Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
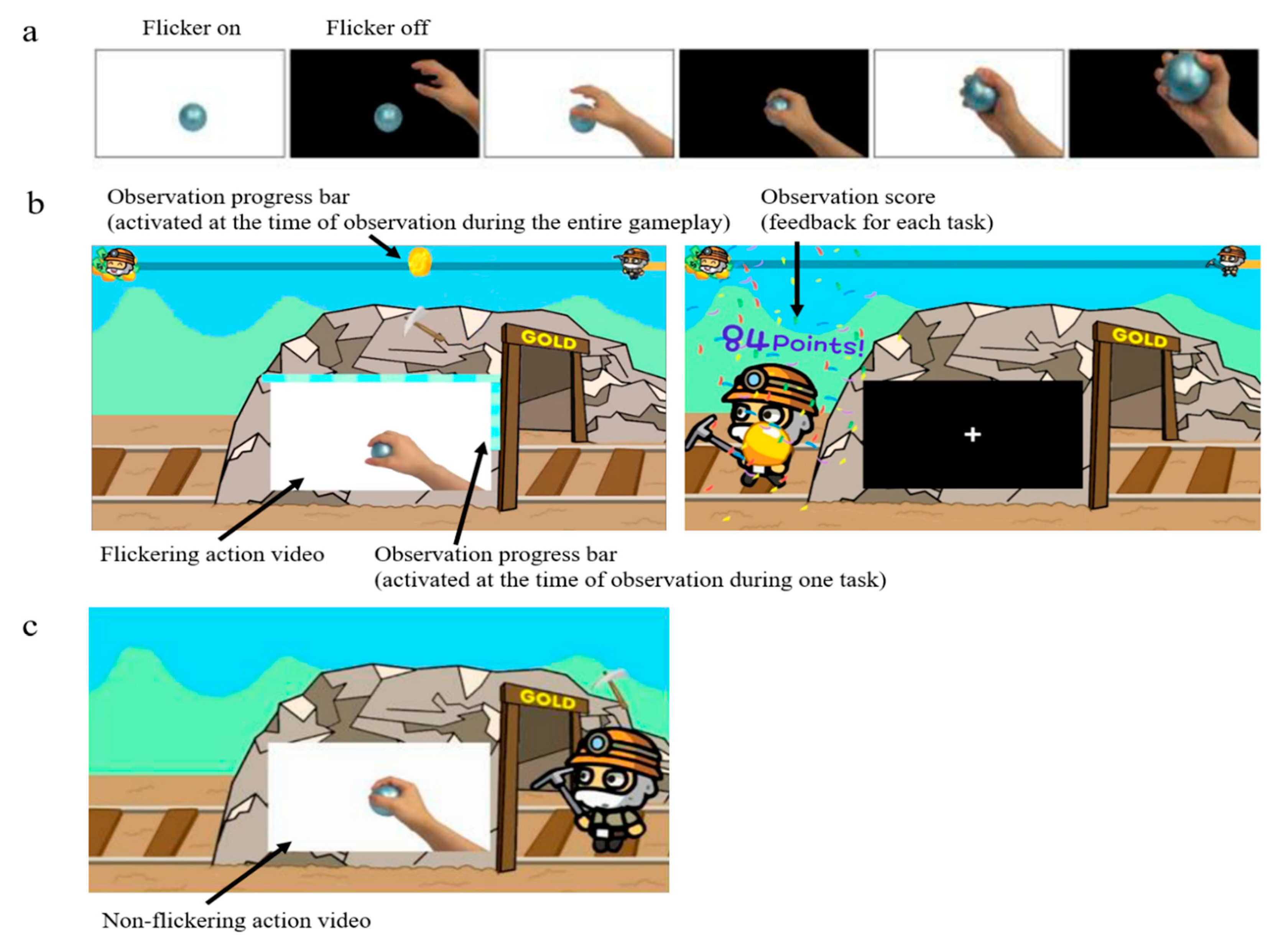
2.2. Video for AO
2.3. Experimental Procedure
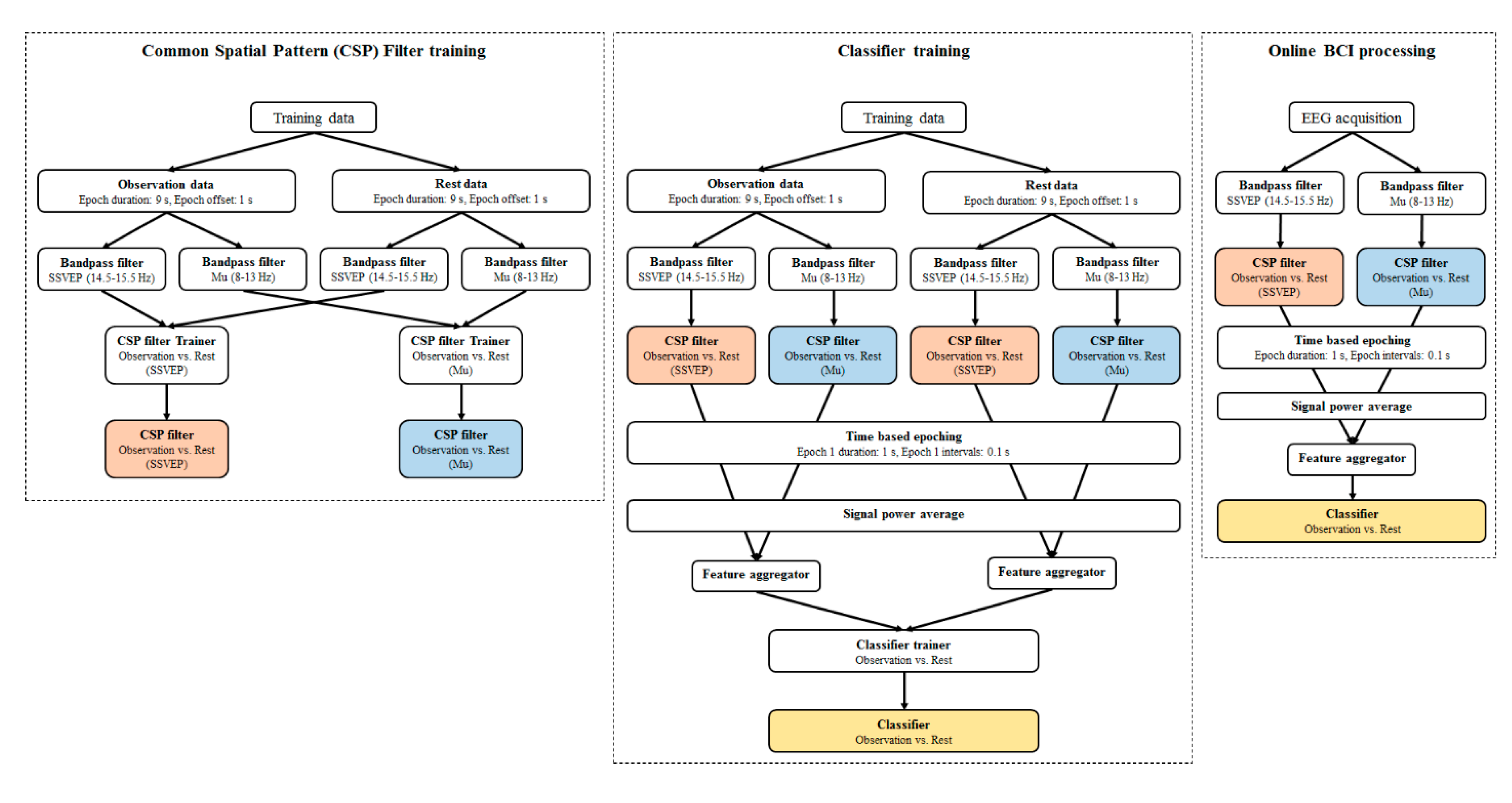
2.4. EEG Data Acquisition and Processing
2.5. Measured Parameters
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
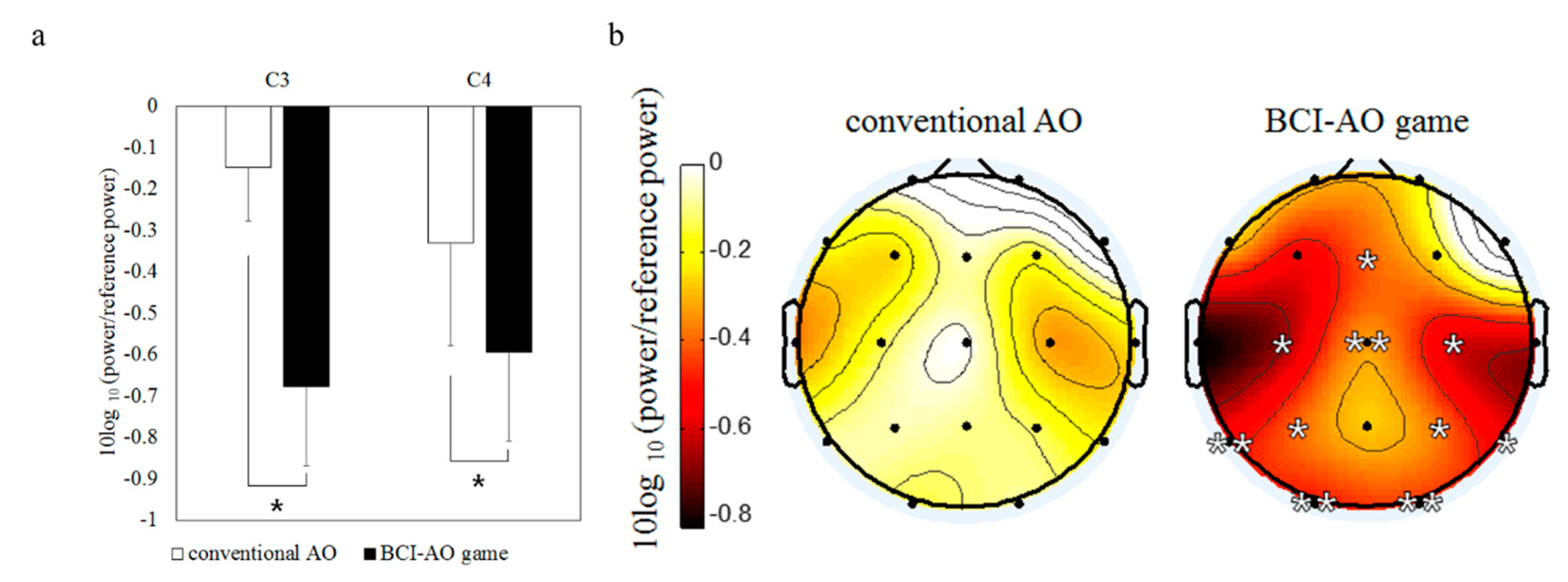
3.2. Mu Suppression According to AO Condition
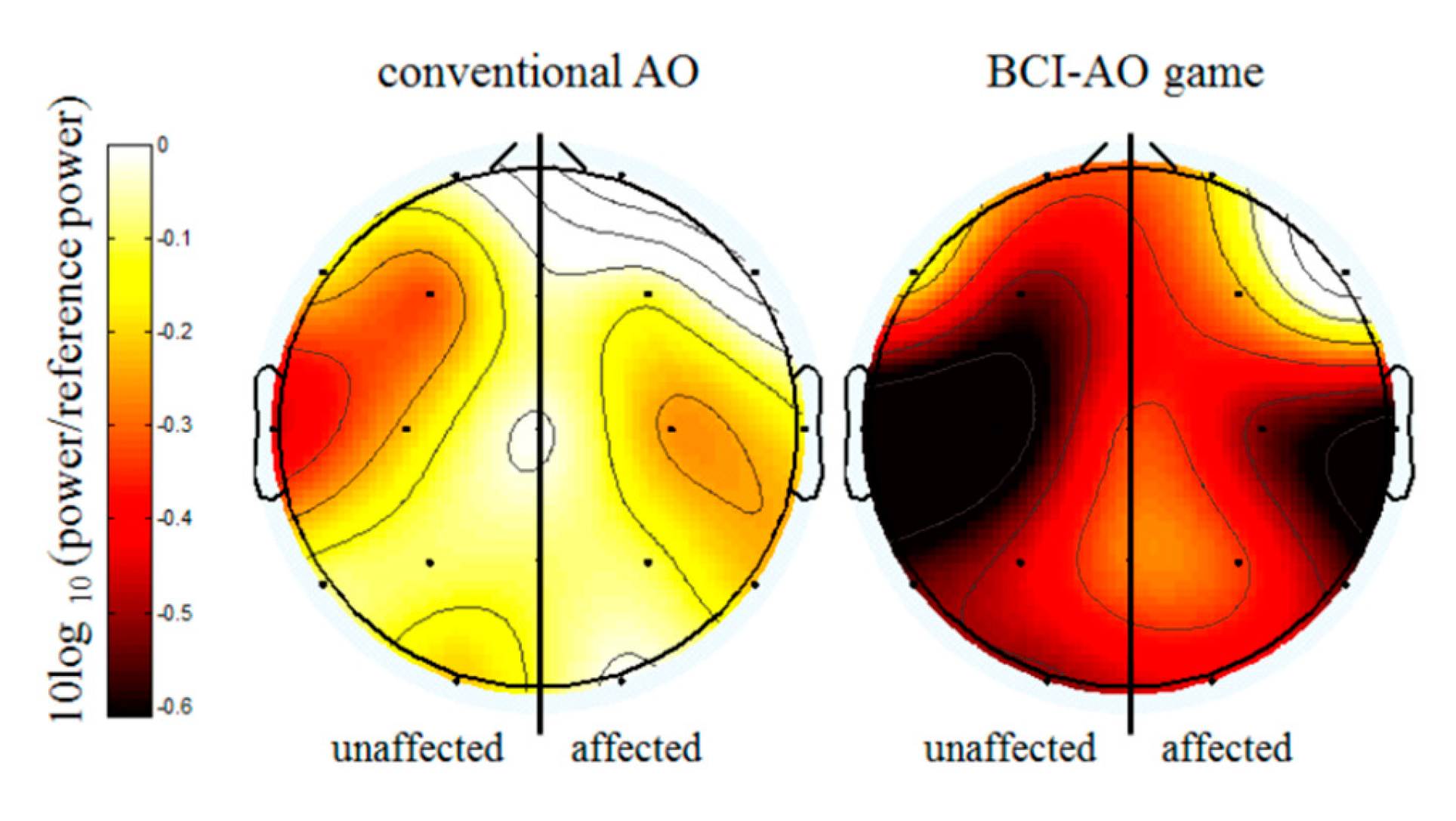
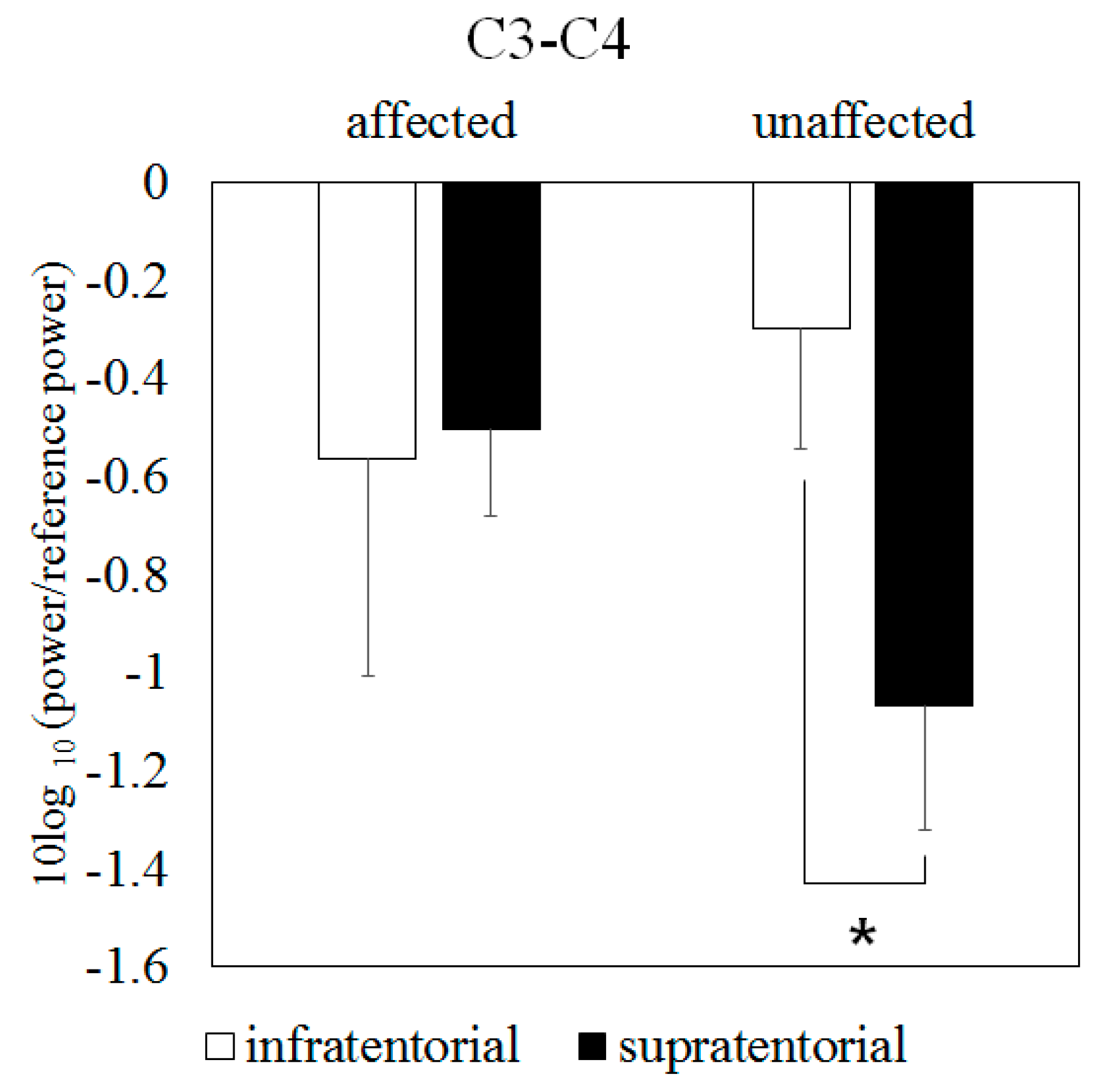
3.3. Mu Suppression According to Affected/Unaffected Hemisphere
3.4. Mu Suppression According to Brain-Lesion Location
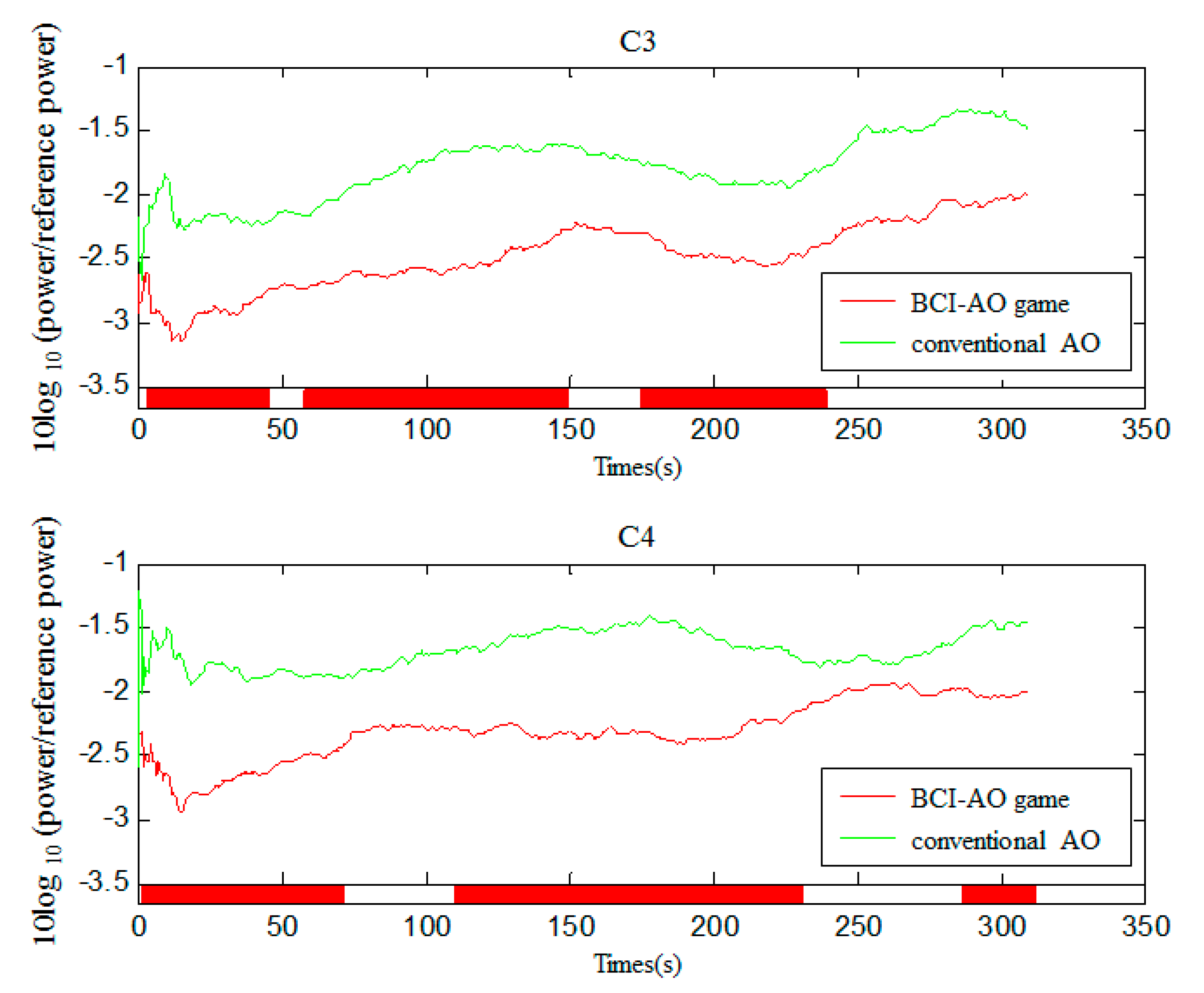
3.5. Mu Suppression According to Time Course
3.6. The Effects of Motor-Impairment Severity on Mu Suppression in the BCI-AO Game
3.7. Comparison of Mu Suppression between Stroke Patients and Healthy Subjects
3.8. Behavioral Results in the BCI-AO Game
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broeks, J.G.; Lankhorst, G.J.; Rumping, K.; Prevo, A.J. The long-term outcome of arm function after stroke: Results of a follow-up study. Disabil. Rehabil. 1999, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gillot, A.J.; Holder-Walls, A.; Kurtz, J.R.; Varley, N.C. Perceptions and experiences of two survivors of stroke who participated in constraint-induced movement therapy home programs. Am. J. Occup. Ther. 2003, 57, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Langton-Hewer, R.; Wood, V.A.; Skilbeck, C.E.; Ismail, H.M. The hemiplegic arm after stroke: Measurement and recovery. J. Neurol. Neurosurg. Psychiatry 1983, 46, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Fumagalli, S.; Pagani, E.; Gatti, R.; Riccitelli, G.C.; Preziosa, P.; Comi, G.; Falini, A.; Filippi, M. Action observation training modifies brain gray matter structure in healthy adult individuals. Brain Imaging Behav. 2017, 11, 1343–1352. [Google Scholar] [CrossRef]
- Shepherd, R.B. Exercise and training to optimize functional motor performance in stroke: Driving neural reorganization? Neural Plast. 2001, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Ono, Y.; Matsubara, M.; Ohmatsu, S.; Yukawa, Y.; Kohno, M.; Tominaga, T. Action observation facilitates motor cortical activity in patients with stroke and hemiplegia. Neurosci. Res. 2018, 133, 7–14. [Google Scholar] [CrossRef]
- Stefan, K.; Cohen, L.G.; Duque, J.; Mazzocchio, R.; Celnik, P.; Sawaki, L.; Ungerleider, L.; Classen, J. Formation of a motor memory by action observation. J. Neurosci. 2005, 25, 9339–9346. [Google Scholar] [CrossRef]
- Cross, E.S.; Hamilton, A.F.; Grafton, S.T. Building a motor simulation de novo: Observation of dance by dancers. Neuroimage 2006, 31, 1257–1267. [Google Scholar] [CrossRef]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V.; Massucci, M.; Schifini, F.; Sale, P. Clinical relevance of action observation in upper-limb stroke rehabilitation: A possible role in recovery of functional dexterity. A randomized clinical trial. Neurorehabilit. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef]
- Harmsen, W.J.; Bussmann, J.B.; Selles, R.W.; Hurkmans, H.L.; Ribbers, G.M. A mirror therapy-based action observation protocol to improve motor learning after stroke. Neurorehabilit. Neural Repair 2015, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Sugg, K.; Muller, S.; Winstein, C.; Hathorn, D.; Dempsey, A. Does action observation training with immediate physical practice improve hemiparetic upper-limb function in chronic stroke? Neurorehabilit. Neural Repair 2015, 29, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.A.; Winstein, C.J.; Aziz-Zadeh, L. The mirror neuron system: A neural substrate for methods in stroke rehabilitation. Neurorehabilit. Neural Repair 2010, 24, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Franceschini, M. Action observation and mirror neuron network: A tool for motor stroke rehabilitation. Eur. J. Phys. Rehabil. Med. 2012, 48, 313–318. [Google Scholar]
- Naro, A.; Calabrò, R.S.; Leo, A.; Russo, M.; Milardi, D.; Cannavò, A.; Manuli, A.; Buda, A.; Casella, C.; Bramanti, P.; et al. Bridging the Gap Towards Awareness Detection in Disorders of Consciousness: An Experimental Study on the Mirror Neuron System. Brain Topogr. 2018, 31, 623–639. [Google Scholar] [CrossRef]
- Chen, W.; Ye, Q.; Ji, X.; Zhang, S.; Yang, X.; Zhou, Q.; Cong, F.; Chen, W.; Zhang, X.; Zhang, B.; et al. Mirror neuron system based therapy for aphasia rehabilitation. Front. Psychol. 2015, 6, 1665. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Bentin, S.; Perry, A.; Liebermann, D.G.; Soroker, N. Mirror-neuron system recruitment by action observation: Effects of focal brain damage on mu suppression. Neuroimage 2014, 87, 127–137. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Johnson, B.W. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology 2004, 41, 152–156. [Google Scholar] [CrossRef]
- Arnstein, D.; Cui, F.; Keysers, C.; Maurits, N.M.; Gazzola, V. Mu-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 2011, 31, 14243–14249. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Brunner, C.; Schlogl, A.; Lopes da Silva, F.H. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage 2006, 31, 153–159. [Google Scholar] [CrossRef]
- Fecteau, S.; Dickler, M.; Pelayo, R.; Kumru, H.; Bernabeu, M.; Opisso Salleras, E.; Tormos, J.M.; Pascual-Leone, A. Cortical excitability during passive action observation in hospitalized adults with subacute moderate to severe traumatic brain injury: A preliminary TMS study. Neurorehabilit. Neural Repair 2015, 29, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Monge-Pereira, E.; Ibanez-Pereda, J.; Alguacil-Diego, I.M.; Serrano, J.I.; Spottorno-Rubio, M.P.; Molina-Rueda, F. Use of electroencephalography brain-computer interface systems as a rehabilitative approach for upper limb function after a stroke: A systematic review. PM R 2017, 9, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Lew, E.; Chavarriaga, R.; Silvoni, S.; Millan Jdel, R. Detection of self-paced reaching movement intention from EEG signals. Front. Neuroeng. 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Martisius, I.; Damasevicius, R. A Prototype SSVEP based real time BCI gaming system. Comput. Intell. Neurosci. 2016, 2016, 3861425. [Google Scholar] [CrossRef] [PubMed]
- Guger, C.; Allison, B.Z.; Großwindhager, B.; Prückl, R.; Hintermüller, C.; Kapeller, C.; Bruckner, M.; Krausz, G.; Edlinger, G. How many people could use an SSVEP BCI? Front. Neurosci. 2012, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Cinetto, A.; Wang, Y.; Chen, X.; Gao, S.; Gao, X. An online hybrid BCI system based on SSVEP and EMG. J. Neural. Eng. 2016, 13, 026020. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, W.; Wang, Y.K.; Hussain, F.K.; Al-Jumaily, A.; Lin, C.T. Effects of repetitive SSVEPs on EEG complexity using multiscale inherent fuzzy entropy. Neurocomputing 2019. [Google Scholar] [CrossRef]
- Lin, C.; Chiu, C.; Singh, A.K.; King, J.; Wang, Y.A. Wireless Multifunctional SSVEP-Based Brain Computer Interface Assistive System. IEEE Trans. Cogn. Dev. Syst. 2018, 11, 375–383. [Google Scholar] [CrossRef]
- Ko, L.W.; Lu, Y.C.; Bustince, H.; Chang, Y.C.; Chang, Y.; Ferandez, J.; Wang, Y.K.; Sanz, J.A.; Dimuro, G.P.; Lin, C.T. Multimodal fuzzy fusion for enhancing the motor-imagery-based brain computer interface. IEEE Comput. Intell. Mag. 2019, 14, 96–106. [Google Scholar] [CrossRef]
- Lim, H.; Ku, J. Flickering exercise video produces mirror neuron system (MNS) activation and steady state visually evoked potentials (SSVEPs). Biomed. Eng. Lett. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- Lim, H.; Ku, J. A brain-computer interface-based action observation game that enhances mu suppression. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Buccino, G.; Solodkin, A.; Small, S.L. Functions of the mirror neuron system: Implications for neurorehabilitation. Cogn. Behav. Neurol. 2006, 19, 55–63. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef]
- Modrono, C.; Navarrete, G.; Rodriguez-Hernandez, A.F.; Gonzalez-Mora, J.L. Activation of the human mirror neuron system during the observation of the manipulation of virtual tools in the absence of a visible effector limb. Neurosci. Lett. 2013, 555, 220–224. [Google Scholar] [CrossRef]
- Nedelko, V.; Hassa, T.; Hamzei, F.; Weiller, C.; Binkofski, F.; Schoenfeld, M.A.; Tüscher, O.; Dettmers, C. Age-independent activation in areas of the mirror neuron system during action observation and action imagery. A fMRI study. Restor. Neurol. Neurosci. 2010, 28, 737–747. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.; Lee, H.S.; Shin, K.H.; Kim, M.J.; Son, E. Differences in brain waves of normal persons and stroke patients during action observation and motor imagery. J. Phys. Ther. Sci. 2014, 26, 215–218. [Google Scholar] [CrossRef]
- Caspers, S.; Zilles, K.; Laird, A.R.; Eickhoff, S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010, 50, 1148–1167. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Gallivan, J.P. Functional coupling between frontoparietal and occipitotemporal pathways during action and perception. Cortex 2018, 98, 8–27. [Google Scholar] [CrossRef]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gallivan, J.P.; Culham, J.C. Neural coding within human brain areas involved in actions. Curr. Opin. Neurobiol. 2015, 33, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.C.; Skouen, J.S.; Ersland, L.; Gruner, R. Plasticity and response to action observation: A longitudinal FMRI study of potential mirror neurons in patients with subacute stroke. Neurorehabilit. Neural Repair 2014, 28, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.A.; Aziz-Zadeh, L.; Wong, S.W.; Liew, S.L.; Winstein, C.J. Modulating the motor system by action observation after stroke. Stroke 2013, 44, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage 2007, 36 (Suppl. 2), T164–T173. [Google Scholar] [CrossRef]
- Capotosto, P.; Babiloni, C.; Romani, G.L.; Corbetta, M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J. Neurosci. 2009, 29, 5863–5872. [Google Scholar] [CrossRef]
- Capotosto, P.; Babiloni, C.; Romani, G.L.; Corbetta, M. Differential contribution of right and left parietal cortex to the control of spatial attention: A simultaneous EEG-rTMS study. Cereb. Cortex. 2012, 22, 446–454. [Google Scholar] [CrossRef]
- Perry, A.; Bentin, S. Mirror activity in the human brain while observing hand movements: A comparison between EEG desynchronization in the mu-range and previous fMRI results. Brain Res. 2009, 1282, 126–132. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Sager, W.; Wege, W. Correlations between CT scan and sensorimotor EEG rhythms in patients with cerebrovascular disorders. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 473–485. [Google Scholar] [CrossRef]
- Stepien, M.; Conradi, J.; Waterstraat, G.; Hohlefeld, F.U.; Curio, G.; Nikulin, V.V. Event-related desynchronization of sensorimotor EEG rhythms in hemiparetic patients with acute stroke. Neurosci. Lett. 2011, 488, 17–21. [Google Scholar] [CrossRef]
- Park, W.; Kwon, G.H.; Kim, Y.H.; Lee, J.H.; Kim, L. EEG response varies with lesion location in patients with chronic stroke. J. Neuroeng. Rehabil. 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Butefisch, C.M.; Wessling, M.; Netz, J.; Seitz, R.J.; Homberg, V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabilit. Neural Repair 2008, 22, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ku, J.; Kim, H.J.; Park, H.K. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann. Rehabil. Med. 2011, 35, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Tangwiriyasakul, C.; Verhagen, R.; Rutten, W.L.; van Putten, M.J. Temporal evolution of event-related desynchronization in acute stroke: A pilot study. Clin. Neurophysiol. 2014, 125, 1112–1120. [Google Scholar] [CrossRef]
- Buetefisch, C.M. Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front. Neurol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Riecker, A.; Groschel, K.; Ackermann, H.; Schnaudigel, S.; Kassubek, J.; Kastrup, A. The role of the unaffected hemisphere in motor recovery after stroke. Hum. Brain Mapp. 2010, 31, 1017–1029. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Joseph, E.; Gopal, J.; Kuah, C.W.K.; Chua, K.S.G. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef]
- Young, B.M.; Nigogosyan, Z.; Nair, V.A.; Walton, L.M.; Song, J.; Tyler, M.E.; Edwards, D.F.; Caldera, K.; Sattin, J.A.; Williams, J.C.; et al. Case report: Post-stroke interventional BCI rehabilitation in an individual with preexisting sensorineural disability. Front. Neuroeng. 2014, 7, 18. [Google Scholar] [CrossRef]
- Lafleur, M.F.; Jackson, P.L.; Malouin, F.; Richards, C.L.; Evans, A.C.; Doyon, J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage 2002, 16, 142–157. [Google Scholar] [CrossRef]
- Rowe, J.B.; Chan, V.; Ingemanson, M.L.; Cramer, S.C.; Wolbrecht, E.T.; Reinkensmeyer, D.J. Robotic assistance for training finger movement using a Hebbian model: A randomized controlled trial. Neurorehabilit. Neural Repair 2017, 31, 769–780. [Google Scholar] [CrossRef]
| Patient No. | Sex | Age | Days Since Onset | Etiology | Site of Lesion | mRS | FMA Upper | MMSE | MBI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | 42 | Hemorrhage | Lt. thalamus (subcortical) | 3 | 54 | 28 | 57 |
| 2 | M | 61 | 27 | Infarction | Lt. med. medullary | 2 | 63 | 29 | 76 |
| 3 | F | 74 | 75 | Infarction | Lt. pontine | 2 | 61 | 27 | 89 |
| 4 | M | 72 | 8 | Infarction | Lt. MCA, PCA (cortical) | 4 | 58 | 28 | 50 |
| 5 | M | 56 | 8 | Infarction | Lt. thalamus (subcortical) | 2 | 62 | 27 | 86 |
| 6 | F | 47 | 8 | Infarction | Rt. Cerebellar | 2 | 62 | 30 | 95 |
| 7 | F | 88 | 9 | Hemorrhage | Rt. MCA (subcortical) | 2 | 62 | 26 | 91 |
| 8 | F | 88 | 16 | Infarction | Lt. pontine | 4 | 59 | 25 | 8 |
| 9 | M | 69 | 26 | Hemorrhage | Rt. MCA (subcortical) | 4 | 28 | 30 | 53 |
| 10 | M | 82 | 30 | Infarction | Rt. Cerebellar | 2 | 61 | 28 | 78 |
| 11 | M | 61 | 24 | Hemorrhage | Rt. MCA (subcortical) | 3 | 64 | 25 | 54 |
| 12 | M | 78 | 14 | Hemorrhage | Rt. MCA (cortical) | 5 | 4 | 25 | 2 |
| 13 | M | 64 | 9 | Hemorrhage | Rt. MCA (subcortical) | 1 | 63 | 26 | 98 |
| 14 | M | 67 | 8 | Infarction | Rt. lat. medullary | 4 | 64 | 30 | 57 |
| 15 | F | 63 | 9 | Hemorrhage | Rt. Pontine | 4 | 62 | 29 | 76 |
| 16 | M | 73 | 50 | Infarction | Lt. MCA (subcortical) | 3 | 48 | 25 | 77 |
| 17 | M | 80 | 51 | Infarction | Lt. MCA (subcortical) | 3 | 50 | 25 | 83 |
| 18 | M | 66 | 52 | Infarction | Rt. PCA (subcortical) | 4 | 32 | 28 | 66 |
| 19 | F | 74 | 26 | Infarction | Lt. Cerebellar | 3 | 63 | 25 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lim, H.; Kim, J.W.; Kang, Y.J.; Ku, J. Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke. Electronics 2019, 8, 1466. https://doi.org/10.3390/electronics8121466
Choi H, Lim H, Kim JW, Kang YJ, Ku J. Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke. Electronics. 2019; 8(12):1466. https://doi.org/10.3390/electronics8121466
Chicago/Turabian StyleChoi, Hyoseon, Hyunmi Lim, Joon Woo Kim, Youn Joo Kang, and Jeonghun Ku. 2019. "Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke" Electronics 8, no. 12: 1466. https://doi.org/10.3390/electronics8121466
APA StyleChoi, H., Lim, H., Kim, J. W., Kang, Y. J., & Ku, J. (2019). Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke. Electronics, 8(12), 1466. https://doi.org/10.3390/electronics8121466





