Incremental Low Rank Noise Reduction for Robust Infrared Tracking of Body Temperature during Medical Imaging
Abstract
1. Introduction
3D Analysis
- Registration of depth to RGB (in order to align depth map with RGB image)
- Applying speeded up robust features speeded up robust features (SURF) [53] to both images to find keypoints;
- Comparing keypoints’ descriptors to find most similar points
- Estimating the required rotation and translation matrices to register keypoints pairs using iterative closest points (ICP) [54]
- Performing the transformations and calculating the final 3D coordinates
- Accumulating the 3D coordinates and their colors in the final point cloud matrix
- Visualize the point cloud using point cloud library (PCL) [55].
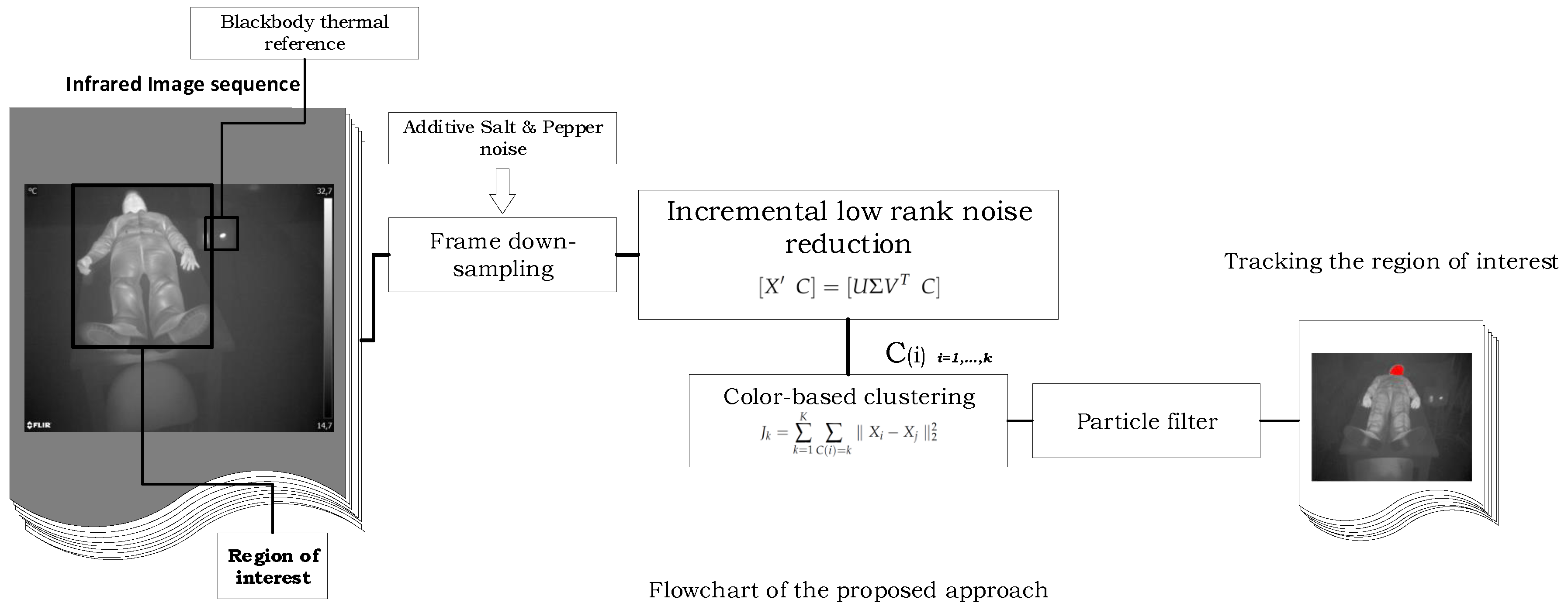
2. Incremental Low Rank Robust Tracking
2.1. Low Rank Noise Reduction
2.2. Incremental SVD
2.3. Clustering and Tracking
2.4. Particle Filter
- -
- Thermal images are gray scale (0,255) corresponds to cold and hot representation;
- -
- Thermal camera’s field of view (FOV) always has ROI;
- -
- The ROI’s temperature is higher than the surrounding temperature;
- -
- The ROI does not have a particular shape and is adjustable in the algorithm with the respect to thermal increases (elevating image intensity);
- -
- The ROI updates during the experiment (simulating medical test) and temperature updates by an upward trend to find hot spots which are cause of the burning in patients.
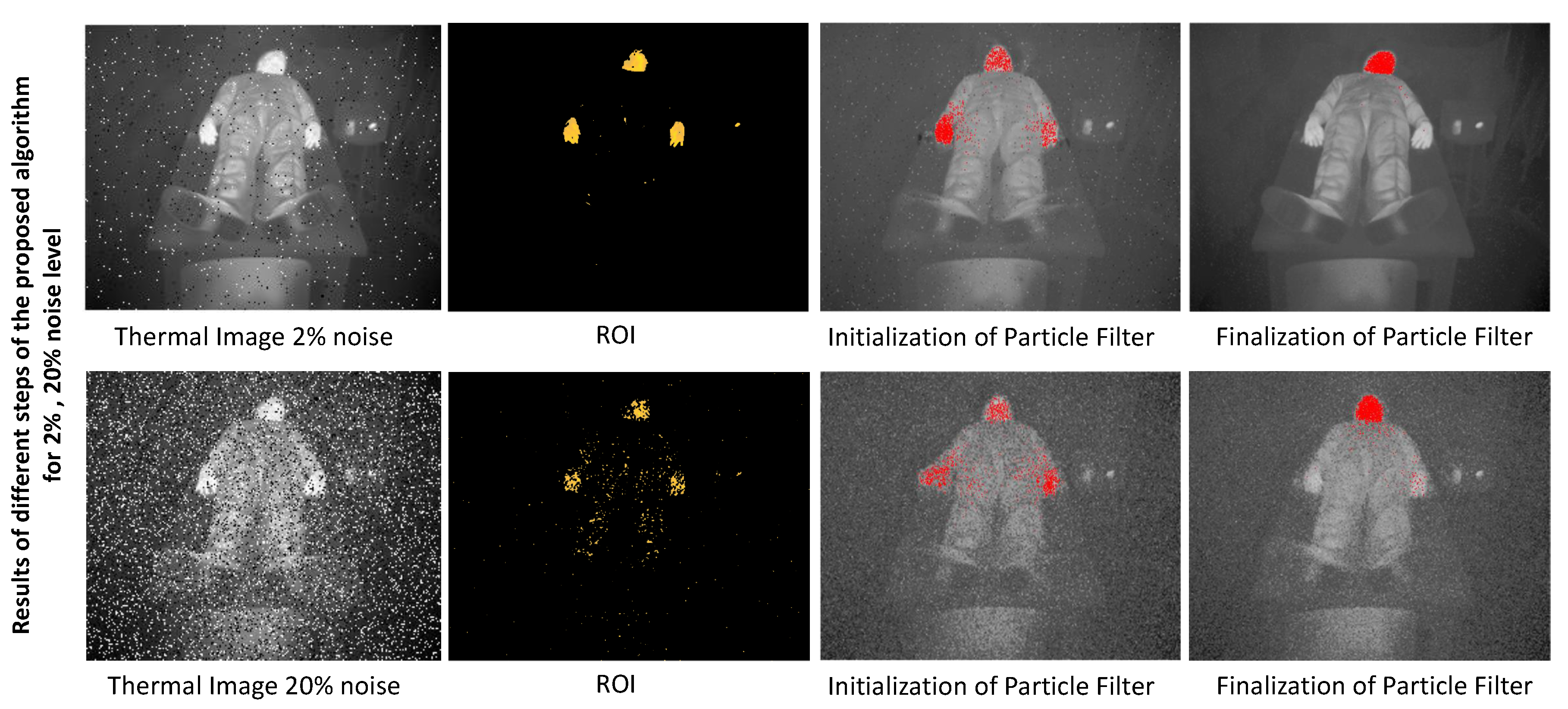
3. Results
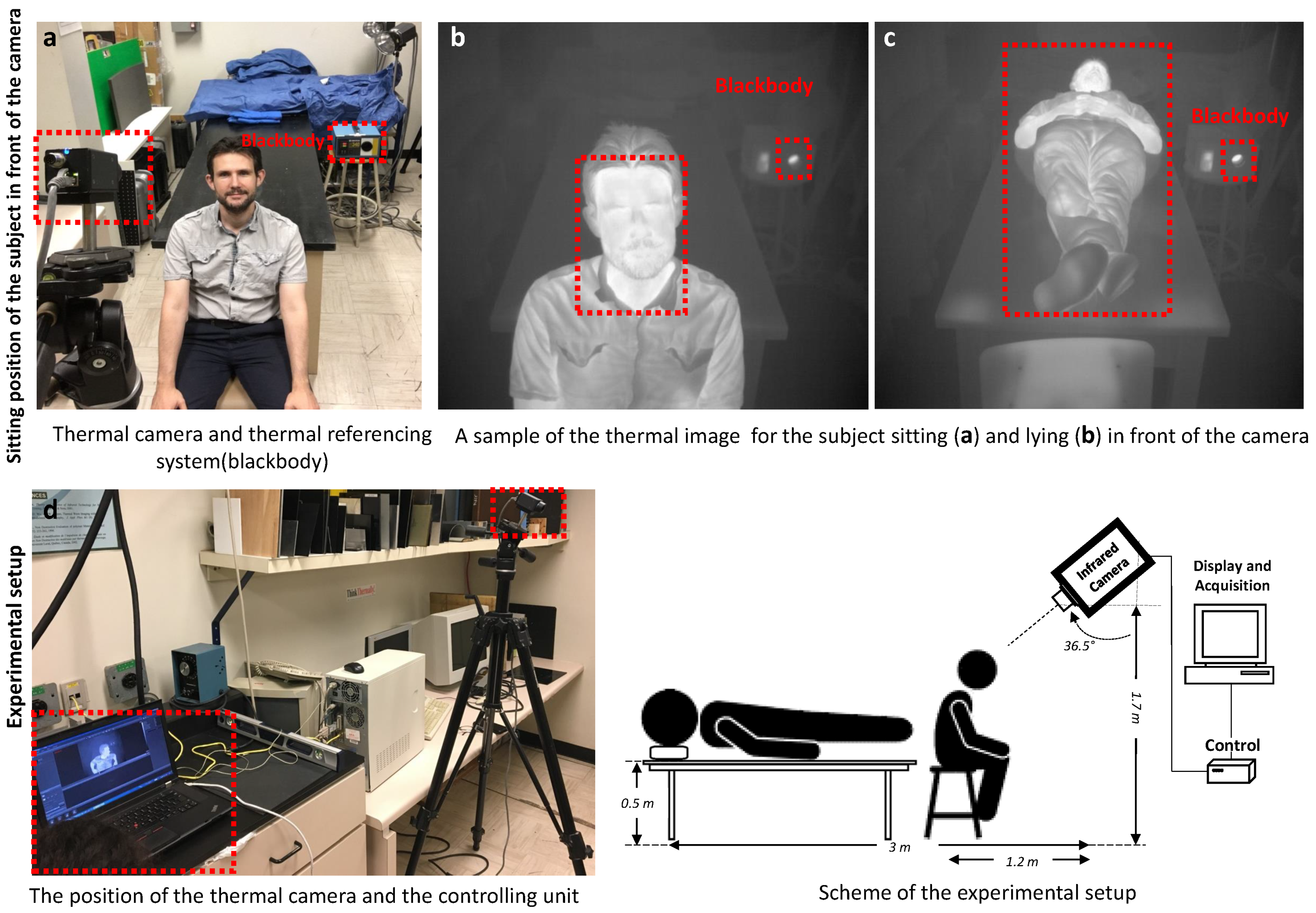
3.1. Experimental Setup and Thermal Image Database
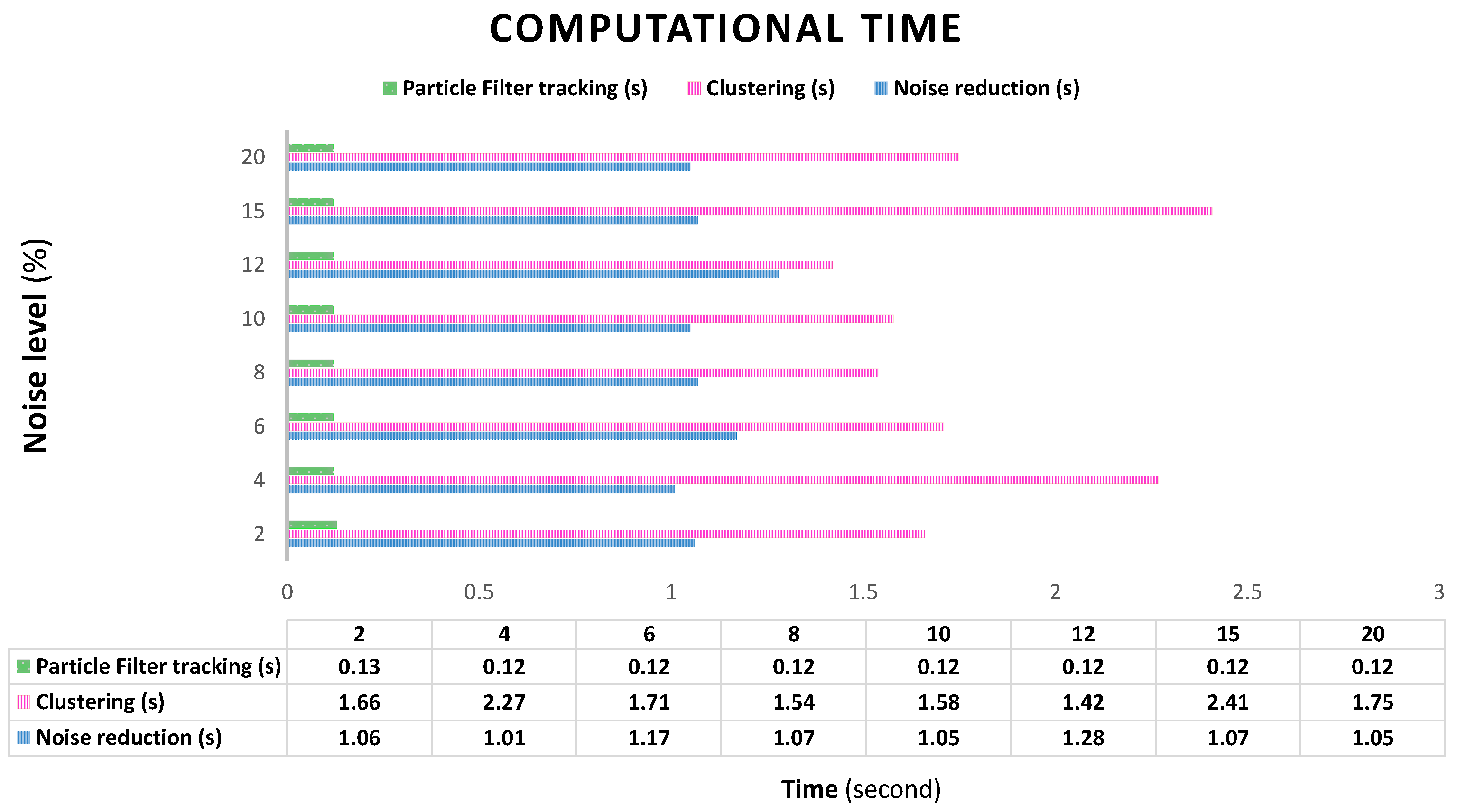
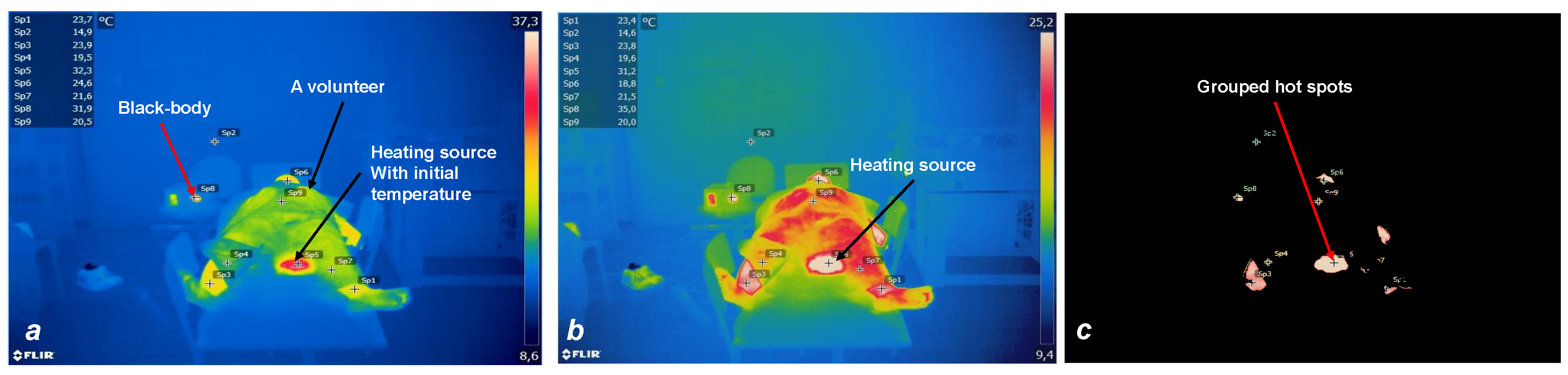
3.2. System Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROI | region of interest |
| SVD | singular value decomposition |
| FOV | field of view |
| 3D | three dimension |
| PCL | point cloud library |
| ICP | iterative closest points |
References
- Jones, B.F. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lu, H.; Zhao, B.; Liu, J.; Zhao, M. Micro-Motion Dynamics and Shape Parameters Estimation Based on an Infrared Signature Model of Spatial Targets. Electronics 2019, 8, 755. [Google Scholar] [CrossRef]
- Abdel-Nasser, M.; Moreno, A.; Puig, D. Breast Cancer Detection in Thermal Infrared Images Using Representation Learning and Texture Analysis Methods. Electronics 2019, 8, 100. [Google Scholar] [CrossRef]
- Etehadtavakol, M.; Ng, E.Y. Potential of Thermography in Pain Diagnosing and Treatment Monitoring. In Application of Infrared to Biomedical Sciences; Springer: New York, NY, USA, 2017; pp. 19–32. [Google Scholar]
- Manohar, S.; Vaartjes, S.E.; van Hespen, J.C.; Klaase, J.M.; van den Engh, F.M.; Steenbergen, W.; Van Leeuwen, T.G. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express 2007, 15, 12277–12285. [Google Scholar] [CrossRef] [PubMed]
- Merla, D.A.; Tsiamyrtzis, P.; Pavlidis, I. Imaging Facial Signs of Neurophysiological Responses. IEEE Trans. Biomed. Eng. 2009, 56, 477–484. [Google Scholar]
- Tabatabaei, N.; Mandelis, A.; Amaechi, B.T. Thermophotonic lock-in imaging of early demineralized and carious lesions in human teeth. J. Biomed. Opt. 2011, 16, 071402. [Google Scholar] [CrossRef]
- Chiang, M.F.; Lin, P.W.; Lin, L.F.; Chiou, H.Y.; Chien, C.W.; Chu, S.F.; Chiu, W.T. Mass screening of suspected febrile patients with remote-sensing infrared thermography: Alarm temperature and optimal distance. J. Formos. Med. Assoc. 2008, 107, 937–944. [Google Scholar] [CrossRef]
- Dempsey, M.F.; Condon, B.; Hadley, D.M. Investigation of the factors responsible for burns during MRI. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2001, 13, 627–631. [Google Scholar] [CrossRef]
- Shellock, F.G.; Kanal, E. Burns associated with the use of monitoring equipment during MR procedures. J. Magn. Reson. Imaging 1996, 6, 271–272. [Google Scholar] [CrossRef]
- Brown, T.; Goldstein, B.; Little, J. Severe burns resulting from magnetic resonance imaging with cardiopulmonary monitoring. Risks and relevant safety precautions. Am. J. Phys. Med. Rehabi. 1993, 72, 166–167. [Google Scholar]
- Bashein, G.; Syrovy, G. Burns associated with pulse oximetry during magnetic resonance imaging. Anesthesiol. J. Am. Soc. Anesthesiol. 1991, 75, 382. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.C.; Stevenson, G.; Suresh, S. Burn associated with temperature monitoring during magnetic resonance imaging. Anesthesiol. J. Am. Soc. Anesthesiol. 1992, 76, 152. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.J.; de Leeuw, R.; Carlson, C.R.; Okeson, J.P.; Miller, C.S.; Andersen, A.H. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain 2006, 122, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, L.; Allen, P.J.; Franconi, F.; Symms, M.R.; Fish, D.K. Recording of EEG during fMRI experiments: Patient safety. Magn. Reson. Med. 1997, 38, 943–952. [Google Scholar] [CrossRef]
- Simi, S.; Ballardin, M.; Casella, M.; De Marchi, D.; Hartwig, V.; Giovannetti, G.; Vanello, N.; Gabbriellini, S.; Landini, L.; Lombardi, M. Is the genotoxic effect of magnetic resonance negligible? Low persistence of micronucleus frequency in lymphocytes of individuals after cardiac scan. Mutat. Res./Fund. Mol. Mech. Mutagenesis 2008, 645, 39–43. [Google Scholar] [CrossRef]
- Bonassi, S.; Znaor, A.; Ceppi, M.; Lando, C.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Ban, S.; Barale, R.; et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007, 28, 625–631. [Google Scholar] [CrossRef]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Lombardi, M.; Landini, L.; Simi, S. Biological effects and safety in magnetic resonance imaging: A review. Int. J. Environ. Res. Public Health 2009, 6, 1778–1798. [Google Scholar] [CrossRef]
- Jin, J. Electromagnetic Analysis and Design in Magnetic Resonance Imaging; Routledge: London, UK, 2018. [Google Scholar]
- Pykett, I.L. NMR imaging in medicine. Sci. Am. 1982, 246, 78–91. [Google Scholar] [CrossRef]
- Rodgers, P.M.; Ward, J.; Baudouin, C.J.; Ridgway, J.P.; Robinson, P.J. Dynamic contrast-enhanced MR imaging of the portal venous system: Comparison with x-ray angiography. Radiology 1994, 191, 741–745. [Google Scholar] [CrossRef]
- Laakman, R.; Kaufman, B.; Han, J.; Nelson, A.; Clampitt, M.; O’Block, A.; Haaga, J.; Alfidi, R. MR imaging in patients with metallic implants. Radiology 1985, 157, 711–714. [Google Scholar] [CrossRef]
- Morgan, D.E.; Kenney, P.J.; Meeks, M.C.; Pile, N.S. MR imaging of breast implants and their complications. AJR Am. J. Roentgenol. 1996, 167, 1271–1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Formica, D.; Silvestri, S. Biological effects of exposure to magnetic resonance imaging: An overview. Biomed. Eng. Online 2004, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G. Radiofrequency energy-induced heating during MR procedures: A review. J. Magn. Reson. Imaging 2000, 12, 30–36. [Google Scholar] [CrossRef]
- Shellock, F.G.; Rothman, B.; Sarti, D. Heating of the scrotum by high-field-strength MR imaging. AJR Am. J. Roentgenol. 1990, 154, 1229–1232. [Google Scholar] [CrossRef]
- Shellock, F.; Crues, J.V. Corneal temperature changes induced by high-field-strength MR imaging with a head coil. Radiology 1988, 167, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Vahlensieck, M. Tattoo-related cutaneous inflammation (burn grade I) in a mid-field MR scanner. Eur. Radiol. 2000, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Wagle, W.A.; Smith, M. Tattoo-induced skin burn during MR imaging. Am. J. Roentgenol. 2000, 174, 1795. [Google Scholar] [CrossRef]
- Kreidstein, M.; Giguere, D.; Freiberg, A. MRI interaction with tattoo pigments: Case report, pathophysiology, and management. Plast. Reconstr. Surg. 1997, 99, 1717–1720. [Google Scholar] [CrossRef]
- Cleveland, R.F., Jr.; Athey, T.W. Specific absorption rate (SAR) in models of the human head exposed to hand-held UHF portable radios. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1989, 10, 173–186. [Google Scholar] [CrossRef]
- International Electrotechnical Commission. Particular Requirements for the Basic Safety and Essential Performance of Magnetic Resonance Equipment for Medical Diagnosis; Technical Report; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2010; p. 60601-2-33. [Google Scholar]
- Adair, E.R.; Berglund, L.G. On the thermoregulatory consequences of NMR imaging. Magn. Reson. Imaging 1986, 4, 321–333. [Google Scholar] [CrossRef]
- Shellock, F.G.; Schaefer, D.J.; Crues, J.V. Alterations in body and skin temperatures caused by MRI. Br. J. Radiol. 1990, 63, 317. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.A.; Van den Bergen, B.; Van de Kamer, J.B.; Raaymakers, B.W.; Kroeze, H.; Bartels, L.W.; Lagendijk, J.J. Simultaneous B homogenization and specific absorption rate hotspot suppression using a magnetic resonance phased array transmit coil. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2007, 57, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, H.; Crozier, S. Calculation of electric fields induced by body and head motion in high-field MRI. J. Magn. Reson. 2003, 161, 99–107. [Google Scholar] [CrossRef]
- Stathopoulos, I.; Skouroliakou, K.; Michail, C.; Valais, I. Dynamic infrared thermography study of blood flow relative to lower limp position. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2015; Volume 637, p. 012027. [Google Scholar]
- Yousefi, B.; Fleuret, J.; Zhang, H.; Maldague, X.P.; Watt, R.; Klein, M. Automated assessment and tracking of human body thermal variations using unsupervised clustering. Appl. Opt. 2016, 55, D162–D172. [Google Scholar] [CrossRef]
- Bilodeau, G.A.; Torabi, A.; Lévesque, M.; Ouellet, C.; Langlois, J.P.; Lema, P.; Carmant, L. Body temperature estimation of a moving subject from thermographic images. Mach. Vis. Appl. 2012, 23, 299–311. [Google Scholar] [CrossRef]
- Kennedy, H.V. Modeling noise in thermal imaging systems. Proc. SPIE 1993, 1969, 66–70. [Google Scholar]
- Watts, M.R.; Shaw, M.J.; Nielson, G.N. Optical resonators: Microphotonic thermal imaging. Nat. Photonics 2007, 1, 632–634. [Google Scholar] [CrossRef]
- Cardone, D.; Pinti, P.; Merla, A. Thermal infrared imaging-based computational psychophysiology for psychometrics. Comput. Math. Methods Med. 2015, 2015. [Google Scholar] [CrossRef]
- Manabe, M.; Yamazaki, H.; Sakai, K. Shape factor simulation for the thermal radiation environment of the human body and the VRML visualization. Build. Environ. 2004, 39, 927–937. [Google Scholar] [CrossRef]
- Badel, E.; Delisee, C.; Lux, J. 3D structural characterisation, deformation measurements and assessment of low-density wood fibreboard under compression: The use of X-ray microtomography. Compos. Sci. Technol. 2008, 68, 1654–1663. [Google Scholar] [CrossRef][Green Version]
- Jaganathan, S.; Tafreshi, H.V.; Pourdeyhimi, B. Modeling liquid porosimetry in modeled and imaged 3-D fibrous microstructures. J. Colloid Interface Sci. 2008, 326, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.S.; Bai, J.; Chen, Y. A high-resolution three-dimensional far-infrared thermal and true-color imaging system for medical applications. Med. Eng. Phys. 2009, 31, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Locke, B.R. Jet fuel toxicity: Skin damage measured by 900-MHz MRI skin microscopy and visualization by 3D MR image processing. Magn. Reson. Imaging 2010, 28, 1030–1048. [Google Scholar] [CrossRef] [PubMed]
- Markelj, P.; Tomaževič, D.; Likar, B.; Pernuš, F. A review of 3D/2D registration methods for image-guided interventions. Med. Image Anal. 2012, 16, 642–661. [Google Scholar] [CrossRef]
- Lagüela, S.; Armesto, J.; Arias, P.; Herráez, J. Automation of thermographic 3D modelling through image fusion and image matching techniques. Autom. Constr. 2012, 27, 24–31. [Google Scholar] [CrossRef]
- Schutt, D.J.; Swindle, M.M.; Helke, K.L.; Bastarrika, G.; Schwarz, F.; Haemmerich, D. Sequential activation of ground pads reduces skin heating during radiofrequency tumor ablation: In vivo porcine results. IEEE Trans. Biomed. Eng. 2009, 57, 746–753. [Google Scholar] [CrossRef]
- Vidas, S.; Moghadam, P. HeatWave: A handheld 3D thermography system for energy auditing. Energy Build. 2013, 66, 445–460. [Google Scholar] [CrossRef]
- Siewert, C.; Dänicke, S.; Kersten, S.; Brosig, B.; Rohweder, D.; Beyerbach, M.; Seifert, H. Difference method for analysing infrared images in pigs with elevated body temperatures. Zeitschrift für Medizinische Physik 2014, 24, 6–15. [Google Scholar] [CrossRef]
- Bay, H.; Tuytelaars, T.; Van Gool, L. Surf: Speeded up robust features. In European Conference on Computer Vision; Springer: New York, NY, USA, 2006; pp. 404–417. [Google Scholar]
- Chen, Y.; Medioni, G. Object modelling by registration of multiple range images. Image Vis. Comput. 1992, 10, 145–155. [Google Scholar] [CrossRef]
- Rusu, R.B.; Cousins, S. Point cloud library (pcl). In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 2011; pp. 1–4. [Google Scholar]
- Golub, G.H.; Van Loan, C.F. Matrix computations; JHU Press: Baltimore, MD, USA, 2012; Volume 3. [Google Scholar]
- Bunch, J.R.; Nielsen, C.P. Updating the singular value decomposition. Numer. Math. 1978, 31, 111–129. [Google Scholar] [CrossRef]
- Brand, M. Incremental singular value decomposition of uncertain data with missing values. In Computer Vision—ECCV 2002; Springer: Berlin/Heidelberg, Germany, 2002; pp. 707–720. [Google Scholar]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Yousefi, P.; Jalab, H.; Ibrahim, R.; Mohd Noor, N.; Ayub, M.; Gani, A. River segmentation using satellite image contextual information and Bayesian classifier. Imaging Sci. J. 2016, 64, 453–459. [Google Scholar] [CrossRef]
- Isard, M.; Blake, A. Condensation—Conditional density propagation for visual tracking. Int. J. Comput. Vis. 1998, 29, 5–28. [Google Scholar] [CrossRef]
- Hoseini, S.; Kabiri, P. A Novel Feature-Based Approach for Indoor Monocular SLAM. Electronics 2018, 7, 305. [Google Scholar] [CrossRef]
- Zhang, F.; Hancock, E.R.; Goodlett, C.; Gerig, G. Probabilistic white matter fiber tracking using particle filtering and von Mises–Fisher sampling. Med. Image Anal. 2009, 13, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Smal, I.; Meijering, E.; Draegestein, K.; Galjart, N.; Grigoriev, I.; Akhmanova, A.; Van Royen, M.; Houtsmuller, A.B.; Niessen, W. Multiple object tracking in molecular bioimaging by Rao-Blackwellized marginal particle filtering. Med. Image Anal. 2008, 12, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Smal, I.; Meijering, E. Quantitative comparison of multiframe data association techniques for particle tracking in time-lapse fluorescence microscopy. Med. Image Anal. 2015, 24, 163–189. [Google Scholar] [CrossRef]
- Pitt, M.K.; Shephard, N. Filtering via simulation: Auxiliary particle filters. J. Am. Stat. Assoc. 1999, 94, 590–599. [Google Scholar] [CrossRef]
- Bilodeau, G.A.; Ghali, R.; Desgent, S.; Langlois, P.; Farah, R.; St-Onge, P.L.; Duss, S.; Carmant, L. Where is the rat? Tracking in low contrast thermographic images. In Proceedings of the 2011 IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Colorado Springs, CO, USA, 2011; pp. 55–60. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, B.; Memarzadeh Sharifipour, H.; Eskandari, M.; Ibarra-Castanedo, C.; Laurendeau, D.; Watts, R.; Klein, M.; Maldague, X.P.V. Incremental Low Rank Noise Reduction for Robust Infrared Tracking of Body Temperature during Medical Imaging. Electronics 2019, 8, 1301. https://doi.org/10.3390/electronics8111301
Yousefi B, Memarzadeh Sharifipour H, Eskandari M, Ibarra-Castanedo C, Laurendeau D, Watts R, Klein M, Maldague XPV. Incremental Low Rank Noise Reduction for Robust Infrared Tracking of Body Temperature during Medical Imaging. Electronics. 2019; 8(11):1301. https://doi.org/10.3390/electronics8111301
Chicago/Turabian StyleYousefi, Bardia, Hossein Memarzadeh Sharifipour, Mana Eskandari, Clemente Ibarra-Castanedo, Denis Laurendeau, Raymond Watts, Matthieu Klein, and Xavier P. V. Maldague. 2019. "Incremental Low Rank Noise Reduction for Robust Infrared Tracking of Body Temperature during Medical Imaging" Electronics 8, no. 11: 1301. https://doi.org/10.3390/electronics8111301
APA StyleYousefi, B., Memarzadeh Sharifipour, H., Eskandari, M., Ibarra-Castanedo, C., Laurendeau, D., Watts, R., Klein, M., & Maldague, X. P. V. (2019). Incremental Low Rank Noise Reduction for Robust Infrared Tracking of Body Temperature during Medical Imaging. Electronics, 8(11), 1301. https://doi.org/10.3390/electronics8111301






