A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking
Abstract
:1. Introduction
1.1. Aim of the Study
1.2. Contributions
- first, providing a classification of stance and swing phases and the prediction of foot-floor-contact signal in more natural walking conditions (similar to everyday walking), overcoming the limitations and the constraints of a controlled environment, such as treadmill walking;
- second, proposing a different approach to process the sEMG signal used to train deep neural networks: while previous studies [13,14,15] processed sEMG signals to extract time/frequency domain features which were used to feed the neural networks, the present study directly used the envelopes of the EMG signal to train the networks, attempting to automatically learn relevant higher level (hidden) features;
- third, improving the reliability of the prediction of gait events (HS and TO) in unseen subjects reported in literature [13], despite the challenging condition of everyday walking. This has been achieved by both enlarging the testing data (four-minute ground walking of 23 different subjects) and decreasing the average error in the prediction of HS and TO timing.
2. Related Works
3. Materials and Methods
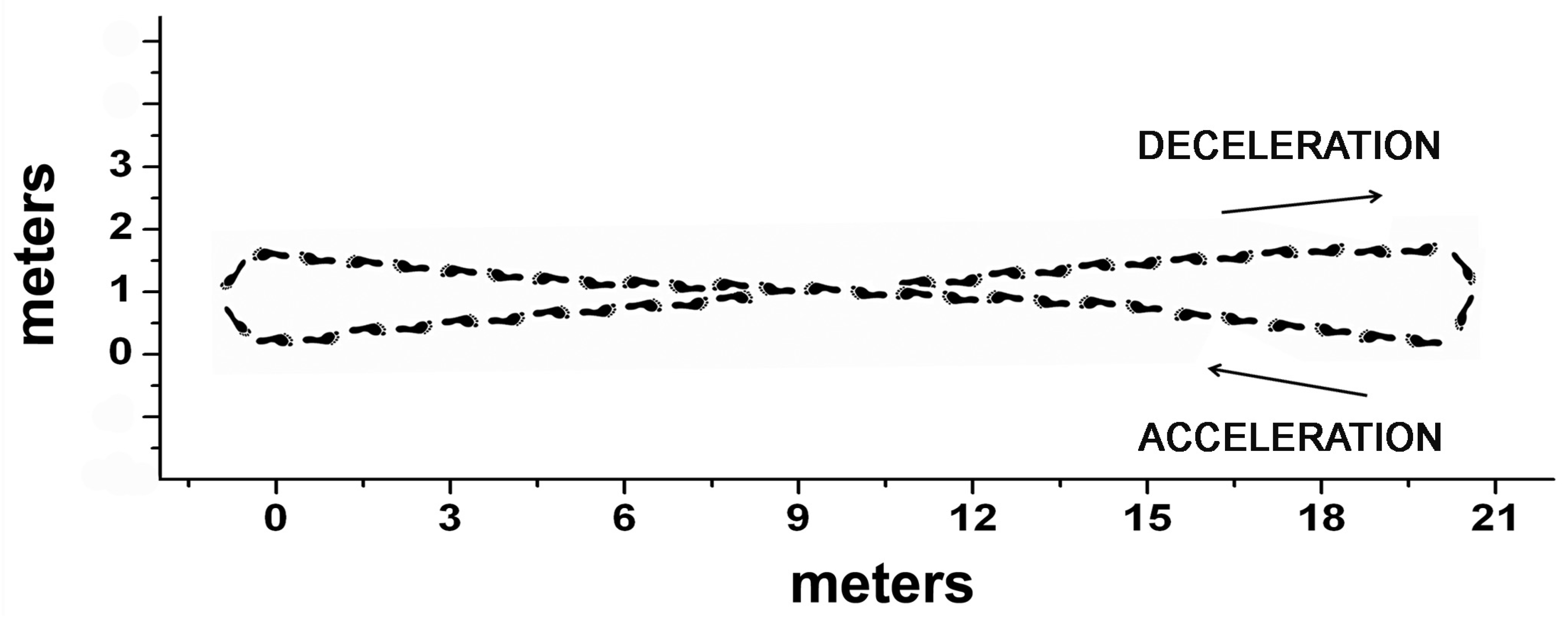
3.1. Dataset
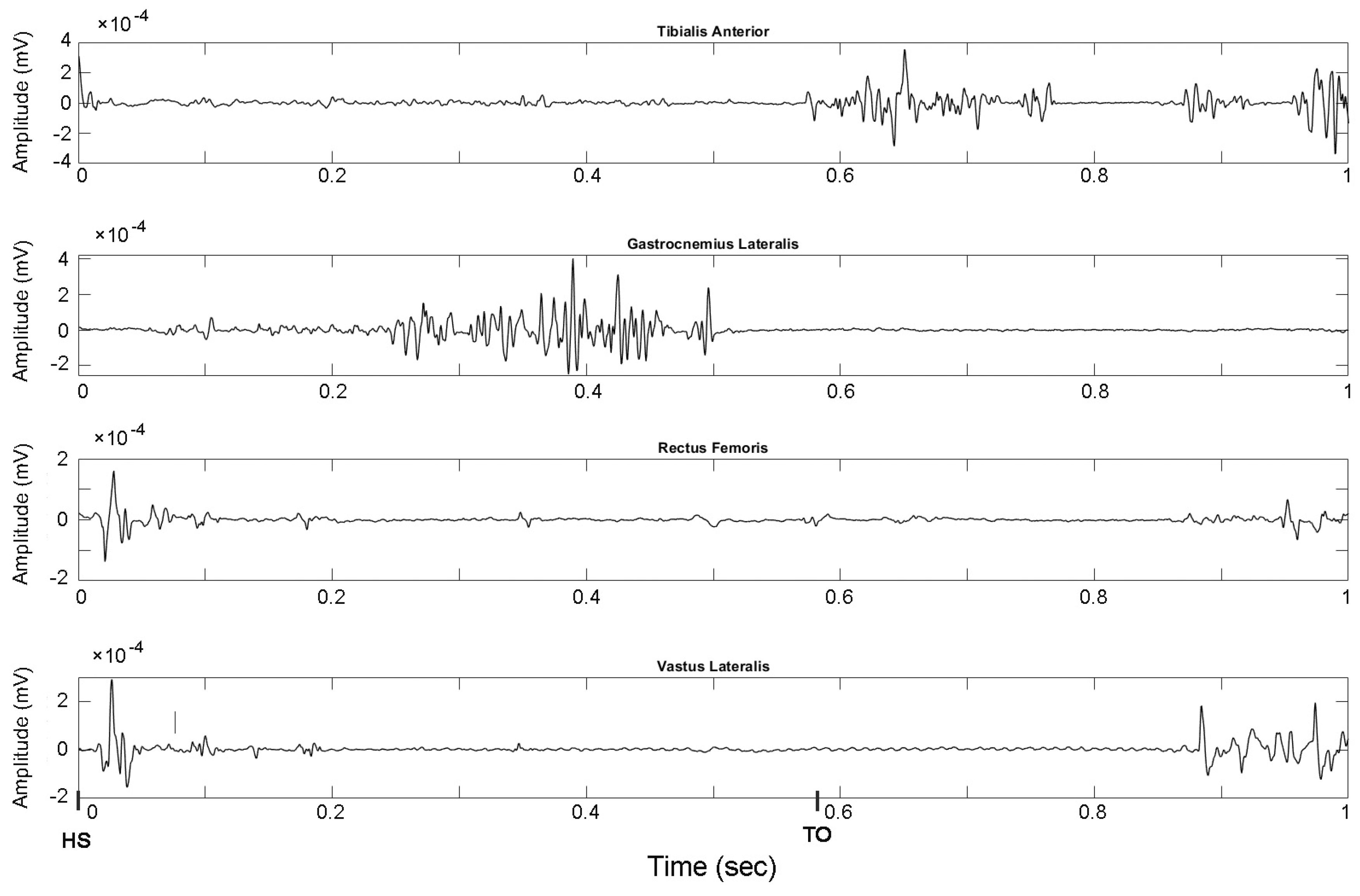
3.2. Signal Acquisition
3.3. Pre-Processing
3.4. Gait Phase Classification
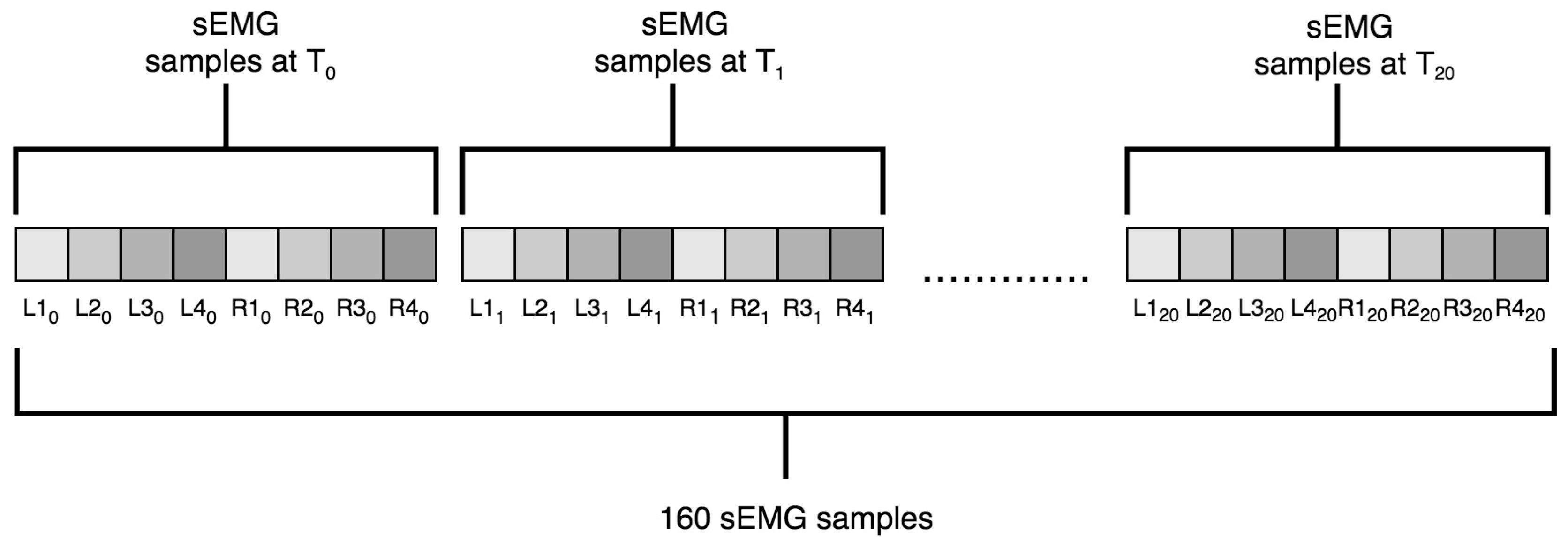
3.4.1. Data Preparation
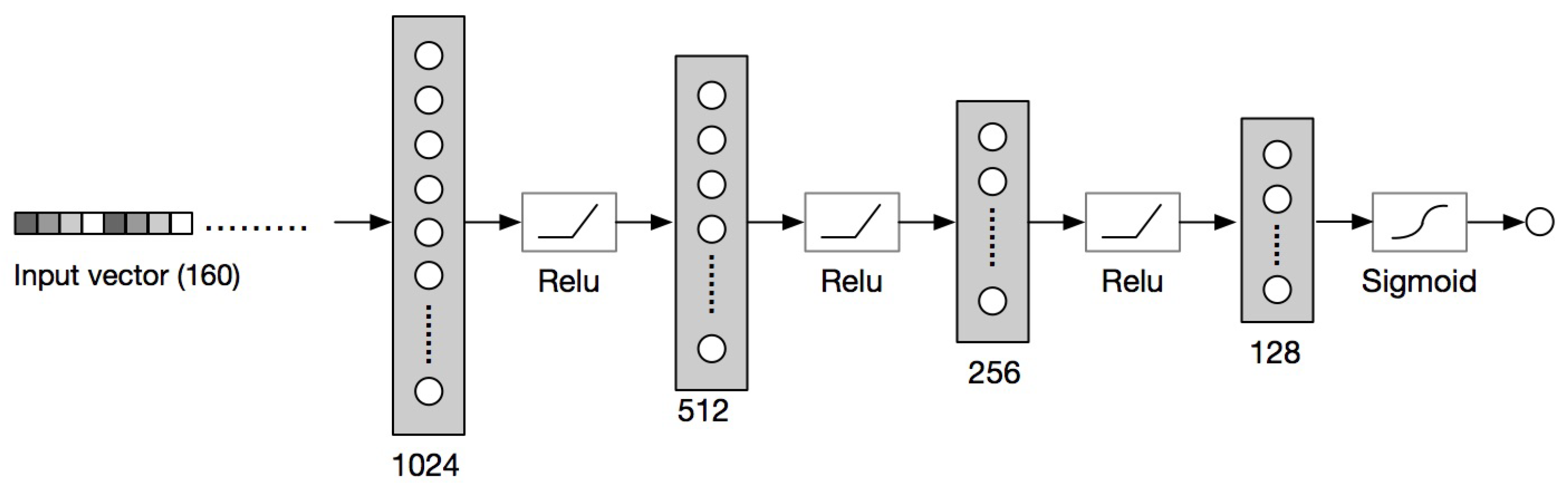
3.4.2. Neural Networks
3.5. Gait Events Timing Detection
3.6. Evaluation Measures
4. Results and Discussion
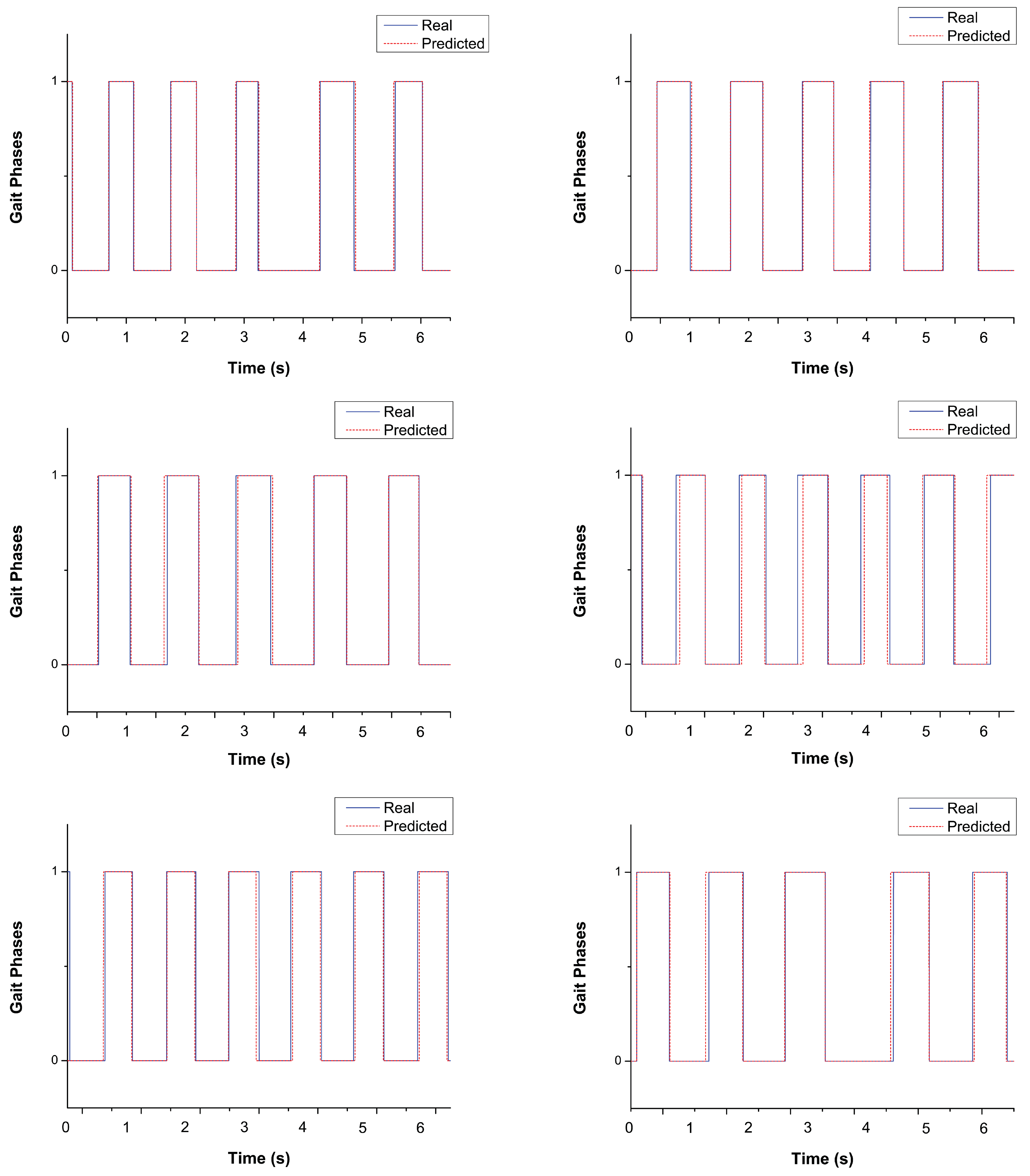
4.1. Gait-Phase Classification
4.2. Comparison with Feature-Based Approach
4.3. Gait Events Detection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loeb, G.E.; Gans, C. Electromyography for Experimentalists; University of Chicago Press: Chicago, IL, USA, 1986. [Google Scholar]
- Perry, J. Gait Analysis: Normal and Pathological Function; Slack Inc.: Thorofare, NJ, USA, 1992. [Google Scholar]
- Špulák, D.; Čmejla, R.; Bačáková, R.; Kračmar, B.; Satrapová, L.; Novotný, P. Muscle activity detection in electromyograms recorded during periodic movements. Comput. Biol. Med. 2014, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Stefano, A.; Allen, R. A simulation model of the surface EMG signal for analysis of muscle activity during the gait cycle. Comput. Biol. Med. 2006, 36, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, A.; Maranesi, E.; Burattini, L.; Fioretti, S.; Di Nardo, F. Co-contraction activity of ankle muscles during walking: A gender comparison. Biomed. Signal Process. Control 2017, 33, 1–9. [Google Scholar] [CrossRef]
- Gurney, J.; Kersting, U.; Rosenbaum, D. Between-day reliability of repeated plantar pressure distribution measurements in a normal population. Gait Posture 2008, 27, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Bovi, G.; Rabuffetti, M.; Mazzoleni, P.; Ferrarin, M. A multiple-task gait analysis approach: Kinematic, kinetic and EMG reference data for healthy young and adult subjects. Gait Posture 2011, 33, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Caldas, R.; Mundt, M.; Potthast, W.; Buarque de Lima Neto, F.; Markert, B. A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait Posture 2017, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Joyseeree, R.; Abou Sabha, R.; Mueller, H. Applying Machine Learning to Gait Analysis Data for Disease Identification. Stud. Health Technol. Inf. 2015, 210, 850–854. [Google Scholar] [CrossRef]
- Zou, Q.; Wang, Y.; Zhao, Y.; Wang, Q.; Shen, C.; Li, Q. Deep Learning Based Gait Recognition Using Smartphones in the Wild. arXiv 2018, arXiv:1811.00338. [Google Scholar]
- Kaczmarczyk, K.; Wit, A.; Krawczyk, M.; Zaborski, J.; Piłsudskii, J. Artificial Neural Networks (ANN) Applied for Gait Classification and Physiotherapy Monitoring in Post Stroke Patients. In Artificial Neural Networks; IntechOpen: Rijeka, Croatia, 2011; Chapter 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zielińska, T. Gait features analysis using artificial neural networks—Testing the footwear effect. Acta Bioeng. Biomech. 2017, 19, 17–32. [Google Scholar]
- Nazmi, N.; Abdul Rahman, M.; Yamamoto, S.I.; Ahmad, S. Walking gait event detection based on electromyography signals using artificial neural network. Biomed. Signal Process. Control 2019, 47, 334–343. [Google Scholar] [CrossRef]
- Ziegier, J.; Gattringer, H.; Mueller, A. Classification of Gait Phases Based on Bilateral EMG Data Using Support Vector Machines. In Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Enschede, The Netherlands, 26–29 August 2018; Volume 2018, pp. 978–983. [Google Scholar] [CrossRef]
- Meng, M.; She, Q.; Gao, Y.; Luo, Z. EMG signals based gait phases recognition using hidden Markov models. In Proceedings of the 2010 IEEE International Conference on Information and Automation, ICIA 2010, Harbin, China, 20–23 June 2010; pp. 852–856. [Google Scholar] [CrossRef]
- Stolze, H.; Kuhtz-Buschbeck, J.; Mondwurf, C.; Boczek-Funcke, A.; Jöhnk, K.; Deuschl, G.; Illert, M. Gait analysis during treadmill and overground locomotion in children and adults. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1997, 105, 490–497. [Google Scholar] [CrossRef]
- Batlkham, B.; Oyunaa, C.; Odongua, N. A Kinematic Comparison of Overground and Treadmill Walking. Value Health 2014, 17, A774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, P.O.; Paolini, G.; Della Croce, U.; Paylo, K.W.; Kerrigan, D.C. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture 2007, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hidler, J. Biomechanics of overground vs. treadmill walking in healthy individuals. J. Appl. Physiol. 2008, 104, 747–755. [Google Scholar] [CrossRef]
- Alanazi, H.; Abdullah, A.; Qureshi, K. A Critical Review for Developing Accurate and Dynamic Predictive Models Using Machine Learning Methods in Medicine and Health Care. J. Med. Syst. 2017, 41, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Hussain, A.; Al-Jumeily, D.; Baker, T.; Khattak, A. Classification of Foetal Distress and Hypoxia Using Machine Learning Approaches. Lect. Notes Comput. Sci. 2018, 10956, 767–776. [Google Scholar] [CrossRef]
- Aljaaf, A.; Al-Jumeily, D.; Haglan, H.; Alloghani, M.; Baker, T.; Hussain, A.; Mustafina, J. Early Prediction of Chronic Kidney Disease Using Machine Learning Supported by Predictive Analytics. In Proceedings of the 2018 IEEE Congress on Evolutionary Computation (CEC), Rio de Janeiro, Brazil, 8–13 July 2018. [Google Scholar]
- Subasi, A.; Yilmaz, M.; Ozcalik, H.R. Classification of EMG signals using wavelet neural network. J. Neurosci. Methods 2006, 156, 360–367. [Google Scholar] [CrossRef]
- Elamvazuthi, I.; Duy, N.; Ali, Z.; Su, S.; Khan, M.; Parasuraman, S. Electromyography (EMG) based Classification of Neuromuscular Disorders using Multi-Layer Perceptron. In Proceedings of the 2015 IEEE International Symposium on Robotics and Intelligent Sensors (IEEE IRIS2015), Langkawi, Malaysia, 18–20 October 2015; Volume 76, pp. 223–228. [Google Scholar] [CrossRef]
- Ibrahimy, M.; Ahsan, M.; Khalifa, O. Design and optimization of levenberg-marquardt based neural network classifier for EMG signals to identify hand motions. Meas. Sci. Rev. 2013, 13, 142–151. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Z. Hand motion pattern classifier based on EMG using wavelet packet transform and LVQ neural networks. In Proceedings of the 2008 IEEE International Symposium on IT in Medicine and Education, ITME 2008, Xiamen, China, 12–14 December 2008; pp. 28–32. [Google Scholar] [CrossRef]
- Oskoei, M.; Hu, H. Support vector machine-based classification scheme for myoelectric control applied to upper limb. IEEE Trans. Biomed. Eng. 2008, 55, 1956–1965. [Google Scholar] [CrossRef]
- Moslem, B.; Diab, M.; Khalil, M.; Marque, C. Classification of multichannel uterine EMG signals by using unsupervised competitive learning. In Proceedings of the 2011 IEEE Workshop on Signal Processing Systems, SiPS 2011, Beirut, Lebanon, 4–7 October 2011; pp. 267–272. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Di Nardo, F.; Mengarelli, A.; Maranesi, E.; Burattini, L.; Fioretti, S. Gender differences in the myoelectric activity of lower limb muscles in young healthy subjects during walking. Biomed. Signal Process. Control 2015, 19, 14–22. [Google Scholar] [CrossRef]
- Agostini, V.; Balestra, G.; Knaflitz, M. Segmentation and classification of gait cycles. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 946–952. [Google Scholar] [CrossRef]
- Khandelwal, S.; Wickstrasm, N. Evaluation of the performance of accelerometer-based gait event detection algorithms in different real-world scenarios using the MAREA gait database. Gait Posture 2017, 51, 84–90. [Google Scholar] [CrossRef]
- Trojaniello, D.; Cereatti, A.; Della Croce, U. Accuracy, sensitivity and robustness of five different methods for the estimation of gait temporal parameters using a single inertial sensor mounted on the lower trunk. Gait Posture 2014, 40, 487–492. [Google Scholar] [CrossRef]
- Toledo-Pérez, D.; Martínez-Prado, M.; Gómez-Loenzo, R.; Paredes-García, W.; Rodríguez-Reséndiz, J. A study of movement classification of the lower limb based on up to 4-EMG channels. Electronics 2019, 8, 259. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait partitioning methods: A systematic review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef]
- Winiarski, S.; Rutkowska-Kucharska, A. Estimated ground reaction force in normal and pathological gait. Acta Bioeng. Biomech. 2009, 11, 53–60. [Google Scholar]
- Ma, Y.; Xie, S.; Zhang, Y. A patient-specific EMG-driven neuromuscular model for the potential use of human-inspired gait rehabilitation robots. Comput. Biol. Med. 2016, 70, 88–98. [Google Scholar] [CrossRef]


| Model Name | Model Structure |
|---|---|
| mlp(128) | |
| mlp(256, 128) | |
| mlp(512, 256, 128) | |
| mlp(1024, 512, 256, 128) | |
| mlp(1024, 1024, 512, 256, 128) |
| Accuracy on US | Accuracy on LS-Test | |
|---|---|---|
| 92.62 ± 2.3 | 93.83 ± 0.28 | |
| 93.01 ± 2.1 | 94.41 ± 0.23 | |
| 93.41 ± 2.3 | 94.83 ± 0.2 | |
| 93.25 ± 2.9 | 94.94 ± 0.3 | |
| 93.03 ± 2.8 | 94.93 ± 0.2 |
| Stance Phase | |||
| Precision | Recall | Score | |
| 92.99 ± 4.5 | 90.50 ± 5.9 | 91.49 ± 2.9 | |
| 93.15 ± 4.4 | 91.22 ± 4.9 | 91.99 ± 2.4 | |
| 93.68 ± 3.9 | 91.57 ± 5.0 | 92.46 ± 2.7 | |
| 93.29 ± 2.9 | 91.78 ± 5.3 | 92.35 ± 3.2 | |
| 92.89 ± 4.7 | 91.53 ± 5.2 | 92.04 ± 3.4 | |
| Swing Phase | |||
| Precision | Recall | Score | |
| 92.49 ± 4.8 | 94.74 ± 3.2 | 93.45 ± 2.0 | |
| 92.97 ± 4.3 | 94.84 ± 3.1 | 93.77 ± 1.9 | |
| 93.24 ± 4.3 | 95.21 ± 2.9 | 94.11 ± 2.1 | |
| 93.32 ± 4.7 | 94.80 ± 3.6 | 93.93 ± 2.7 | |
| 93.29 ± 4.0 | 94.47 ± 3.7 | 93.77 ± 2.5 | |
| Stance Phase | |||
| Precision | Recall | Score | |
| 94.15 ± 0.3 | 91.72 ± 0.8 | 92.92 ± 0.3 | |
| 94.48 ± 0.6 | 92.75 ± 0.8 | 93.60 ± 0.3 | |
| 94.63 ± 0.5 | 93.59 ± 0.5 | 94.11 ± 0.2 | |
| 94.80 ± 0.5 | 93.67 ± 0.9 | 94.22 ± 0.4 | |
| 94.50 ± 0.5 | 93.99 ± 0.6 | 94.24 ± 0.3 | |
| Swing phase | |||
| Precision | Recall | Score | |
| 93.60 ± 0.5 | 95.50 ± 0.3 | 94.54 ± 0.2 | |
| 94.37 ± 0.5 | 95.72 ± 0.6 | 95.04 ± 0.2 | |
| 94.99 ± 0.3 | 95.81 ± 0.4 | 95.40 ± 0.2 | |
| 95.06 ± 0.7 | 95.94 ± 0.4 | 95.50 ± 0.2 | |
| 95.28 ± 0.5 | 95.68 ± 0.4 | 95.48 ± 0.2 | |
| HS | ||||
| MAE | Precision | Recall | ||
| 21.6 ± 7.0 | 99.67 ± 0.5 | 99.50 ± 2.9 | 99.04 ± 2.6 | |
| 56.7 ± 31.9 | 99.19 ± 1.5 | 96.40 ± 9.4 | 97.56 ± 2.6 | |
| TO | ||||
| MAE | Precision | Recall | ||
| 38.1 ± 15.2 | 99.07 ± 1.5 | 97.90 ± 3.5 | 98.40 ± 2.4 | |
| 64.4 ± 42.7 | 98.45 ± 2.6 | 95.67 ± 9.9 | 96.84 ± 6.9 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morbidoni, C.; Cucchiarelli, A.; Fioretti, S.; Di Nardo, F. A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics 2019, 8, 894. https://doi.org/10.3390/electronics8080894
Morbidoni C, Cucchiarelli A, Fioretti S, Di Nardo F. A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics. 2019; 8(8):894. https://doi.org/10.3390/electronics8080894
Chicago/Turabian StyleMorbidoni, Christian, Alessandro Cucchiarelli, Sandro Fioretti, and Francesco Di Nardo. 2019. "A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking" Electronics 8, no. 8: 894. https://doi.org/10.3390/electronics8080894
APA StyleMorbidoni, C., Cucchiarelli, A., Fioretti, S., & Di Nardo, F. (2019). A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics, 8(8), 894. https://doi.org/10.3390/electronics8080894








