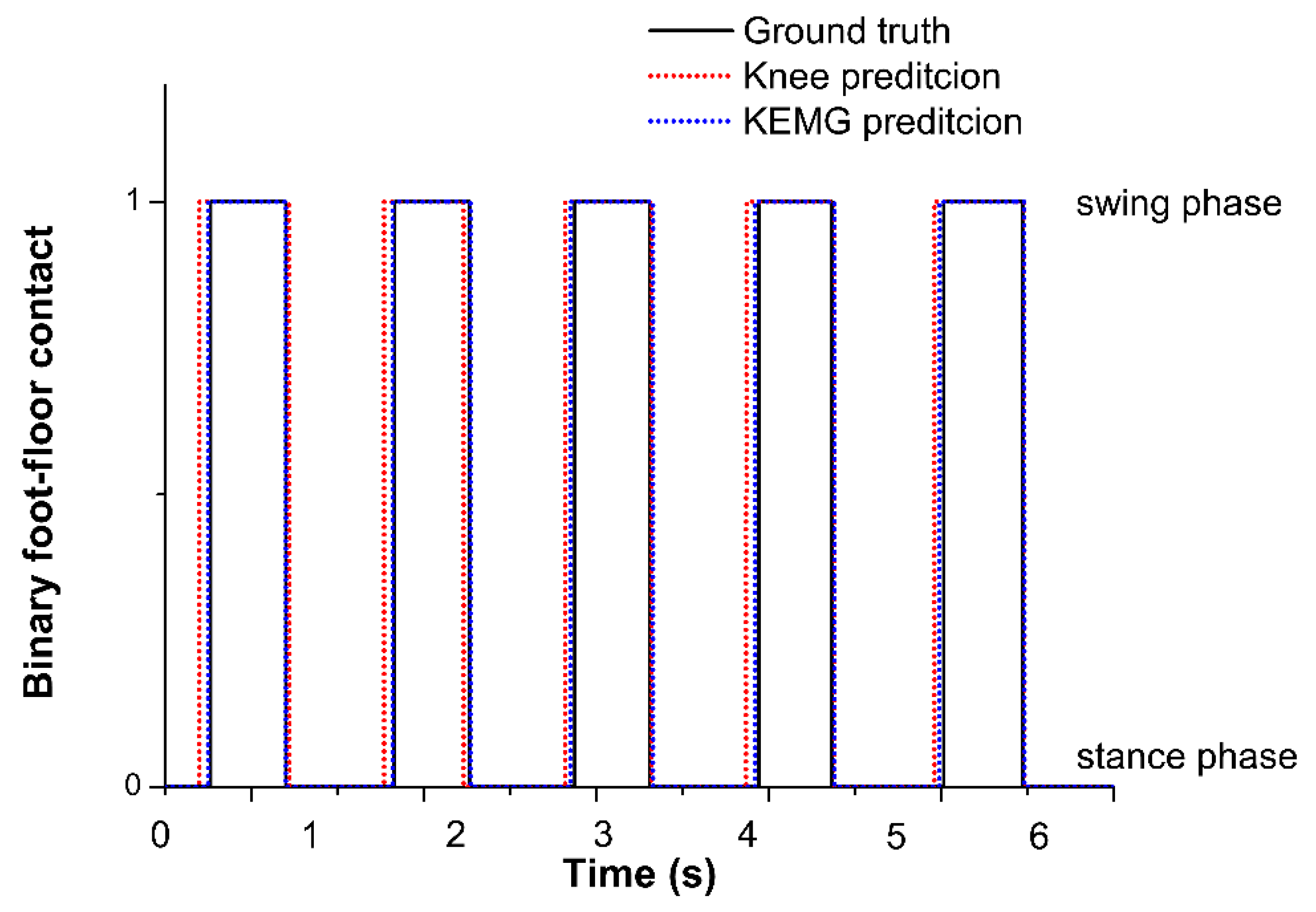
3.7. Gait-Event Identification
3.8. Statistics
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pappas, I.P.I.; Popovic, M.R.; Keller, T.; Dietz, V.; Morari, M. A reliable gait phase detection system. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Rueterbories, J.; Spaich, E.G.; Larsen, B.; Andersen, O.K. Methods for gait event detection and analysis in ambulatory systems. Med. Eng. Phys. 2010, 32, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Soh, C.B.; Gunawan, E.; Low, K.; Thomas, R. Assessment of foot trajectory for human gait phase detection using wireless ultrasonic sensor network. IEEE Trans. Neural Syst. Rehabil. 2016, 24, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.; Chen, B.; Wei, K.; Wang, Q. Lower limb wearable capacitive sensing and its applications to recognizing human gaits. Sensors (Basel) 2013, 13, 13334–13355. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, M.; Schmidt, K.; Duarte, J.E.; Neuner, L.; Koginov, G.; Riener, R. Stance and Swing Detection Based on the Angular Velocity of Lower Limb Segments during Walking. Front. Neurorobot. 2019, 24, 13–57. [Google Scholar] [CrossRef] [PubMed]
- Miller, A. Gait event detection using a multilayer neural network. Gait Posture 2009, 29, 542–545. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait Partitioning Methods: A Systematic Review. Sensors (Basel) 2016, 16, 66. [Google Scholar] [CrossRef]
- Novak, D.; Gorsic, M.; Podobnik, J.; Munih, M. Toward real-time automated detection of turns during gait using wearable inertial measurement units. Sensors (Basel) 2014, 14, 18800–18822. [Google Scholar] [CrossRef]
- Osis, S.T.; Hettinga, B.A.; Ferber, R. Predicting ground contact events for a continuum of gait types: An application of targeted machine learning using principal component analysis. Gait Posture 2016, 46, 86–90. [Google Scholar] [CrossRef]
- Liu, D.X.; Wu, X.; Du, W.; Wang, C.; Xu, T. Gait Phase Recognition for Lower-Limb Exoskeleton with Only Joint Angular Sensors. Sensors (Basel) 2016, 16, 1579. [Google Scholar] [CrossRef]
- Pacini Panebianco, G.; Bisi, M.C.; Stagni, R.; Fantozzi, S. Analysis of the performance of 17 algorithms from a systematic review: Influence of sensor position, analysed variable and computational approach in gait timing estimation from IMU measurements. Gait Posture 2018, 66, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kidziński, Ł.; Delp, S.; Schwartz, M. Automatic real-time gait event detection in children using deep neural networks. PLoS ONE 2019, 14, e0211466. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, N.; Abdul Rahman, M.; Yamamoto, S.I.; Ahmad, S. Walking gait event detection based on electromyography signals using artificial neural network. Biomed. Signal Process. Control 2019, 47, 334–343. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Fioretti, S.; Di Nardo, F. A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics 2019, 8, 894. [Google Scholar] [CrossRef]
- Mohammed, S.; Same, A.; Oukhellou, L.; Kong, K.; Huo, W.; Amirat, Y. Recognition of gait cycle phases using wearable sensors. Robot. Auton. Syst. 2016, 75, 50–59. [Google Scholar] [CrossRef]
- Morettini, M.; Peroni, C.; Sbrollini, A.; Marcantoni, I.; Burattini, L. Classification of drug-induced hERG potassium-channel block from electrocardiographic T-wave features using artificial neural networks. Ann. Noninvasive Electrocardiol. 2019, 24, e12679. [Google Scholar] [CrossRef]
- Di Nardo, F.; Mengarelli, A.; Ghetti, G.; Fioretti, S. Statistical analysis of EMG signal acquired from tibialis anterior during gait. IFMBE Proc. 2014, 41, 619–622. [Google Scholar] [CrossRef]
- Gurney, J.; Kersting, U.; Rosenbaum, D. Between-day reliability of repeated plantar pressure distribution measurements in a normal population. Gait Posture 2008, 27, 706–709. [Google Scholar] [CrossRef]
- Bovi, G.; Rabuffetti, M.; Mazzoleni, P.; Ferrarin, M. A multiple-task gait analysis approach: Kinematic, kinetic and EMG reference data for healthy young and adult subjects. Gait Posture 2011, 33, 6–13. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, K.; Sun, S.; Gao, Z.; Zhang, L.; Yang, Z. An upper-limb power-assist exoskeleton using proportional myoelectric control. Sensors (Basel) 2014, 14, 6677–6694. [Google Scholar] [CrossRef]
- Joshi, C.D.; Lahiri, U.; Thakor, N.V. Classification of gait phases from lower limb EMG: Application to exoskeleton orthosis. In Proceedings of the 2013 IEEE Point-of-Care Healthcare Technologies (PHT), Bangalore, India, 16–18 January 2013; pp. 228–231. [Google Scholar] [CrossRef]
- Jung, J.; Heo, W.; Yang, H.; Park, H. A neural network-based gait phase classification method using sensors equipped on lower limb exoskeleton robots. Sensors (Basel) 2015, 15, 27738–27759. [Google Scholar] [CrossRef] [PubMed]
- Ziegier, J.; Gattringer, H.; Mueller, A. Classification of Gait Phases Based on Bilateral EMG Data Using Support Vector Machines. In Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Enschede, The Netherlands, 26–29 August 2018; pp. 978–983. [Google Scholar] [CrossRef]
- Meng, M.; She, Q.; Gao, Y.; Luo, Z. EMG signals based gait phases recognition using hidden Markov models. In Proceedings of the 2010 IEEE International Conference on Information and Automation, ICIA 2010, Harbin, China, 20–23 June 2010; pp. 852–856. [Google Scholar] [CrossRef]
- Morbidoni, C.; Principi, L.; Mascia, G.; Strazza, A.; Verdini, F.; Cucchiarelli, A.; Di Nardo, F. Gait Phase Classification from Surface EMG Signals Using Neural Networks. IFMBE Proc. 2020, 76, 75–82. [Google Scholar] [CrossRef]
- Agostini, V.; Balestra, G.; Knaflitz, M. Segmentation and Classification of Gait Cycles. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, P.; Moser, D.; Ghoussayni, S.; Ewins, D. Detection of gait events using an F-Scan in-shoe pressure measurement system. Gait Posture 2008, 28, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Crea, S.; de Rossi, S.M.M.; Donati, M.; Reberšek, P.; Novak, D.; Vitiello, N.; Lenzi, T.; Podobnik, J.; Munih, M.; Carrozza, M.C. Development of gait segmentation methods for wearable foot pressure sensors. In Proceedings of the 34th IEEE Engineering in Medicine and Biology Society (EMBS), San Diego, CA, USA, 28 August–1 September 2012; pp. 5018–5021. [Google Scholar] [CrossRef]
- Senanayake, C.M.; Senanayake, S.M.N.A. Computational intelligent gait-phase detection system to identify pathological gait. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Fontecha, J.; Hervás, R.; Bravo, J. An ambulatory system for gait monitoring based on wireless sensorized insoles. Sensors (Basel) 2015, 15, 16589–16613. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography, SENIAM. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Khandelwal, S.; Wickstrasm, N. Evaluation of the performance of accelerometer-based gait event detection algorithms in different real-world scenarios using the MAREA gait database. Gait Posture 2017, 51, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, H.; Steger, R.; Huang, L.H. Hybrid control of the Berkeley Lower Extremity Exoskeleton (BLEEX). Int. J. Robot. Res. 2006, 25, 561–573. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, S.; Zhang, Y. A patient-specific EMG-driven neuromuscularmodel for the potential use of human-inspired gait rehabilitation robots. Comput. Biol. Med. 2016, 70, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Azimi, V.; Nguyen, T.T.; Sharifi, M.; Fakoorian, S.A.; Simon, D. Robust Ground Reaction Force Estimation and Control of Lower-Limb Prostheses: Theory and Simulation. IEEE Trans. Syst. Man Cybern. Syst. 2018, 99, 1–12. [Google Scholar] [CrossRef]
- Watanabe, T.; Endo, S.; Morita, R. Development of a prototype of portable FES rehabilitation system for relearning of gait for hemiplegic subjects. Healthc. Technol. Lett. 2016, 3, 284–289. [Google Scholar] [CrossRef] [PubMed]
| Classification accuracy in LS-test (%) | |||
|---|---|---|---|
| Fold | Knee | KEMG | Reference |
| 1 | 90.7 | 95.6 | 94.9 |
| 2 | 90.8 | 95.1 | 94.6 |
| 3 | 90.8 | 95.1 | 94.4 |
| 4 | 91.3 | 96.0 | 94.8 |
| 5 | 91.2 | 95.6 | 95.0 |
| 6 | 90.9 | 95.4 | 94.9 |
| 7 | 90.8 | 95.7 | 95.0 |
| 8 | 90.8 | 95.5 | 94.7 |
| 9 | 90.5 | 95.8 | 94.8 |
| 10 | 90.4 | 95.2 | 94.6 |
| 11 | 91.3 | 96.0 | 95.0 |
| 12 | 89.9 | 95.9 | 94.8 |
| 13 | 91.4 | 95.9 | 94.8 |
| 14 | 91.5 | 95.2 | 95.0 |
| 15 | 90.6 | 95.8 | 94.8 |
| 16 | 91.2 | 95.5 | 94.7 |
| 17 | 91.0 | 95.6 | 94.7 |
| 18 | 90.2 | 95.5 | 94.8 |
| 19 | 90.8 | 95.4 | 94.8 |
| 20 | 90.7 | 95.5 | 94.8 |
| 21 | 91.8 | 96.3 | 95.3 |
| 22 | 91.5 | 95.8 | 95.0 |
| 23 | 90.8 | 95.6 | 94.8 |
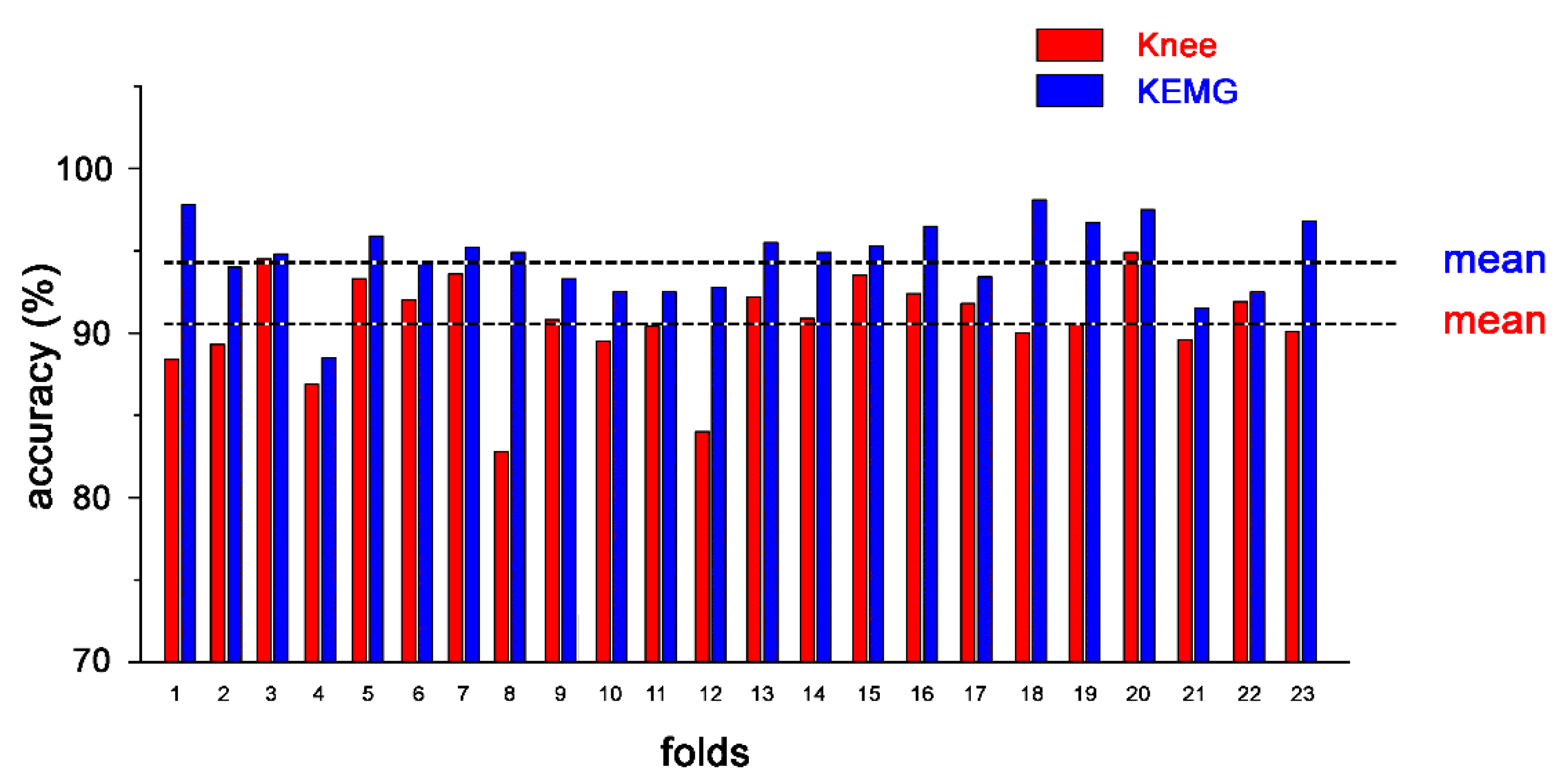
| Mean ± SD | 90.9 ± 0.4 | 95.6 ± 0.3 | 94.8 ± 0.2 |
| Classification Accuracy in US (%) | |||
|---|---|---|---|
| Fold | Knee | KEMG | Reference |
| 1 | 88.4 | 97.8 | 95.4 |
| 2 | 89.3 | 94.0 | 91.8 |
| 3 | 94.5 | 94.8 | 93.1 |
| 4 | 86.9 | 88.5 | 90.0 |
| 5 | 93.3 | 95.9 | 93.1 |
| 6 | 92.0 | 94.2 | 92.5 |
| 7 | 93.6 | 95.2 | 95.3 |
| 8 | 82.8 | 94.9 | 90.3 |
| 9 | 90.8 | 93.3 | 93.5 |
| 10 | 89.5 | 92.5 | 93.0 |
| 11 | 90.4 | 92.5 | 91.5 |
| 12 | 84.0 | 92.8 | 92.6 |
| 13 | 92.2 | 95.5 | 87.6 |
| 14 | 90.9 | 94.9 | 94.5 |
| 15 | 93.5 | 95.3 | 93.3 |
| 16 | 92.4 | 96.5 | 95.8 |
| 17 | 91.8 | 93.4 | 94.5 |
| 18 | 90.0 | 98.1 | 96.1 |
| 19 | 90.5 | 96.7 | 96.0 |
| 20 | 94.9 | 97.5 | 97.3 |
| 21 | 89.6 | 91.5 | 90.6 |
| 22 | 91.9 | 92.5 | 94.3 |
| 23 | 90.1 | 96.8 | 96.3 |
| Mean ± SD | 90.6 ± 2.9 | 94.6 ± 2.3 | 93.4 ± 2.4 |
| HS | MAE (ms) | Precision | Recall | F1-score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold | Knee | KEMG | Ref | Knee | KEMG | Ref | Knee | KEMG | Ref | Knee | KEMG | Ref |
| 1 | 31.8 | 9.9 | 22.3 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 |
| 2 | 38.4 | 34.0 | 31.3 | 1.00 | 1.00 | 0.99 | 0.99 | 0.98 | 0.97 | 1.00 | 0.99 | 0.98 |
| 3 | 13.4 | 20.6 | 29.4 | 1.00 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 |
| 4 | 27.9 | 15.0 | 16.0 | 1.00 | 1.00 | 1.00 | 0.95 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 |
| 5 | 17.4 | 14.5 | 25.0 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| 6 | 18.2 | 16.4 | 22.5 | 1.00 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| 7 | 15.2 | 25.2 | 25.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 8 | 71.1 | 24.7 | 25.2 | 1.00 | 1.00 | 0.99 | 0.99 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 |
| 9 | 53.0 | 20.2 | 19.4 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | 0.99 | 0.99 | 1.00 | 0.99 |
| 10 | 28.5 | 30.4 | 38.2 | 1.00 | 1.00 | 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| 11 | 17.6 | 9.6 | 14.3 | 1.00 | 1.00 | 1.00 | 0.98 | 0.98 | 0.97 | 0.99 | 0.99 | 0.99 |
| 12 | 25.2 | 7.1 | 10.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13 | 31.6 | 18.2 | 29.0 | 1.00 | 1.00 | 1.00 | 0.94 | 0.98 | 0.86 | 0.97 | 0.99 | 0.92 |
| 14 | 47.9 | 15.5 | 18.0 | 1.00 | 1.00 | 1.00 | 0.73 | 0.99 | 0.98 | 0.85 | 1.00 | 0.99 |
| 15 | 33.1 | 17.2 | 21.8 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| 16 | 29.6 | 15.9 | 18.7 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 17 | 38.8 | 37.0 | 27.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 | 29.7 | 9.6 | 13.1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 19 | 26.5 | 19.6 | 12.7 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 20 | 14.0 | 10.4 | 13.4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 21 | 18.5 | 20.3 | 22.4 | 0.94 | 0.99 | 0.98 | 0.96 | 0.97 | 0.98 | 0.95 | 0.98 | 0.98 |
| 22 | 21.2 | 25.7 | 26.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 |
| 23 | 28.8 | 15.0 | 14.8 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| Mean | 29.4 | 18.8* | 21.6 | 1.00 | 1.00 | 1.00 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 | 0.99 |
| SD | 13.7 | 7.9 | 7.0 | 0.01 | 0.01 | 0.01 | 0.04 | 0.01 | 0.03 | 0.03 | 0.01 | 0.02 |
| TO | MAE (ms) | Precision | Recall | F1-score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold | Knee | KEMG | Ref | Knee | KEMG | Ref | Knee | KEMG | Ref | Knee | KEMG | Ref |
| 1 | 116.4 | 16.8 | 26.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 126.8 | 23.4 | 40.1 | 0.99 | 0.99 | 0.96 | 0.98 | 0.97 | 0.94 | 0.98 | 0.98 | 0.95 |
| 3 | 125.3 | 31.9 | 22.6 | 1.00 | 0.98 | 0.99 | 1.00 | 0.99 | 1.00 | 1.00 | 0.98 | 0.99 |
| 4 | 99.6 | 104.0 | 80.8 | 0.99 | 0.99 | 1.00 | 0.93 | 0.99 | 0.99 | 0.96 | 0.99 | 0.99 |
| 5 | 99.6 | 28.6 | 30.1 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | 0.99 | 0.99 | 1.00 | 1.00 |
| 6 | 56.4 | 47.1 | 47.4 | 1.00 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| 7 | 97.9 | 33.2 | 31.2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 8 | 170.5 | 18.7 | 54.1 | 0.96 | 0.99 | 0.97 | 0.95 | 0.99 | 0.97 | 0.96 | 0.99 | 0.97 |
| 9 | 67.9 | 50.6 | 44.2 | 1.00 | 1.00 | 1.00 | 0.97 | 0.99 | 0.98 | 0.98 | 1.00 | 0.99 |
| 10 | 87.0 | 51.6 | 34.0 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 |
| 11 | 107.8 | 53.3 | 59.0 | 0.93 | 0.94 | 0.96 | 0.91 | 0.93 | 0.93 | 0.92 | 0.93 | 0.94 |
| 12 | 67.6 | 64.7 | 59.1 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| 13 | 76.7 | 20.6 | 39.9 | 1.00 | 1.00 | 0.98 | 0.93 | 0.98 | 0.84 | 0.96 | 0.99 | 0.90 |
| 14 | 72.3 | 33.5 | 26.4 | 1.00 | 1.00 | 1.00 | 0.73 | 1.00 | 0.98 | 0.85 | 1.00 | 0.99 |
| 15 | 59.4 | 37.7 | 55.0 | 0.99 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 |
| 16 | 135.8 | 20.2 | 30.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 17 | 94.5 | 32.6 | 28.2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 | 119.4 | 12.3 | 23.9 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 19 | 122.1 | 17.8 | 30.5 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 0.99 | 0.99 | 1.00 | 0.99 |
| 20 | 119.2 | 19.1 | 17.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 21 | 90.9 | 38.3 | 37.6 | 0.96 | 0.98 | 0.96 | 0.97 | 0.95 | 0.95 | 0.96 | 0.96 | 0.96 |
| 22 | 117.4 | 49.5 | 35.2 | 0.97 | 0.98 | 0.99 | 0.97 | 0.98 | 0.98 | 0.97 | 0.98 | 0.99 |
| 23 | 59.5 | 21.3 | 23.8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mean | 99.5 | 35.9* | 38.1 | 0.99 | 0.99 | 0.99 | 0.97 | 0.99 | 0.98 | 0.98 | 0.99 | 0.98 |
| SD | 28.9 | 20.6 | 14.2 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.04 | 0.04 | 0.01 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, F.; Morbidoni, C.; Cucchiarelli, A.; Fioretti, S. Recognition of Gait Phases with a Single Knee Electrogoniometer: A Deep Learning Approach. Electronics 2020, 9, 355. https://doi.org/10.3390/electronics9020355
Di Nardo F, Morbidoni C, Cucchiarelli A, Fioretti S. Recognition of Gait Phases with a Single Knee Electrogoniometer: A Deep Learning Approach. Electronics. 2020; 9(2):355. https://doi.org/10.3390/electronics9020355
Chicago/Turabian StyleDi Nardo, Francesco, Christian Morbidoni, Alessandro Cucchiarelli, and Sandro Fioretti. 2020. "Recognition of Gait Phases with a Single Knee Electrogoniometer: A Deep Learning Approach" Electronics 9, no. 2: 355. https://doi.org/10.3390/electronics9020355
APA StyleDi Nardo, F., Morbidoni, C., Cucchiarelli, A., & Fioretti, S. (2020). Recognition of Gait Phases with a Single Knee Electrogoniometer: A Deep Learning Approach. Electronics, 9(2), 355. https://doi.org/10.3390/electronics9020355








