Wireless Epidermal Electromyogram Sensing System
Abstract
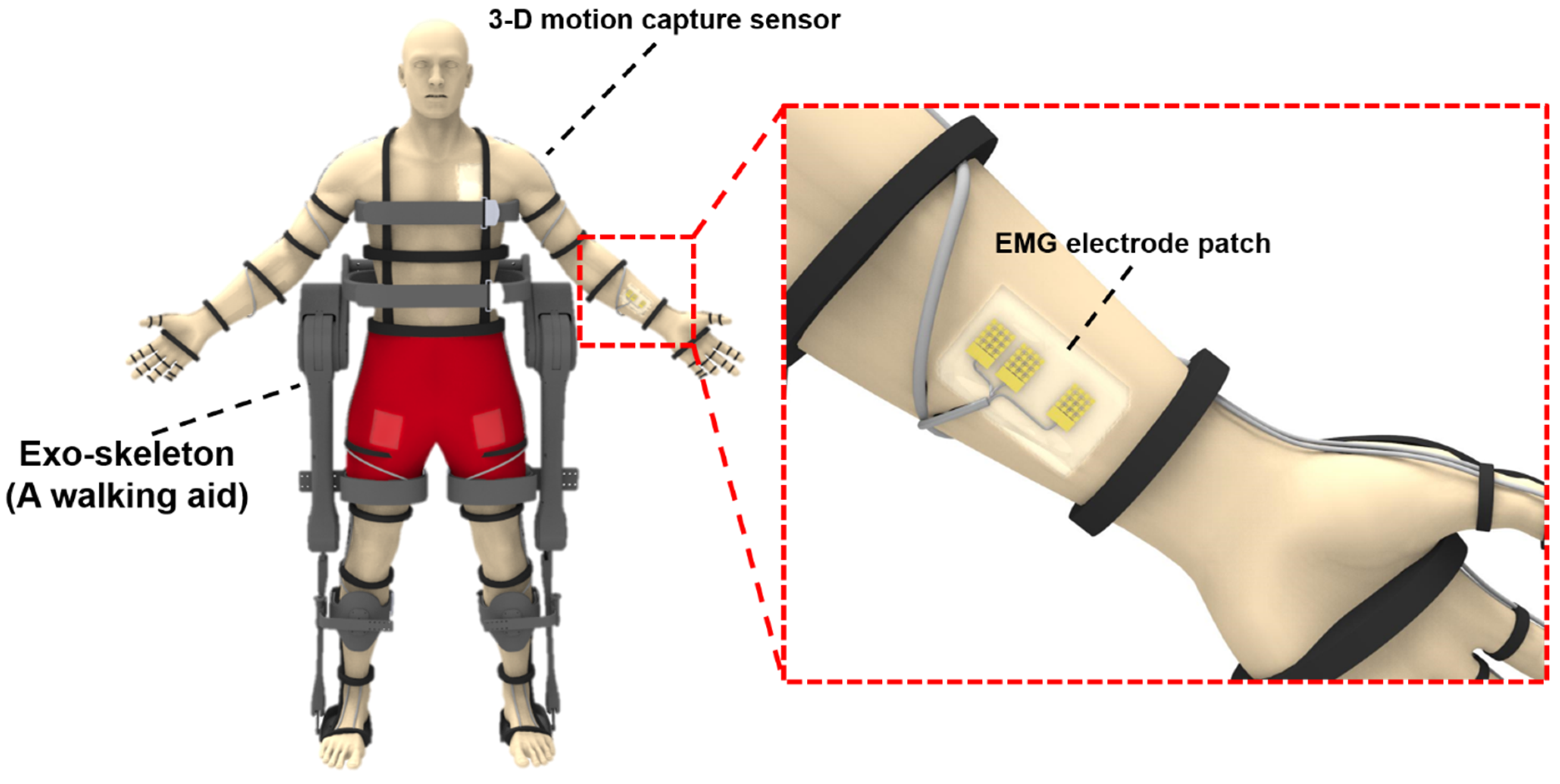
1. Introduction
2. Materials and Methods
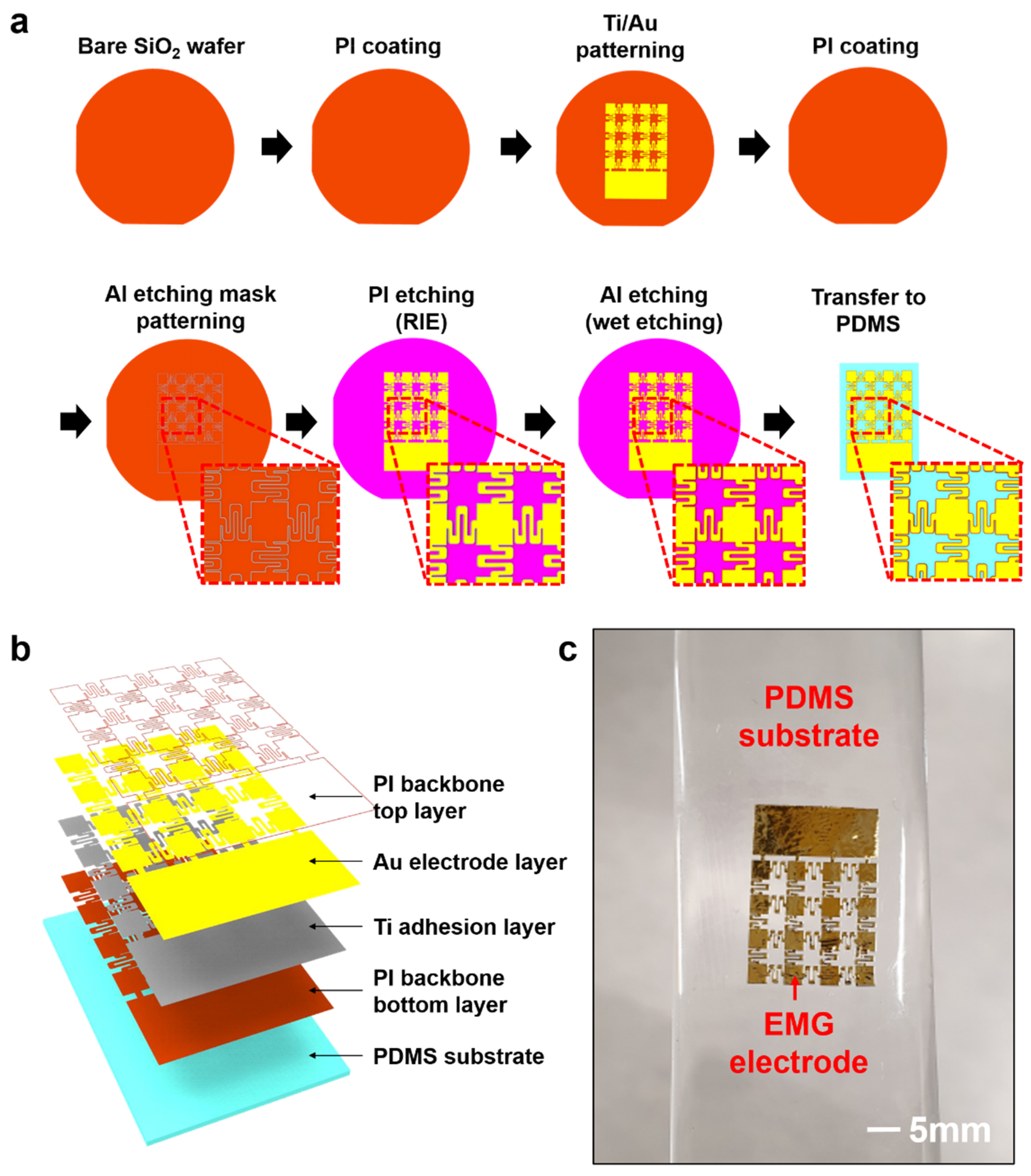
2.1. Fabrication and Transfer Printing of Stretchable EMG Electrode
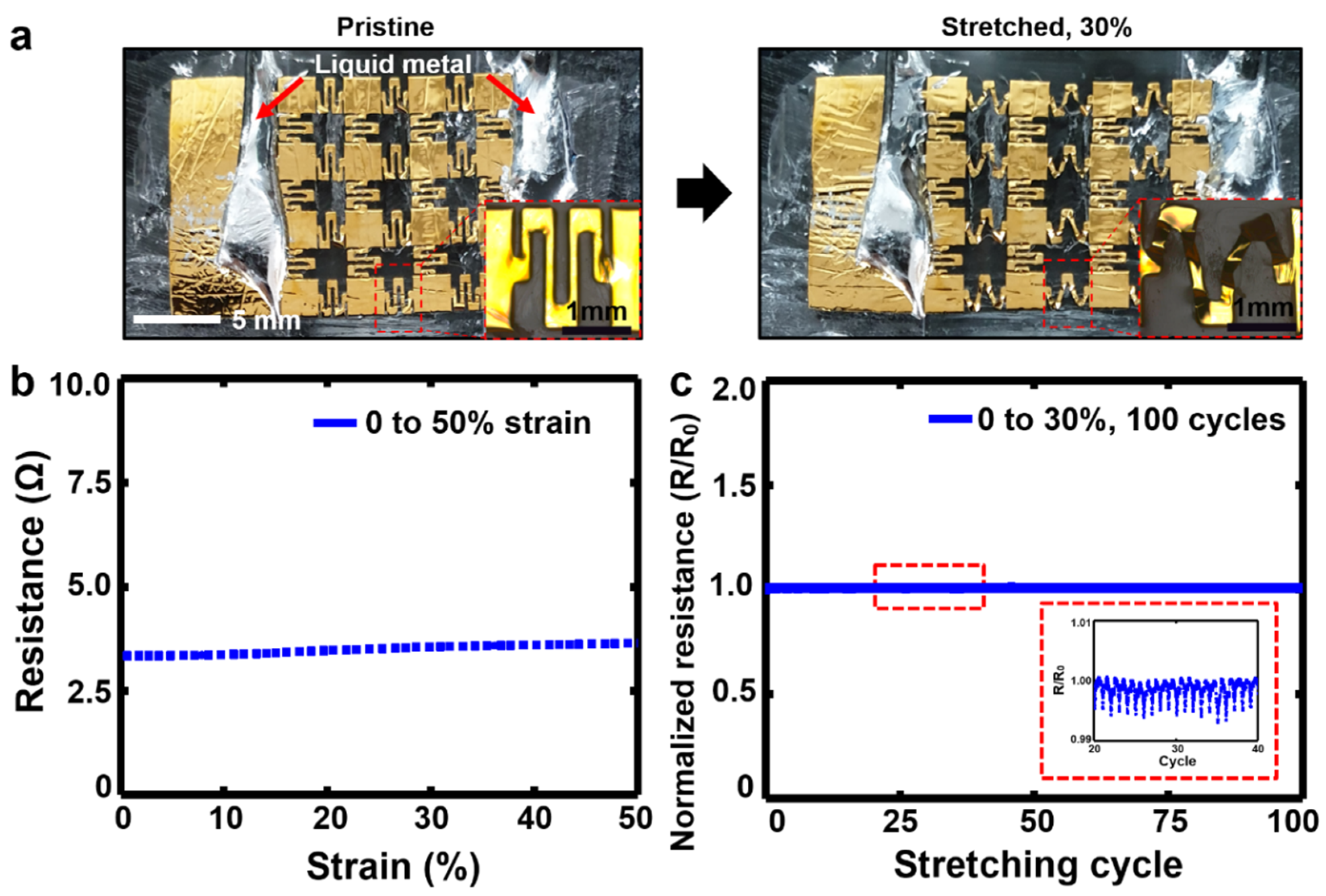
2.2. Mechanical and Electrical Characterizations of the Stretchable EMG Electrodes
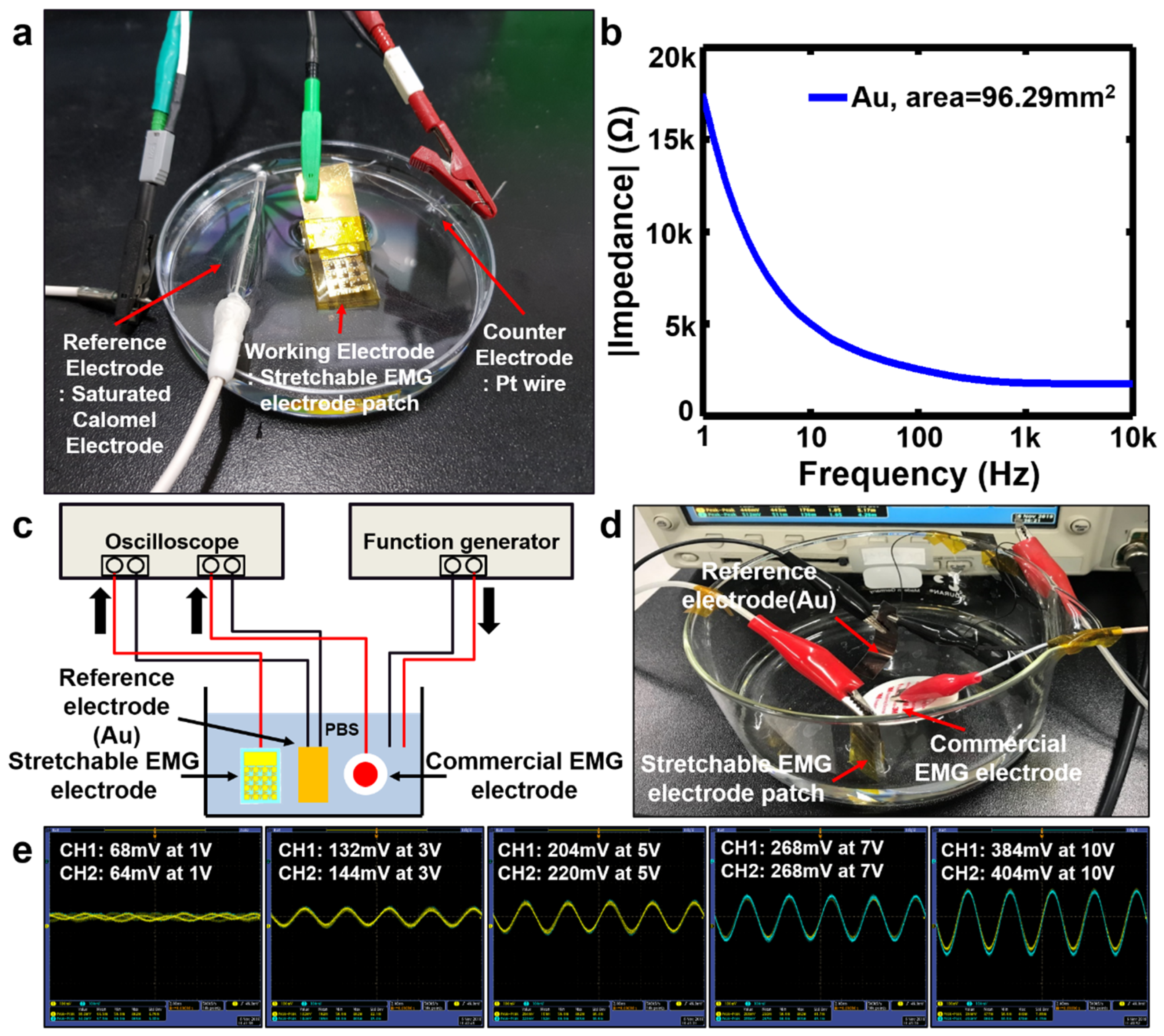
2.3. In Vitro Demonstration of the Electrical Performance of the Stretchable EMG Electrodes
2.3.1. Electrochemical Impedance Characterization
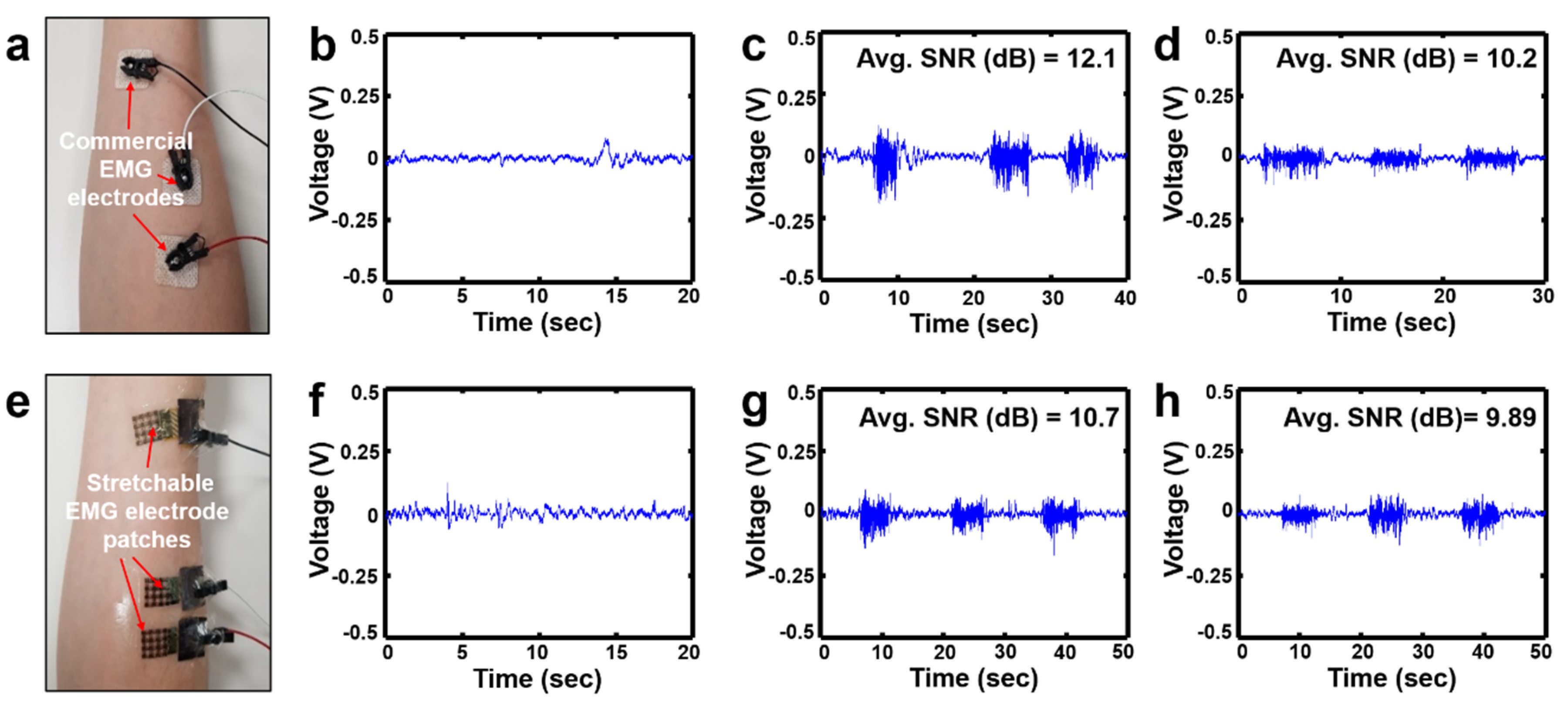
2.3.2. Assessment of Signal Acquisition Performance
2.4. In vivo EMG Recording
2.4.1. Sample Preparation for Epidermal EMG Sensor
2.4.2. Assessment of the EMG Recording Performance
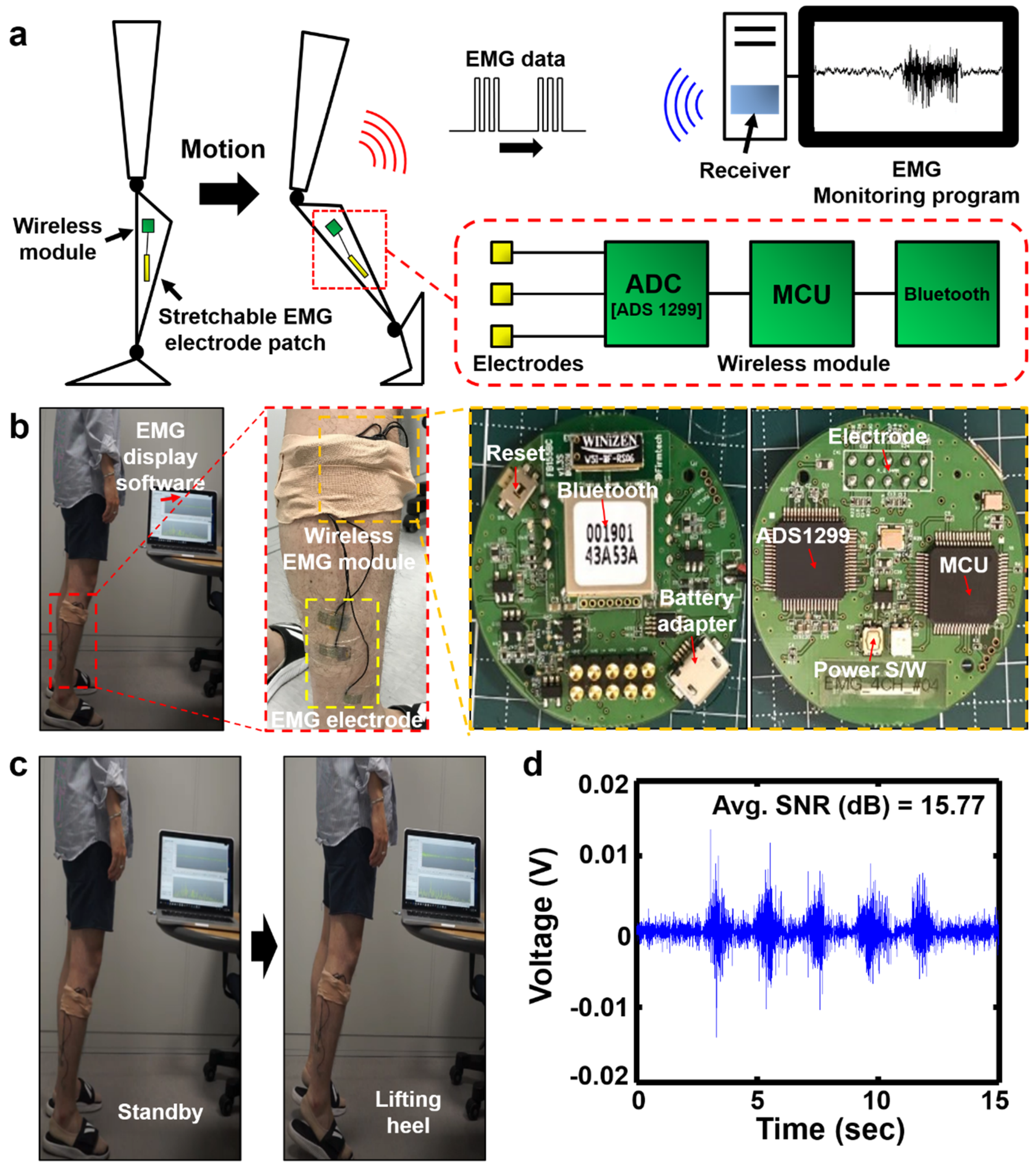
2.5. Wireless EMG Monitoring
3. Results
3.1. Mechanical and Electrical Properties of the Stretchable EMG Electrodes
3.2. In Vitro Electrical Performance of the Stretchable EMG Electrodes
3.3. In Vivo Vital Sign Recording Using the Stretchable EMG Sensor
3.4. Demonstration of Wireless EMG Signal Monitoring
4. Discussion and Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Fiers, P.; Witte, K.A.; Jackson, R.W.; Poggensee, K.L.; Atkeson, C.G.; Collins, S.H. Human in the-roop optimization of exoskeleton assistance during walking. Science 2017, 356, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Kim, M.; Kuindersma, S.; Walsh, C.J. Human-in-the-loop optimization of hip assistance with a soft exosuit during walking. Sci. Rob. 2018, 3, 15. [Google Scholar] [CrossRef]
- Quinlivan, B.T.; Lee, S.; Malcolm, P.; Rossi, D.M.; Grimmer, M.; Siviy, C.; Karavas, N.; Wagner, D.; Asbeck, A.; Galiana, I.; et al. Assistance magnitude versus metabolic cost reductions for a tethered multiarticular soft exosuit. Sci. Rob. 2017, 2. [Google Scholar] [CrossRef]
- Choi, H.; Seo, K.; Hyung, S.; Shim, Y.; Lim, S.-C. Compact Hip-Force sensor for a Gait-Assistance exoskeleton system. Sensors 2018, 18, 566. [Google Scholar] [CrossRef]
- Malcolm, P.; Derave, W.; Galle, S.; Clercq, D. A simple exoskeleton that assists plantarflexion can reduce the metabolic cost of human walking. PLoS ONE 2013, 8, e56137. [Google Scholar] [CrossRef]
- Grimmer, M.; Quinlivan, B.T.; Lee, S.; Malcolm, P.; Rossi, D.M.; Sivily, C.; Walsh, C.J. Comparison of the human-exosuit interaction using ankle moment andankle positive power inspired walking assistance. J. Biomech. 2019, 83, 76–84. [Google Scholar] [CrossRef]
- Lee, G.; Ding, Y.; Bujanda, I.G.; Karavas, N.; Zhou, Y.M.; Walsh, C.J. Improved assistive profile tracking of soft exosuits for walking and jogging with off-board actuation. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 1699–1706. [Google Scholar]
- Lee, S.; Karavas, N.; Quinlivan, B.T.; Ryan, D.L.; Perry, D.; Eckert-Erdheim, A.; Murphy, P.; Goldy, T.G.; Menard, N.; Athanassiu, M.; et al. Autonomous Multi-Joint Soft Exosuit for Assistance with Walking Overground. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018; pp. 2812–2819. [Google Scholar]
- Asbeck, A.T.; Rossi, S.M.M.; Holt, K.G.; Walsh, C.J. A Biologically Inspired Soft Exosuit for Walking Assistance. Int. J. Rob. Res. 2015, 34, 744–762. [Google Scholar] [CrossRef]
- Panizzolo, F.A.; Galiana, I.; Asbeck, A.T.; Siviy, C.; Schmidt, K.; Holt, K.G.; Walsh, C.J. A biologically-inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking. J. Neuroeng. Rehabil. 2016, 13, 43. [Google Scholar] [CrossRef]
- Lerner, Z.F.; Gasparri, G.M.; Bair, M.O.; Lawson, J.L.; Luque, J.; Harvey, T.A.; Lerner, A.T. An untethered ankle exoskeleton improves walking economy in a pilot study of individuals with cerebral palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1895–1993. [Google Scholar] [CrossRef]
- Lerner, Z.F.; Damiano, D.L.; Bulea, T.C. A robotic exoskeleton to treat crouch gait from cerebral palsy: Initial kinematic and neuromuscular evaluation. In Proceedings of the IEEE Engineering in Medicine and Biology Society (EMBC), Lake Buena Vista, FL, USA, 16–20 August 2016; pp. 2214–2217. [Google Scholar]
- Lee, S.-H.; Lee, H.-J.; Chang, W.H.; Choi, B.-O.; Lee, J.; Kim, J.; Ryu, G.-H.; Kim, Y.-H. Gait performance and foot pressure distribution during wearable robot-assisted gait in elderly adults. J. Neuroeng. Rehabil. 2017, 14, 123. [Google Scholar] [CrossRef]
- Mooney, L.M.; Herr, H.M. Biomechanical walking mechanisms underlying the metabolic reduction caused by an autonomous exoskeleton. J. Neuroeng. Rehabil. 2016, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Goher, K. Working Aids for older adults: Review of end-user needs. Asian Soc. Sci. 2016, 12, 12. [Google Scholar] [CrossRef]
- Martini, E.; Crea, S.; Vitiello, N. Gait training using a robotic hip exoskeleton improves metabolic gait efficiency in the elderly. Sci. Rep. 2019, 9, 7157. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Curran, C.; DasMahapatra, P. Mobility assessment using wearable technology in patients with late-onset Pompe disease. NPJ Digit. Med. 2019, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, T.; Carpinella, I.; Ferrarin, M. Human kinematic, kinetic and EMG data during different walking and stair ascending and descending tasks. Sci. Data 2019, 6, 309. [Google Scholar] [CrossRef] [PubMed]
- Diller, S.; Majidi, C.; Collins, S. Exoskeleton walking with a lightweight, low power electroadhesive clutch and spring for exoskeleton actuation. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016. [Google Scholar]
- Lerner, Z.F.; Damiano, D.L.; Bulea, T.C. Relationship between assistive torque and knee biomechanics during exoskeleton walking in individuals with crouch gait. In Proceedings of the IEEE International Conference on Rehabilitation Robotics (ICORR) QEII Centre, London, UK, 17–20 July 2017. [Google Scholar]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Ma, R.; Kim, D.-H.; McCormick, M.; Coleman, T.; Rogers, J.A. A Stretchable Electrode Array for Non-invasive, Skin-Mounted Measurement of Electrocardiography (ECG), Electromyography (EMG) and Electroencephalography (EEG). In Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar]
- Huang, Z.; Hao, Y.; Li, Y.; Hu, H.; Wang, C.; Nomoto, A.; Pan, T.; Gu, Y.; Chen, Y.; Zhang, T.; et al. Three-dimensional integrated stretchable electronics. Nat. Elec. 2018, 1, 473–480. [Google Scholar] [CrossRef]
- Kim, J.; Son, D.; Lee, M.; Song, C.; Song, J.K.; Koo, J.H.; Lee, D.J.; Shim, J.H.; Kim, J.H.; Lee, M.; et al. A wearable multiplexed silicon nonvolatile memory array using nanocrystal charge confinement. Sci. Adv. 2016, 2, e1501101. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, H.J.; Ghaffari, R.; Hyeon, T.; Kim, D.-H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Koo, J.H.; Kim, D.C.; Shim, H.J.; Kim, T.H.; Kim, D.-H. Flexible and Stretchable Smart Display: Materials, Fabrication, Device Design, and System Integration. Adv. Funct. Mater. 2018, 28, 1801834. [Google Scholar] [CrossRef]
- Kim, D.-H.; Rogers, J.A. Stretchable Electronics: Materials, Strategies and Devices. Adv. Mater. 2008, 20, 4887–4892. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, J.K.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T.; et al. Stretchable Heater Using Ligand-Exchanged Silver Nanowire Nanocomposite for Wearable Articular Thermotherapy. ACS Nano 2015, 9, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Yeo, W.H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Comm. 2014, 5, 3266. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Kim, D.-H.; Xiao, J.; Kim, B.H.; Park, S.I.; Panilaitis, B.; Ghaffari, R.; Yao, J.; Li, M.; Liu, Z.; et al. Waterproof AlInGaP Optoelectronics on Flexible Tubing, Sutures, Gloves and Other Unusual Substrates with Application Examples in Biomedicine and Robotics. Nat. Mater. 2010, 9, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Li, Y.; Song, J.; Yu, C. Biaxially stretchable ultrathin Si enabled by serpentine structures on prestrained elastomers. Adv. Mater. Technol. 2019, 4, 1800489. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and Mechanics for Stretchable Electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef]
- Choi, M.K.; Yang, J.; Kim, D.C.; Dai, Z.; Kim, J.; Seung, H.; Kale, V.S.; Sung, S.J.; Park, C.R.; Lu, N.; et al. Extremely Vivid, Highly Transparent, and Ultrathin Quantum Dot Light-Emitting Diodes. Adv. Mater. 2017, 30, 1703279. [Google Scholar] [CrossRef]
- Krishnan, S.; Shi, Y.; Webb, R.C.; Ma, Y.; Bastien, P.; Crawford, K.E.; Wang, A.; Feng, X.; Manco, M.; Kurniawan, J.; et al. Multimodal epidermal devices for hydration monitoring. Microsyst. Nanoeng. 2017, 3, 17014. [Google Scholar] [CrossRef]
- Lopes, P.A.; Paisana, H.; Almeida, A.T.; Majidi, C.; Tavakoli, M. Hydroprinted Electronics: Ultrthin stretchable Ag-In-Ga E-skin for bioelectronics and human-machine interaction. ACS Appl. Mater. Interfaces 2018, 10, 38760–38768. [Google Scholar] [CrossRef]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.-G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.-H.; Bao, Z. Highly skin-conformal microhairy sensor for pulse signal amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Roberts, T.; Zhang, Y.; Ismailova, E.; Herve, T.; Malliaras, G.G.; Graat, J.B.; Inal, S.; Saadaoui, M. Fully printed all-polymer tattoo/textile electronics for electromyography. Flex. Print Electron. 2018, 3, 034004. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Bao, Z. Nanomaterials in Skin-Inspired Electronics; Toward Soft and Robust Skin-like Electronic Nanosystems. ACS Nano 2018, 12, 11731. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Sim, K.; Ershad, F.; Yang, P.; Thukral, A.; Rao, Z.; Kim, H.J.; Liu, Y.; Wang, X.; Gu, G.; et al. Stretchable elastic synaptic transistors for neurologically integrated soft engineering systems. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Rao, Z.; Kim, H.J.; Thukral, A.; Shim, H.; Yu, C. Fully rubbery integrated electronics from high effective mobility intrinsically stretchable semiconductors. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent, elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Sekitani, T.; Nakajima, H.; Maeda, H.; Fukushima, T.; Aida, T.; Hata, K.; Someya, T. Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 2009, 8, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.C.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-Healable Thin Film Electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Niu, S.; Wang, L.; Lopez, J.; Chen, S.; Cai, Y.; Du, R.; Liu, Y.; Lai, J.C.; Liu, L.; et al. An Elastic Autonomous Self-Healing Capacitive Sensor Based on a Dynamic Dual Crosslinked Chemical System. Adv. Mater. 2018, 30, 1801435. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, H.; Allec, S.I.; Wong, B.M.; Nguyen, D.S.; Wang, C. A Highly Stretchy, Transparent Elastomer with the Capability to Automatically Self-Heal Underwater. Adv. Mater. 2018, 30, 1804602. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 2018, 30, 1706846. [Google Scholar] [CrossRef]
- Kang, J.; Tok, J.B.H.; Bao, Z. Self-healing soft electronics. Nat. Elec. 2019, 2, 144–150. [Google Scholar] [CrossRef]
- Son, D.; Kang, J.; Vardoulis, O.; Kim, Y.; Matsuhisa, N.; Oh, J.Y.; To, J.W.; Mun, J.; Katsumata, T.; Liu, Y.; et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 2018, 13, 1057–1065. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.S.; Kang, J.H.; Hong, J.Y.; Seong, D.-H.; Kim, H.J.; Kim, J.; Mun, J.; Youn, I.; Kim, J.; et al. An Ultrastretchable and Self-Healable Nanocomposite Conductor Enabled by Autonomously Percolative Electrical Pathways. ACS Nano 2019, 13, 6531–6539. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Vardoulis, O.; Mun, J.W.; Matsuhisa, N.; Kim, Y.; Kim, J.; Tok, J.B.H.; Bao, Z. Modular and Reconfigurable Stretchable Electronic Systems. Adv. Mater. Technol. 2018, 4, 1800417. [Google Scholar] [CrossRef]
- Khatib, M.; Huynh, T.P.; Deng, Y.; Horev, Y.D.; Saliba, W.; Wu, W.; Haick, H. A Freestanding Stretchable and Multifunctional Transistor with Intrinsic Self-Healing Properties of all Device Components. Small 2019, 15, 1803939. [Google Scholar]
- Rogel, R.; Borgne, B.L.; Mohammed-Brahim, T.; Jacques, E.; Harnois, M. Spontaneous buckling of multiaxially flexible and stretchable interconnects using PDMS/fibrous composite substrates. Adv. Mater. Interfaces 2017, 4, 1600946. [Google Scholar] [CrossRef]
- Linghu, C.; Zhang, S.; Wang, C.; Song, J. Transfer printing techniques for flexible and stretchable inorganic electronics. NPJ Flex. Electron. 2018, 2, 26. [Google Scholar] [CrossRef]
- Le Borgne, B.; Liu, S.; Morvan, X.; Crand, S.; Sporea, R.A.; Lu, N.; Harnois, M. Water Transfer Printing Enhanced by Water-Induced Pattern Expansion: Toward Large-Area 3D Electronics. Adv. Mater. Technol. 2019, 4, 1800600. [Google Scholar] [CrossRef]
- Wiedemann, L.G.; McDaid, A.J. On the function and robustness of skin-electrode interfaces for high-density electromyography: Towards ubiquitous integration with robotics devices. In Proceedings of the 2017 IEEE Life Sciences Conference (LSC), Sydney, Australia, 13–15 December 2017; pp. 137–140. [Google Scholar]
- Hegyi, A.; Csala, D.; Péter, A.; Finni, T.; Cronin, N.J. High-density electromyography activity in various hamstring exercises. Scand. J. Med. Sci. Sports 2019, 29, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Rasool, G.; Afsharipour, B.; Suresh, N.L.; Rymer, W.Z. Spatial Analysis of Multichannel Surface EMG in Hemiplegic Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1802–1811. [Google Scholar] [CrossRef]
- Constantinescu, G.; Jeong, J.; Li, X.; Scott, D.K.; Jang, K.; Chung, H.; Rogers, J.; Rieger, J. Epidermal electronics for electromyography: An application to swallowing therapy. Med. Eng. Phys. 2016, 38, 807–812. [Google Scholar] [CrossRef]
- Posada-Quintero, H.; Noh, Y.; Eaton-Robb, C.; Florain, J.; Chon, K. Feasibility Testing of Hydrophobic Carbon Electrodes for Acquisition of Underwater Surface Electromyography Data. Ann. Biomed Eng. 2018, 46, 1397–1405. [Google Scholar] [CrossRef]
- Lee, J.W.; Wang, S.; Albers, C.; Dunne, L.E. Garment-based EMG system for intra-spacesuit biomechanics analysis. Assoc. Comput. Mach. 2018, 272–277. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Ní Anniadh, A.; Bruyere, K.; Otténio, M.; Xie, H.; Gilchrist, M.D. Dynamic Tensile Properties of Human Skin. Proc. Int. Res. Counc. Biomech. Inj. Conf. 2012, 40, 494–502. [Google Scholar]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yoon, J.; Lee, D.; Seong, D.; Lee, S.; Jang, M.; Choi, J.; Yu, K.J.; Kim, J.; Lee, S.; et al. Wireless Epidermal Electromyogram Sensing System. Electronics 2020, 9, 269. https://doi.org/10.3390/electronics9020269
Lee S, Yoon J, Lee D, Seong D, Lee S, Jang M, Choi J, Yu KJ, Kim J, Lee S, et al. Wireless Epidermal Electromyogram Sensing System. Electronics. 2020; 9(2):269. https://doi.org/10.3390/electronics9020269
Chicago/Turabian StyleLee, Sungjun, Jiyong Yoon, Daewoong Lee, Duhwan Seong, Sangkyu Lee, Minsu Jang, Junho Choi, Ki Jun Yu, Jinseok Kim, Sangyoup Lee, and et al. 2020. "Wireless Epidermal Electromyogram Sensing System" Electronics 9, no. 2: 269. https://doi.org/10.3390/electronics9020269
APA StyleLee, S., Yoon, J., Lee, D., Seong, D., Lee, S., Jang, M., Choi, J., Yu, K. J., Kim, J., Lee, S., & Son, D. (2020). Wireless Epidermal Electromyogram Sensing System. Electronics, 9(2), 269. https://doi.org/10.3390/electronics9020269






