Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification
Abstract
1. Introduction
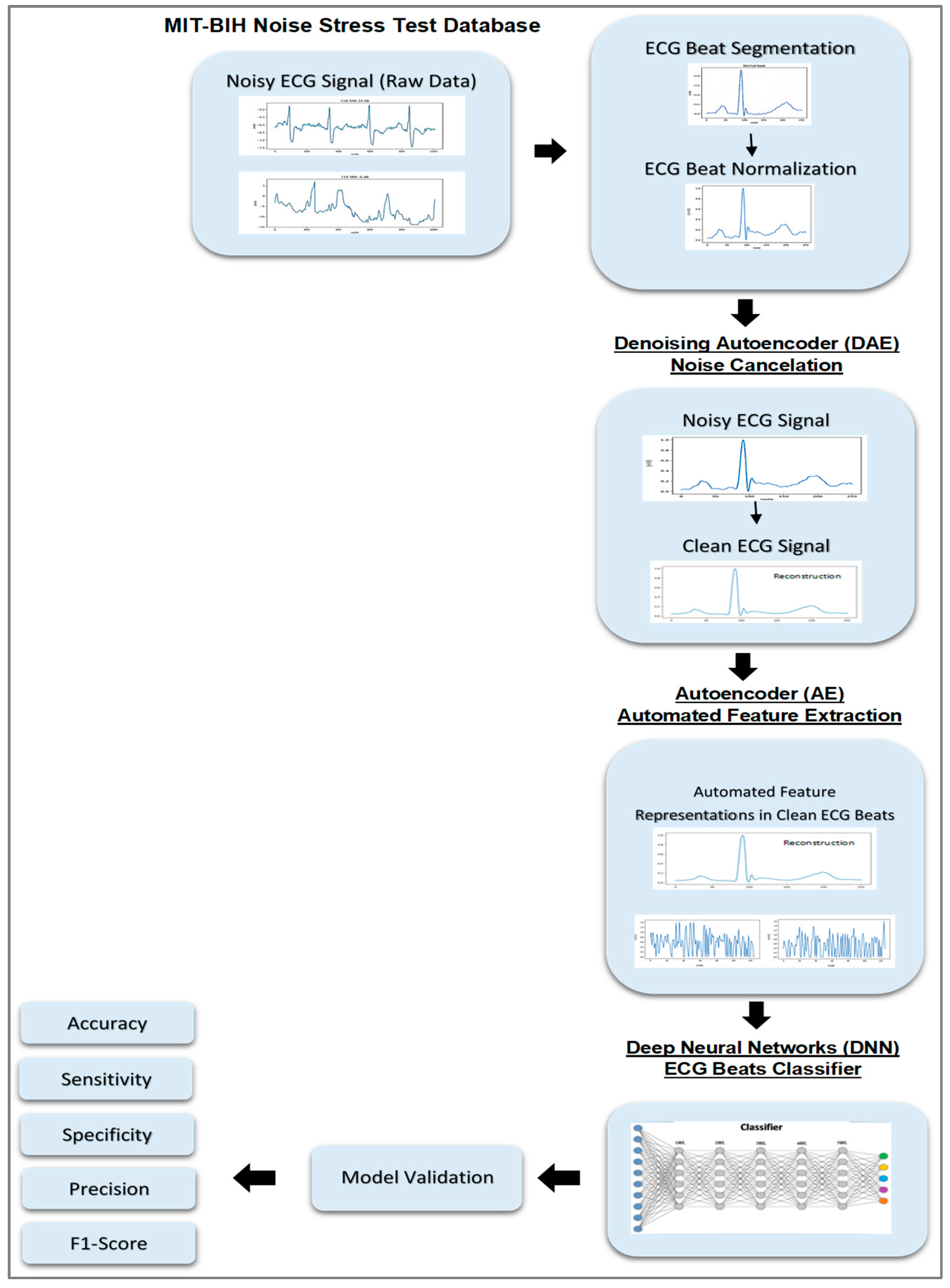
2. Research Method
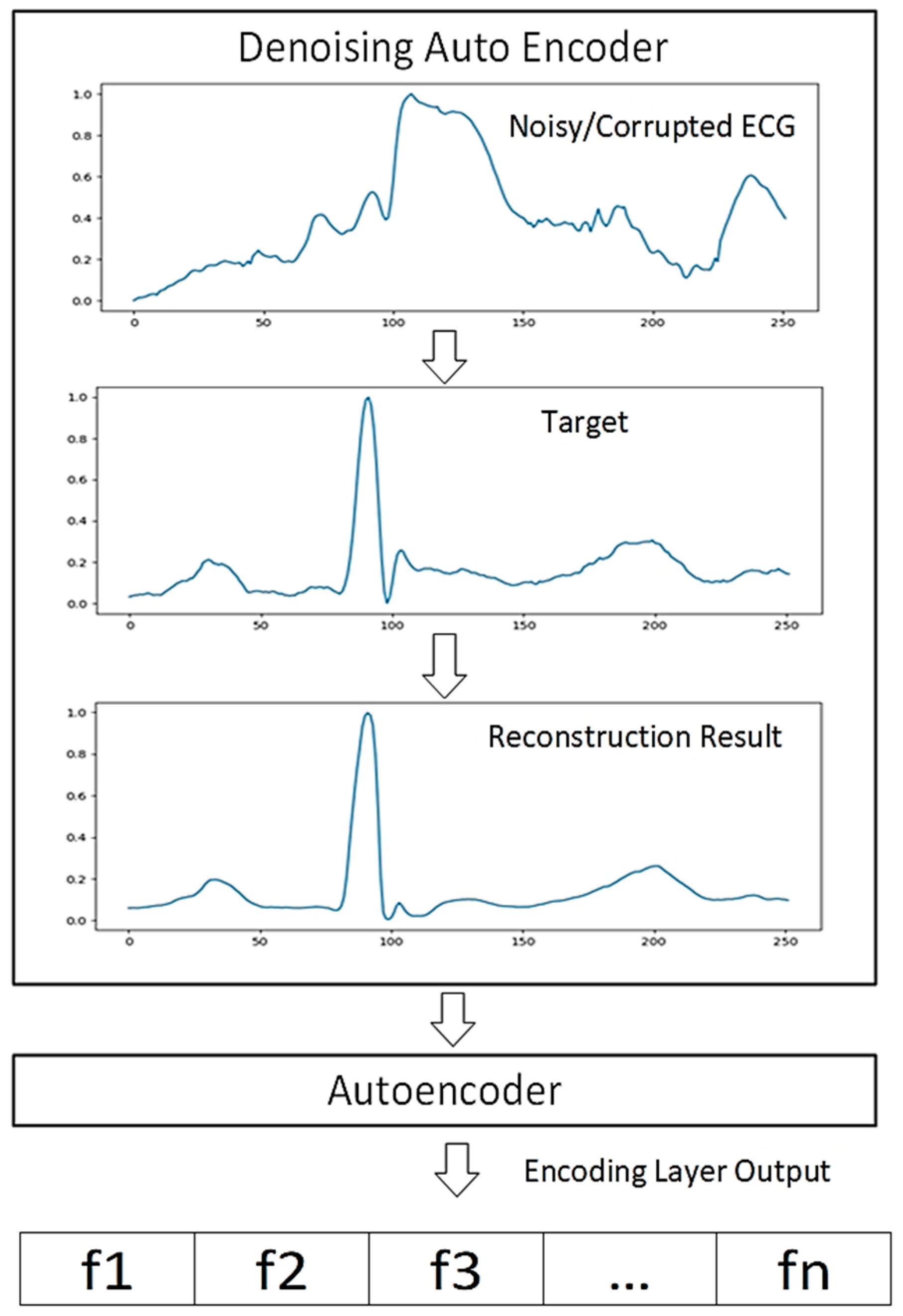
2.1. Autoencoder
2.2. Proposed Deep Learning Structure
2.3. Experimental Result
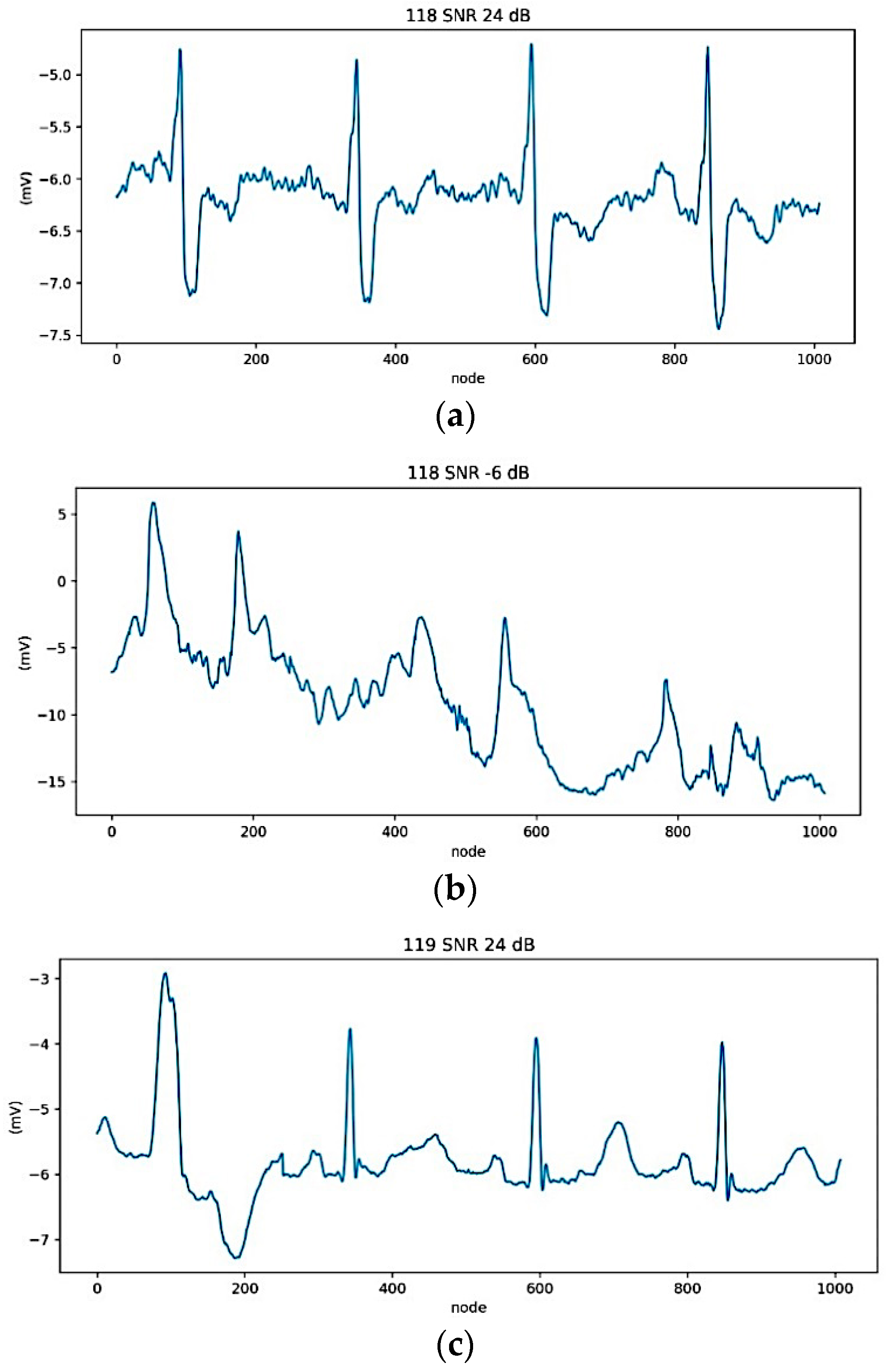
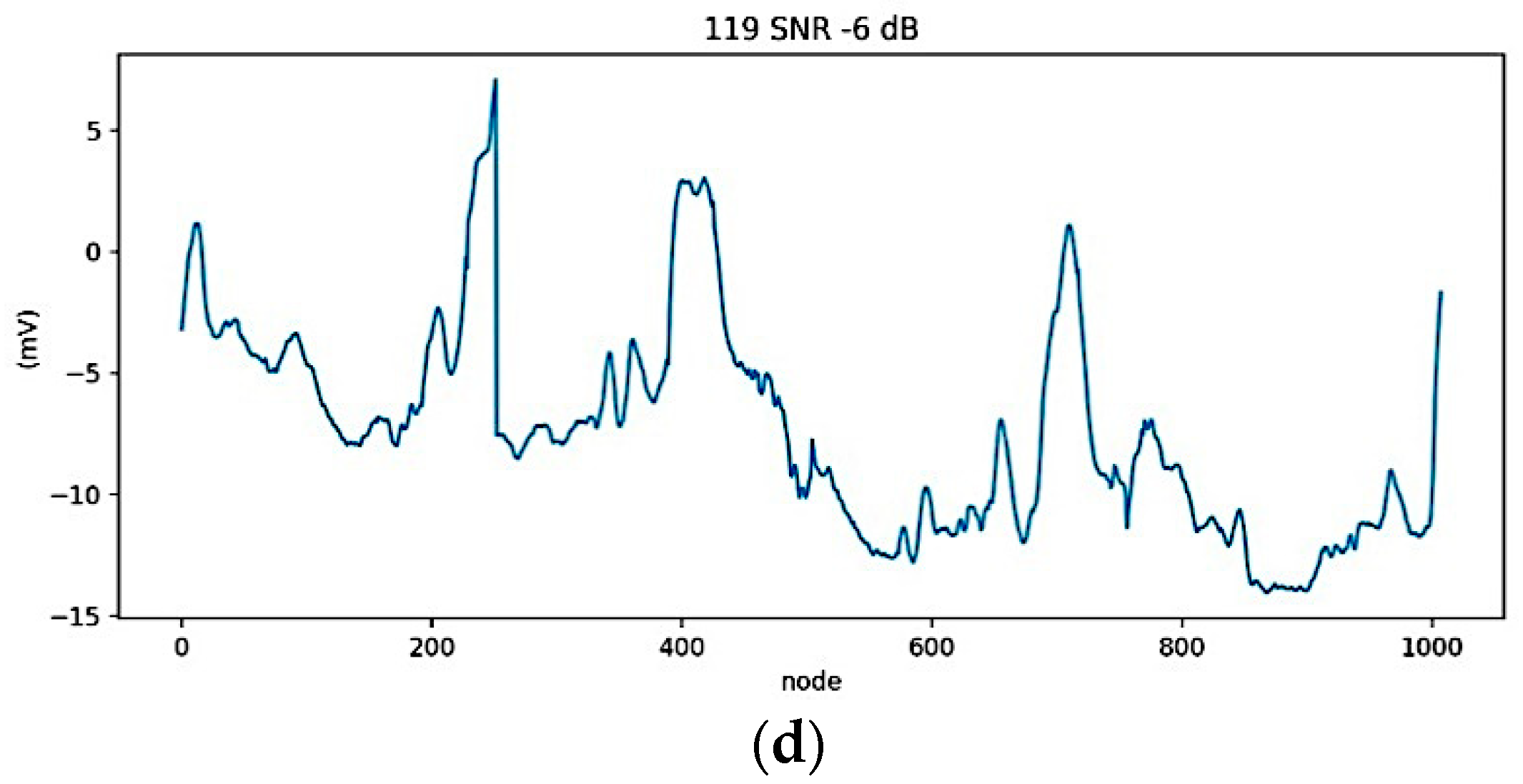
2.3.1. Data Preparation
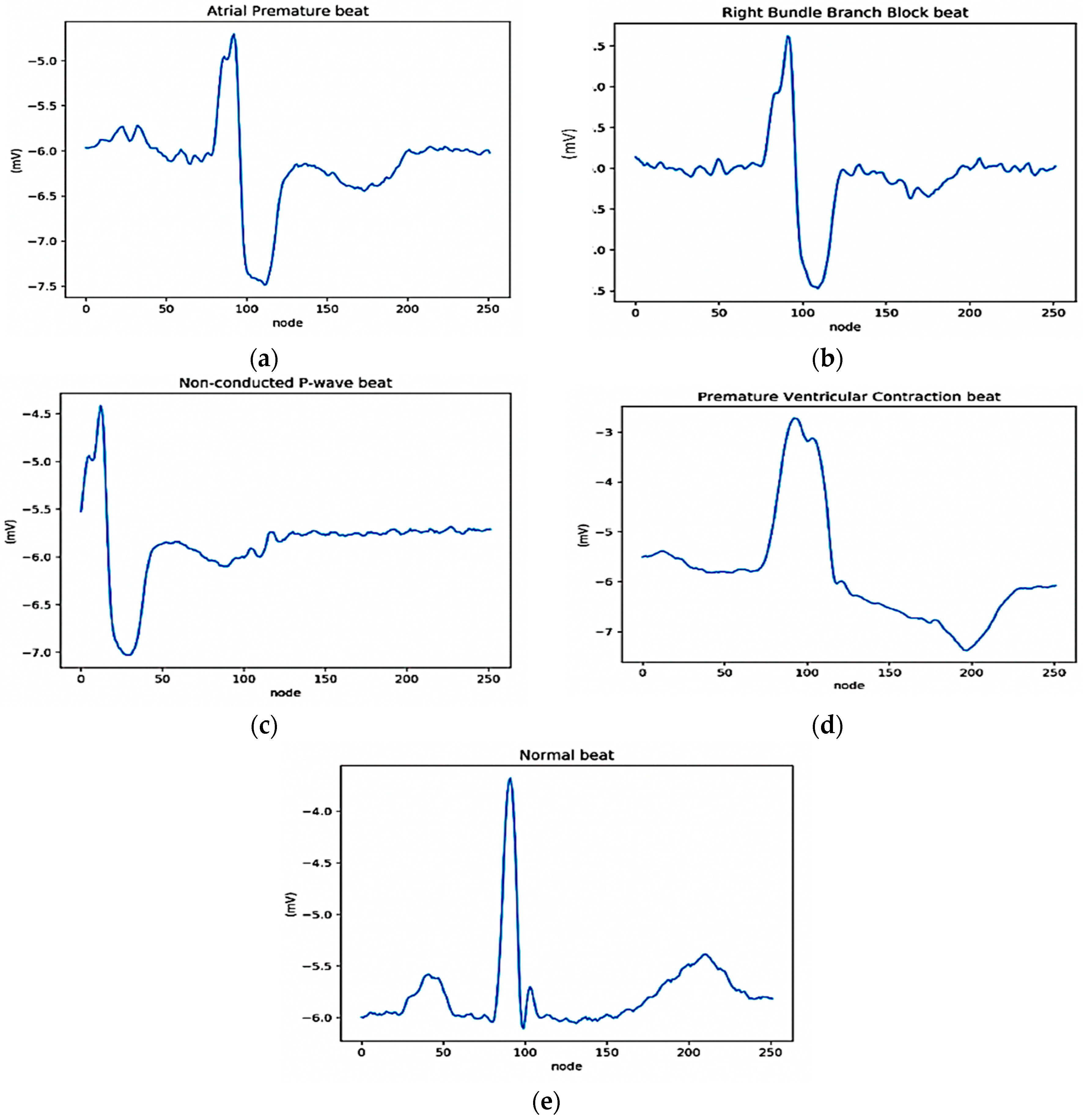
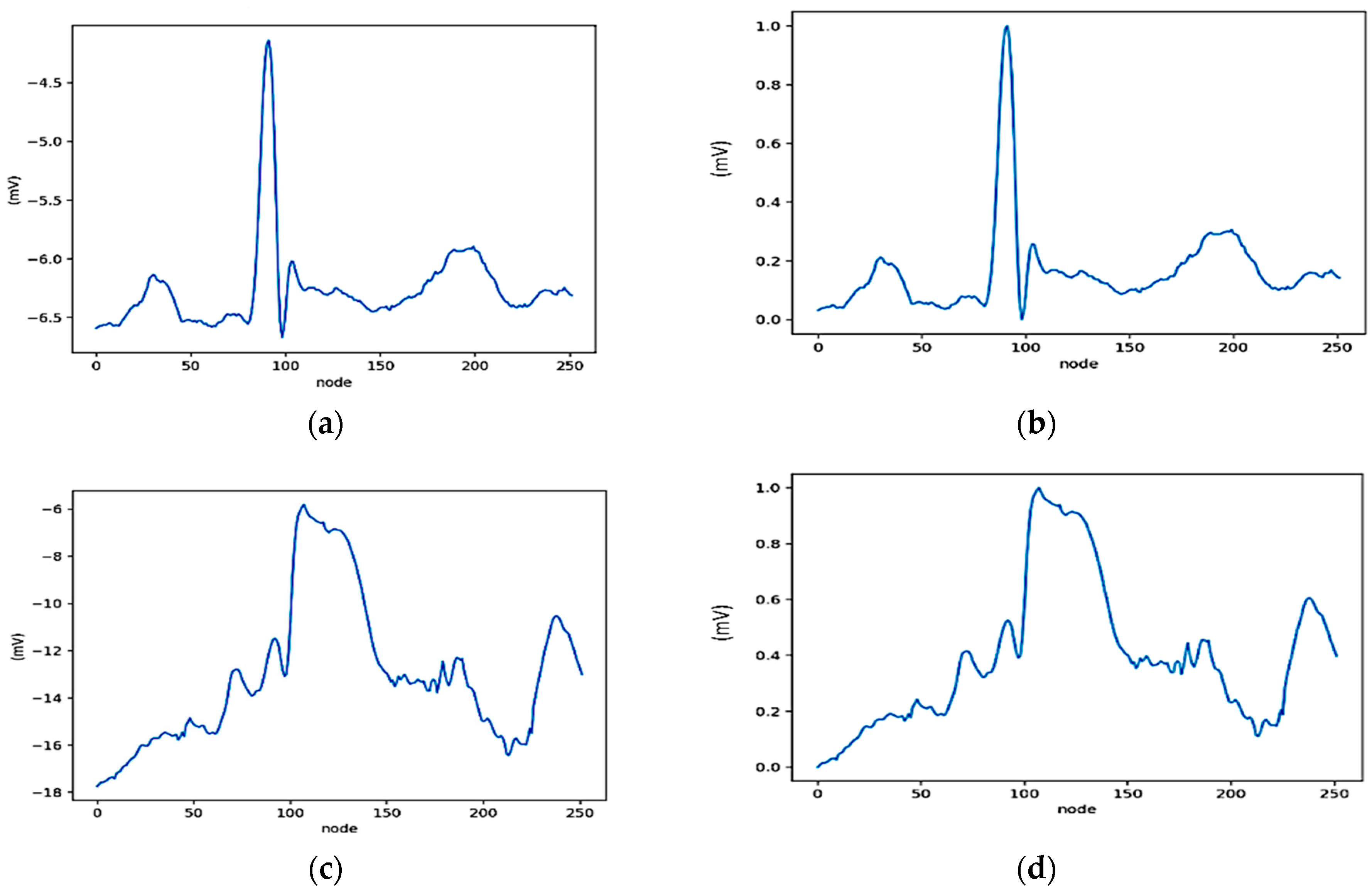
2.3.2. ECG Segmentation and Normalization
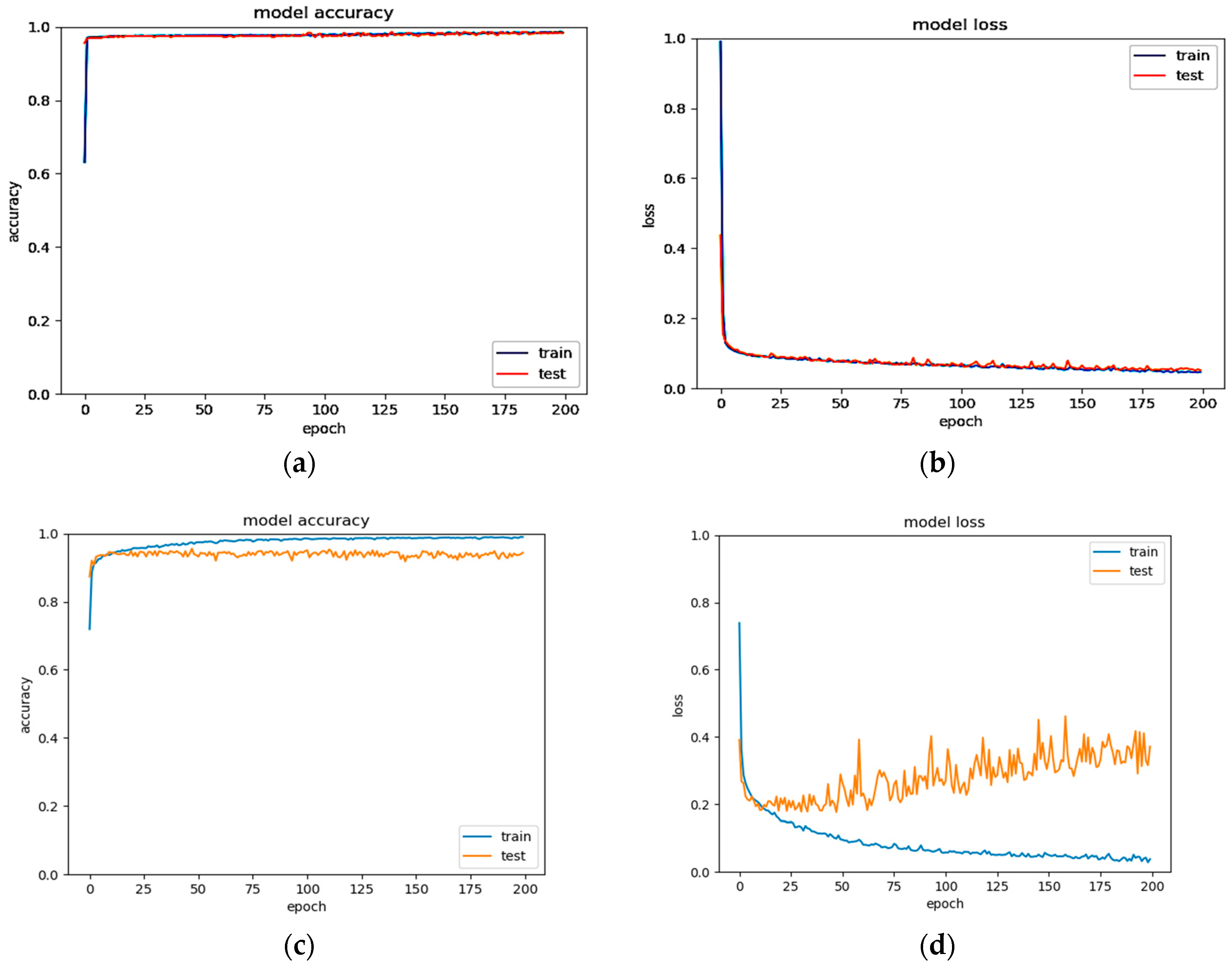
2.3.3. Pre-Training and Fine-Tuning
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guaragnella, C.; Rizzi, M.; Giorgio, A. Marginal Component Analysis of ECG Signals for Beat-to-Beat Detection of Ventricular Late Potentials. Electronics 2019, 8, 1000. [Google Scholar] [CrossRef]
- Castillo, E.; Morales, D.P.; Garcia, A.; Martinez-Marti, F.; Parrilla, L.; Palma, A.J. Noise suppression in ECG signals through efficient one-step wavelet processing techniques. J. Appl. Math. 2013, 2013. [Google Scholar] [CrossRef]
- Antczak, K. Deep Recurrent Neural Networks for ECG Signal Denoising. arXiv 2018, arXiv:1807.11551. [Google Scholar]
- Wang, D.; Si, Y.; Yang, W.; Zhang, G.; Li, J. A Novel Electrocardiogram Biometric Identification Method Based on Temporal-Frequency Autoencoding. Electronics 2019, 8, 667. [Google Scholar] [CrossRef]
- Meireles, A.J.M. ECG Denoising Based on Adaptive Signal Processing Technique. Master’s Thesis, Instituto Politécnico do Porto, Instituto Superior de Engenharia do Porto, Porto, Portugal, 2011. [Google Scholar]
- Joshi, V.; Verma, A.R.; Singh, Y. De-noising of ECG signal using Adaptive Filter based on MPSO. Procedia Comput. Sci. 2015, 57, 395–402. [Google Scholar] [CrossRef]
- Sharma, I.; Mehra, R.; Singh, M. Adaptive filter design for ECG noise reduction using LMS algorithm. In Proceedings of the 2015 4th International Conference on Reliability, Infocom Technologies and Optimization (ICRITO) (Trends and Future Directions), Noida, India, 2–4 September 2015; pp. 1–6. [Google Scholar]
- Aqil, M.; Jbari, A.; Bourouhou, A. ECG Signal Denoising by Discrete Wavelet Transform. Int. J. Online Eng. 2017, 13. [Google Scholar] [CrossRef]
- Srivastava, M.; Anderson, C.L.; Freed, J.H. A new wavelet denoising method for selecting decomposition levels and noise thresholds. IEEE Access 2016, 4, 3862–3877. [Google Scholar] [CrossRef]
- Kong, Q.; Song, Q.; Hai, Y.; Gong, R.; Liu, J.; Shao, X. Denoising signals for photoacoustic imaging in frequency domain based on empirical mode decomposition. Optik (Stuttg) 2018, 160, 402–414. [Google Scholar] [CrossRef]
- Nguyen, P.; Kim, J.-M. Adaptive ECG denoising using genetic algorithm-based thresholding and ensemble empirical mode decomposition. Inf. Sci. (N. Y.) 2016, 373, 499–511. [Google Scholar] [CrossRef]
- Yoon, D.; Lim, H.S.; Jung, K.; Kim, T.Y.; Lee, S. Deep Learning-Based Electrocardiogram Signal Noise Detection and Screening Model. Healthc. Inform. Res. 2019, 25, 201–211. [Google Scholar] [CrossRef]
- Vincent, P.; Larochelle, H.; Bengio, Y.; Manzagol, P.-A. Extracting and composing robust features with denoising autoencoders. In Proceedings of the 25th International Conference on Machine Learning, Helsinki, Finland, 5–9 July 2008; pp. 1096–1103. [Google Scholar]
- Chiang, H.-T.; Hsieh, Y.-Y.; Fu, S.-W.; Hung, K.-H.; Tsao, Y.; Chien, S.-Y. Noise Reduction in ECG Signals Using Fully Convolutional Denoising Autoencoders. IEEE Access 2019, 7, 60806–60813. [Google Scholar] [CrossRef]
- Xiong, P.; Wang, H.; Liu, M.; Liu, X. Denoising autoencoder for eletrocardiogram signal enhancement. J. Med. Imaging Heal. Inform. 2015, 5, 1804–1810. [Google Scholar] [CrossRef]
- Xiong, P.; Wang, H.; Liu, M.; Lin, F.; Hou, Z.; Liu, X. A stacked contractive denoising auto-encoder for ECG signal denoising. Physiol. Meas. 2016, 37, 2214. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Ma, L.; Yang, X. Stacked denoise autoencoder based feature extraction and classification for hyperspectral images. J. Sens. 2016, 2016. [Google Scholar] [CrossRef]
- Yang, J.; Nguyen, M.N.; San, P.P.; Li, X.L.; Krishnaswamy, S. Deep convolutional neural networks on multichannel time series for human activity recognition. In Proceedings of the Twenty-Fourth International Joint Conference on Artificial Intelligence, Buenos Aires, Argentina, 25–31 July 2015. [Google Scholar]
- Budiman, A.; Fanany, M.I.; Basaruddin, C. Stacked denoising autoencoder for feature representation learning in pose-based action recognition. In Proceedings of the 2014 IEEE 3rd Global Conference on Consumer Electronics (GCCE), Tokyo, Japan, 7–10 October 2014; pp. 684–688. [Google Scholar]
- Nurmaini, S.; Partan, R.U.; Rachmatullah, M.N. Deep Neural Networks Classifiers on The Electrocardiogram Signal for Intelligent Interpretation System. Sriwij. Int. Conf. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Martis, R.J.; Acharya, U.R.; Min, L.C. ECG beat classification using PCA, LDA, ICA and discrete wavelet transform. Biomed. Signal Process. Control 2013, 8, 437–448. [Google Scholar] [CrossRef]
- Qin, Q.; Li, J.; Zhang, L.; Yue, Y.; Liu, C. Combining low-dimensional wavelet features and support vector machine for arrhythmia beat classification. Sci. Rep. 2017, 7, 6067. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.J.M.; Stoks, J.; Bennis, F.C.; Vink, A.S.; Garde, A.; Wilde, A.A.M.; Pison, L.; Postema, P.G.; Delhaas, T. Support vector machine-based assessment of the T-wave morphology improves long QT syndrome diagnosis. EP Eur. 2018, 20, iii113–iii119. [Google Scholar] [CrossRef]
- Faziludeen, S.; Sankaran, P. ECG beat classification using evidential K-nearest neighbours. Procedia Comput. Sci. 2016, 89, 499–505. [Google Scholar] [CrossRef]
- Hejazi, M.; Al-Haddad, S.A.R.; Singh, Y.P.; Hashim, S.J.; Aziz, A.F.A. Multiclass support vector machines for classification of ECG data with missing values. Appl. Artif. Intell. 2015, 29, 660–674. [Google Scholar] [CrossRef]
- Yu, S.-N.; Chen, Y.-H. Noise-tolerant electrocardiogram beat classification based on higher order statistics of subband components. Artif. Intell. Med. 2009, 46, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Rajagopalan, C.; Clifford, G.D. A machine learning approach to multi-level ECG signal quality classification. Comput. Methods Programs Biomed. 2014, 117, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Übeyli, E.D. ECG beats classification using multiclass support vector machines with error correcting output codes. Digit. Signal Process. 2007, 17, 675–684. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kon, Y.; Lee, S.; Okada, Y. Detection and localization of myocardial infarction based on a convolutional autoencoder. Knowl. Based Syst. 2019, 178, 123–131. [Google Scholar] [CrossRef]
- Nurmaini, S.; Partan, R.U.; Caesarendra, W.; Dewi, T.; Rahmatullah, M.N.; Darmawahyuni, A.; Bhayyu, V.; Firdaus, F. An Automated ECG Beat Classification System Using Deep Neural Networks with an Unsupervised Feature Extraction Technique. Appl. Sci. 2019, 9, 2921. [Google Scholar] [CrossRef]
- Ochiai, K.; Takahashi, S.; Fukazawa, Y. Arrhythmia Detection from 2-lead ECG using Convolutional Denoising Autoencoders. In Proceedings of the KDD’18 Deep Learning Day, London, UK, 19–23 August 2018. [Google Scholar]


| Beats | Record of 118 | Record of 119 | Total |
|---|---|---|---|
| N | None | 1543 | 1543 |
| V | 16 | 444 | 460 |
| R | 2165 | None | 2165 |
| A | 96 | None | 96 |
| P | 10 | None | 10 |
| Total | 2287 | 1987 | 4274 |
| Model | DAEs | AEs | DNNs |
|---|---|---|---|
| 1 | (252-252-252) (ReLU-Sigmoid) | Autoencoder (252-126-252) (ReLU-Sigmoid) | (126-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| 2 | (252-252-252) (ReLU-Sigmoid) | Deep Autoencoder (252-126-63-126-252) (ReLU-ReLU-ReLU-Sigmoid) | (63-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| 3 | (252-252-252) (ReLU-Sigmoid) | Deep Autoencoder (252-126-63-32-63-126-252) (ReLU-ReLU-ReLU-ReLU-ReLU-Sigmoid) | (32-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| 4 | (252-126-252) (ReLU-Sigmoid) | Autoencoder (252-126-252) (ReLU-Sigmoid) | (126-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| 5 | (252-126-252) (ReLU-Sigmoid) | Deep Autoencoder (252-126-63-126-252) (ReLU-ReLU-ReLU-Sigmoid) | (63-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| 6 | (252-126-252) (ReLU-Sigmoid) | Deep Autoencoder (252-126-63-32-63-126-252) (ReLU-ReLU-ReLU-ReLU-ReLU-Sigmoid) | (32-100-100-100-100-100-5) (ReLU-ReLU-ReLU-ReLU-ReLU-Softmax) |
| Training | Validation Model (%) | |||||
|---|---|---|---|---|---|---|
| Metrics | 1 | 2 | 3 | 4 | 5 | 6 |
| Accuracy | 99.49 | 99.39 | 99.26 | 99.14 | 99.03 | 98.93 |
| Sensitivity | 95.94 | 96.83 | 93.00 | 94.59 | 98.90 | 98.84 |
| Specificity | 99.68 | 99.66 | 99.52 | 99.55 | 99.49 | 99.40 |
| Precision | 91.23 | 87.62 | 87.66 | 77.80 | 78.06 | 73.51 |
| F1-Score | 93.13 | 90.35 | 89.58 | 81.10 | 79.08 | 76.18 |
| Testing | Model Validation (%) | |||||
|---|---|---|---|---|---|---|
| Metrics | 1 | 2 | 3 | 4 | 5 | 6 |
| Accuracy | 99.34 | 99.06 | 99.06 | 98.97 | 98.22 | 98.41 |
| Sensitivity | 93.83 | 79.54 | 93.56 | 79.07 | 56.97 | 57.49 |
| Specificity | 99.57 | 99.33 | 99.35 | 99.43 | 98.75 | 98.94 |
| Precision | 89.81 | 87.63 | 89.42 | 79.90 | 59.19 | 59.37 |
| F1-Score | 91.44 | 81.80 | 91.11 | 79.48 | 58.04 | 58.40 |
| Class | A | R | P | V | N |
|---|---|---|---|---|---|
| A | 49 | 37 | 0 | 0 | 0 |
| R | 11 | 1937 | 0 | 0 | 0 |
| P | 0 | 0 | 9 | 0 | 0 |
| V | 0 | 0 | 0 | 413 | 1 |
| N | 0 | 0 | 0 | 0 | 1389 |
| Class | A | R | P | V | N |
|---|---|---|---|---|---|
| A | 5 | 5 | 0 | 0 | 0 |
| R | 2 | 215 | 0 | 0 | 0 |
| P | 0 | 0 | 1 | 0 | 0 |
| V | 0 | 0 | 0 | 46 | 0 |
| N | 0 | 0 | 0 | 0 | 154 |
| Architecture | Performance Metrics (%) | ||||
|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Precision | F1-Score | |
| DAEs–DNNs (without AEs) | 96.95 | 85.23 | 97.67 | 78.67 | 80.52 |
| DAEs–AEs–DNNs (proposed model) | 99.34 | 93.83 | 99.57 | 89.81 | 91.44 |
| Authors | Dataset | Noise Cancelation | Feature Extraction | Classifier | Case | Accuracy (%) |
|---|---|---|---|---|---|---|
| Yu et al. [26] | MITDB | DWT | Correlation coefficient | FBNNs | Beats classification | 97.50 |
| Li et al. [27] | MITDB | Filter | Variance | SVM | ECG signal quality | 80.26 |
| Ochiai et al. [31] | MITDB | DAEs | CNNs | CNNs 1D/2D | Beats classification | 95.70 |
| Nurmaini et al. [30] | MITDB | DWT | AEs | DNNs | Beats classification | 99.73 |
| Proposed | MITDB with NSTDB | DAEs | AEs | DNNs | Beats classification | 99.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurmaini, S.; Darmawahyuni, A.; Sakti Mukti, A.N.; Rachmatullah, M.N.; Firdaus, F.; Tutuko, B. Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification. Electronics 2020, 9, 135. https://doi.org/10.3390/electronics9010135
Nurmaini S, Darmawahyuni A, Sakti Mukti AN, Rachmatullah MN, Firdaus F, Tutuko B. Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification. Electronics. 2020; 9(1):135. https://doi.org/10.3390/electronics9010135
Chicago/Turabian StyleNurmaini, Siti, Annisa Darmawahyuni, Akhmad Noviar Sakti Mukti, Muhammad Naufal Rachmatullah, Firdaus Firdaus, and Bambang Tutuko. 2020. "Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification" Electronics 9, no. 1: 135. https://doi.org/10.3390/electronics9010135
APA StyleNurmaini, S., Darmawahyuni, A., Sakti Mukti, A. N., Rachmatullah, M. N., Firdaus, F., & Tutuko, B. (2020). Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification. Electronics, 9(1), 135. https://doi.org/10.3390/electronics9010135







