An Interference Suppression Method for Non-Contact Bioelectric Acquisition
Abstract
1. Introduction
2. Methods and Models
2.1. Traditional Capacitive Driven-Right-Leg Model for Eliminating Common Mode Interference
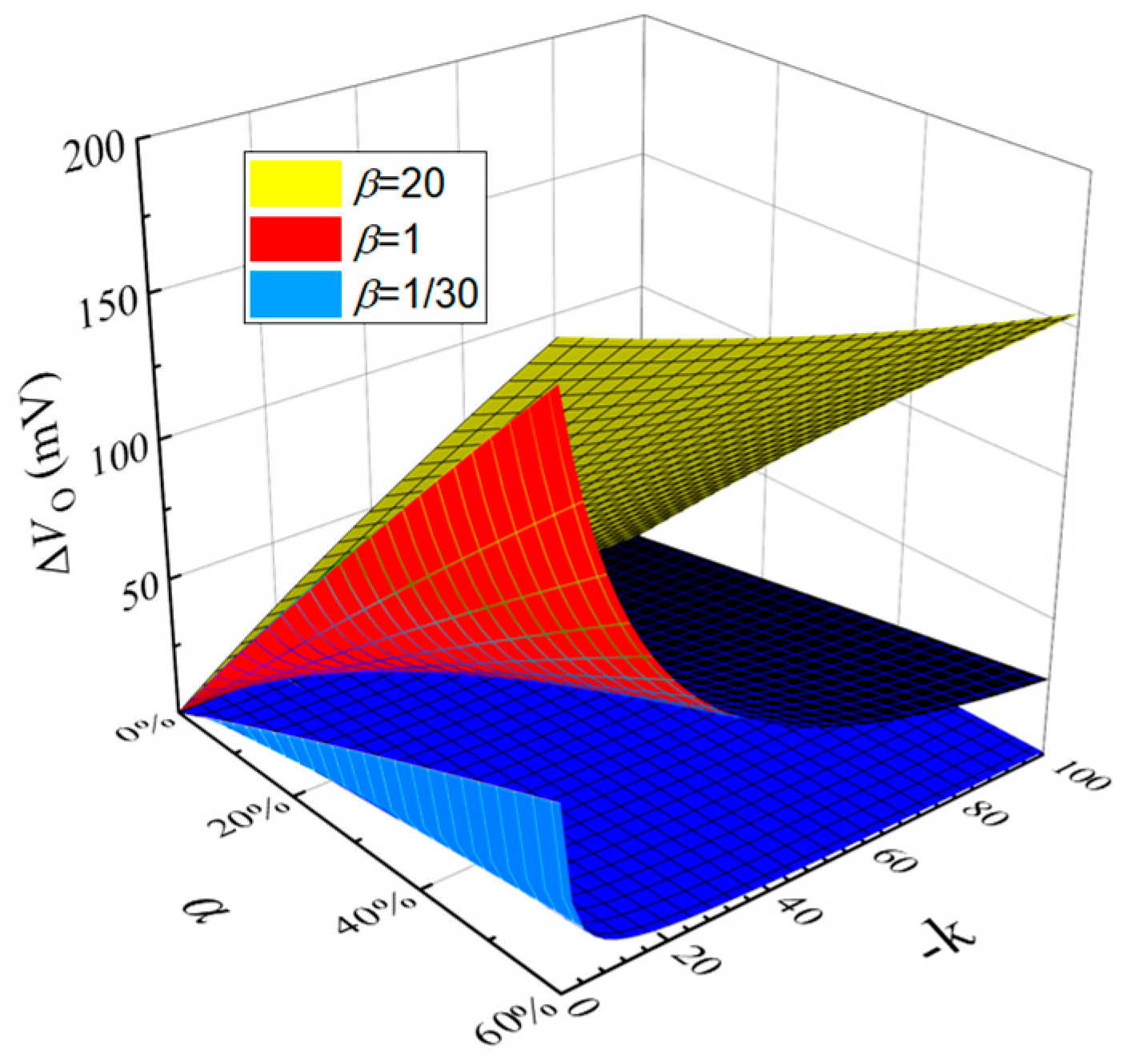
2.2. Noise Neutralization Method to Eliminate Common Mode Interference Model
3. Experiments
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | Analog to digital converter |
| Ag/AgCl | Silver/silver chloride |
| C-DRL | Capacitive driven-right-leg |
| CMI | Common mode interference |
| CMRR | Common mode rejection ratio |
| DRL | Driven-right-leg |
| ECG | Electrocardiography |
| MCU | Micro control unit |
| PDMC | Pseudo difference mode components |
References
- Spinelli, E.; Haberman, M. Insulating electrodes: A review on biopotential front ends for dielectric skin-electrode interfaces. Physiol. Meas. 2010, 31, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Leicht, L.; Eilebrecht, B.; Weyer, S.; Leonhardt, S.; Teichmann, D. Closed-loop control of humidification for artifact reduction in capacitive ECG measurements. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 300–313. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.; Yu, X.; Neu, W.; Leonhardt, S.; Teichmann, D. A Novel 12-Lead ECG T-Shirt with Active Electrodes. Electronics 2016, 5, 75. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.B. Capacitive biopotential measurement for electrophysiological signal acquisition: A review. IEEE Sens. J. 2016, 16, 2832–2853. [Google Scholar] [CrossRef]
- Oehler, M.; Ling, V.; Melhorn, K.; Schilling, M. A multichannel portable ECG system with capacitive sensors. Physiol. Meas. 2008, 29, 783–793. [Google Scholar] [CrossRef]
- Wannenburg, J.; Malekian, R.; Hancke, G.P. Wireless capacitive based ECG sensing for feature extraction and mobile health monitoring. IEEE Sens. J. 2018, 18, 6023–6032. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Hou, Z.; Xue, X. Simultaneously capturing electrocardiography and impedance plethysmogram signals from human feet by capacitive coupled electrode system. IEEE Sens. J. 2017, 17, 5654–5662. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef]
- Tomasini, M.; Benatti, S.; Milosevic, B.; Farella, E.; Benini, L. Power line interference removal for high-quality continuous biosignal monitoring with low-power wearable devices. IEEE Sens. J. 2016, 16, 3887–3895. [Google Scholar] [CrossRef]
- Sullivan, T.J.; Deiss, S.R.; Jung, T.P.; Cauwenberghs, G. A brain-machine interface using dry-contact, low-noise EEG sensors. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS 2008), Seattle, WA, USA, 18–21 May 2008; pp. 1986–1989. [Google Scholar]
- Kim, K.K.; Park, K.S. Effective coupling impedance for power line interference in capacitive-coupled ECG measurement system. In Proceedings of the International Conference on Information Technology and Applications in Biomedicine (ITAB 2008), Shenzhen, China, 30–31 May 2008; pp. 256–258. [Google Scholar]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG measurement on a chair without conductive contact. IEEE Trans. Biomed. Eng. 2006, 53, 956–959. [Google Scholar] [PubMed]
- Lim, Y.G.; Chung, G.S.; Park, K.S. Capacitive driven-right-leg grounding in indirect-contact ECG measurement. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology (EMBS 2010), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1250–1253. [Google Scholar]
- Kim, K.K.; Lim, Y.G.; Park, K.S. Common mode noise cancellation for electrically non-contact ECG measurement system on a chair. In Proceedings of the IEEE Engineering in Medicine and Biology 27th Annual Conference (EMBS 2005), Shanghai, China, 17–18 January 2006; pp. 5881–5883. [Google Scholar]
- Pallas-Areny, R. On the reduction of interference due to common mode voltage in two-electrode biopotential amplifiers. IEEE Trans. Biomed. Eng. 1986, 33, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.B.; Webster, J.G. Reduction of interference due to common mode voltage in biopotential amplifiers. IEEE Trans. Biomed. Eng. 1983, 30, 58–62. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Sim, K.; Kim, K.; Park, K.S. Noise reduction for non-contact electrocardiogram measurement in daily life. In Proceedings of the 36th Annual Computers in Cardiology Conference (CinC 2009), Park City, UT, USA, 13–16 September 2009; pp. 493–496. [Google Scholar]
- Guermandi, M.; Scarselli, E.F.; Guerrieri, R. A driving right leg circuit (DgRL) for improved common mode rejection in bio-potential acquisition systems. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 507–517. [Google Scholar] [CrossRef]
- Steffen, M.; Aleksandrowicz, A.; Leonhardt, S. Mobile noncontact monitoring of heart and lung activity. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 250–257. [Google Scholar] [CrossRef]
- Winter, B.B.; Webster, J.G. Driven-right-leg circuit design. IEEE Trans. Biomed. Eng. 1983, 30, 62–66. [Google Scholar] [CrossRef]
- Villegas, A.; McEneaney, D.; Escalona, O. Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics 2019, 8, 1300. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Y.; Zhang, L.; Yan, F.; Gu, X.; Wu, R. A novel front-end design for bioelectrical signal wearable acquisition. IEEE Sens. J. 2019, 19, 8009–8018. [Google Scholar] [CrossRef]
- Xu, J.; Mitra, S.; Matsumoto, A.; Patki, S.; VanHoof, C.; Makinwa, K.A.A.; Yazicioglu, R.F. A wearable 8-channel active-electrode EEG/ETI acquisition system for body area networks. IEEE J. Solid-State Circuits 2014, 49, 2005–2016. [Google Scholar] [CrossRef]
- Sakuma, J.; Anzai, D.; Wang, J. Performance of human body communication-based wearable ECG with capacitive coupling electrodes. Healthc. Technol. Lett. 2016, 3, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Serteyn, A.; Vullings, R.; Meftah, M.; Bergmans, J.W.M. Motion artifacts in capacitive ECG measurements: Reducing the combined effect of DC voltages and capacitance changes using an injection signal. IEEE Trans. Biomed. Eng. 2015, 62, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Min, S.D.; Yun, Y.H.; Lee, S.; Lee, M. Adaptive motion artifacts reduction using 3-axis accelerometer in e-textile ECG measurement system. J. Med. Syst. 2008, 32, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Couto, P. A neural network approach to ECG denoising. arXiv 2012, arXiv:1212.5217. [Google Scholar]
- AAMI; ANSI. Diagnostic Electrocardiographic Devices; Standard ANSI/AAMI EC11; AAMI: Washington, DC, USA, 2007. [Google Scholar]
- Wu, R.; Tang, Y.; Li, Z.; Zhang, L.; Yan, F. A novel high input impedance front-end for capacitive biopotential measurement. Med. Biol. Eng. Comput. 2018, 56, 1343–1355. [Google Scholar] [CrossRef] [PubMed]














| Simulation of Electrode Mismatch | Input Signal Amplitude of Electrode 1 | CMRR | |
|---|---|---|---|
| C-DRL Method | Proposed Method | ||
| α = 0 | P: 500 mV N: 500 mV | 91.45 dB | 90.36 dB |
| α = 20% | P: 500 mV N: 400 mV | 14.40 dB 2 | 90.22 dB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Chang, R.; Zhang, L.; Yan, F. An Interference Suppression Method for Non-Contact Bioelectric Acquisition. Electronics 2020, 9, 293. https://doi.org/10.3390/electronics9020293
Tang Y, Chang R, Zhang L, Yan F. An Interference Suppression Method for Non-Contact Bioelectric Acquisition. Electronics. 2020; 9(2):293. https://doi.org/10.3390/electronics9020293
Chicago/Turabian StyleTang, Yue, Ronghui Chang, Limin Zhang, and Feng Yan. 2020. "An Interference Suppression Method for Non-Contact Bioelectric Acquisition" Electronics 9, no. 2: 293. https://doi.org/10.3390/electronics9020293
APA StyleTang, Y., Chang, R., Zhang, L., & Yan, F. (2020). An Interference Suppression Method for Non-Contact Bioelectric Acquisition. Electronics, 9(2), 293. https://doi.org/10.3390/electronics9020293






